Auricularia auricula Melanin Protects against Alcoholic Liver Injury and Modulates Intestinal Microbiota Composition in Mice Exposed to Alcohol Intake
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of AAM
2.2. Structural Characterization of AAM by Ultraviolet-Visible, Fourier Transform Infrared (FT-IR), Gel Permeation Chromatography (GPC) and Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3. Animal Experimental Design
2.4. Biochemical Assays of the Serum and Liver Samples
2.5. Hematoxylin–Eosin (H&E) Staining of Liver and Ileum Section
2.6. High Throughput Sequencing Analysis of Intestinal Flora
2.7. Determination of Short Chain Fatty Acids (SCFAs)
2.8. Metabolomics Analysis of Liver
2.9. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Statistical
3. Results and Discussion
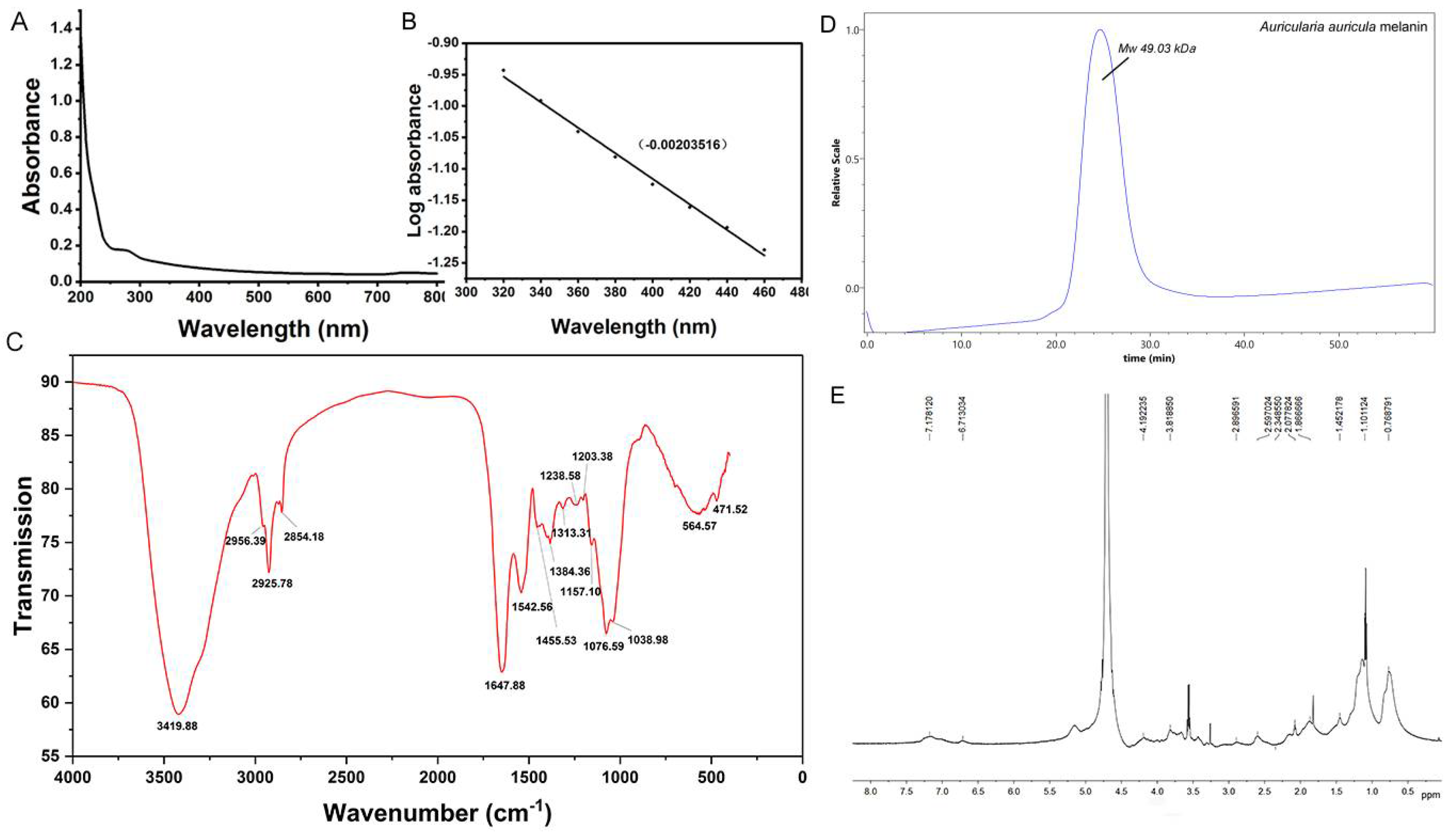
3.1. Morphological Analysis of AAM
3.1.1. UV-Visible Absorption Spectra of AAM
3.1.2. FT-IR Spectrometric Analysis and Molecular Weight Determination of AAM
H-NMR Spectrum and Elemental Analysis of AAM
3.2. Effects of AAM on Body Weight Growth and Liver Index
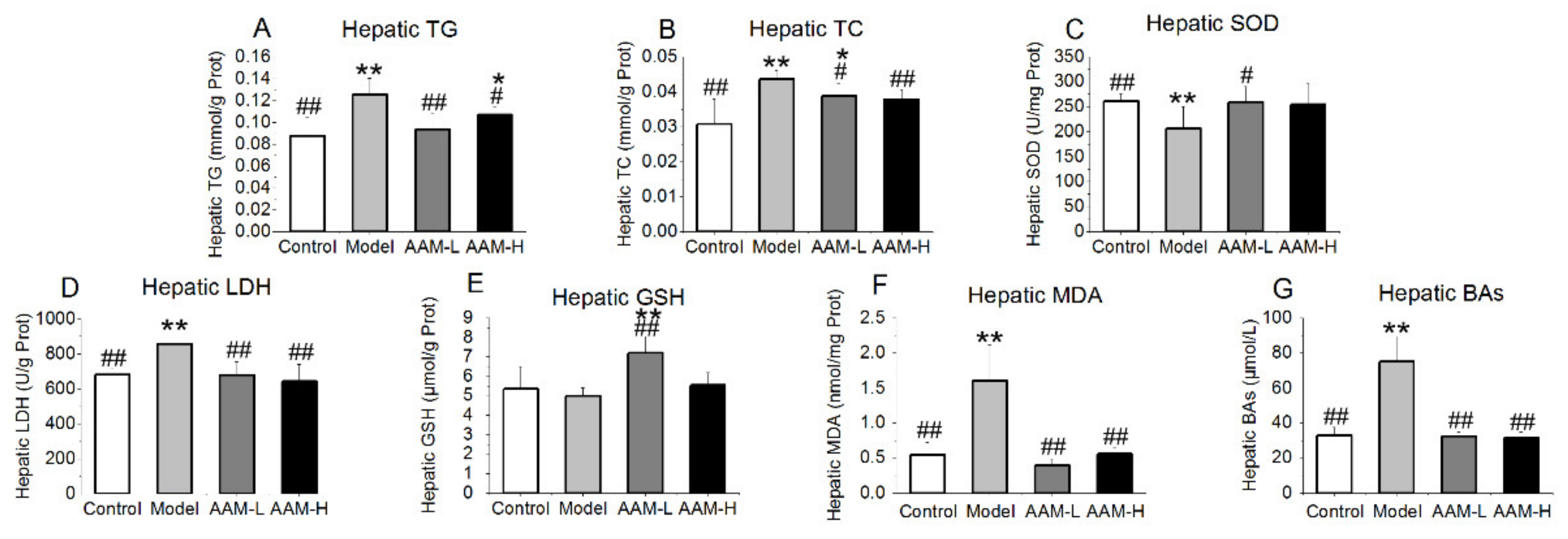
3.3. Effects of AAM on Serum Biochemical Profile
3.4. Effects of AAM on Liver Biochemical Parameters
3.5. Effects of AAM on Histopathological Features of Liver and Small Intestine
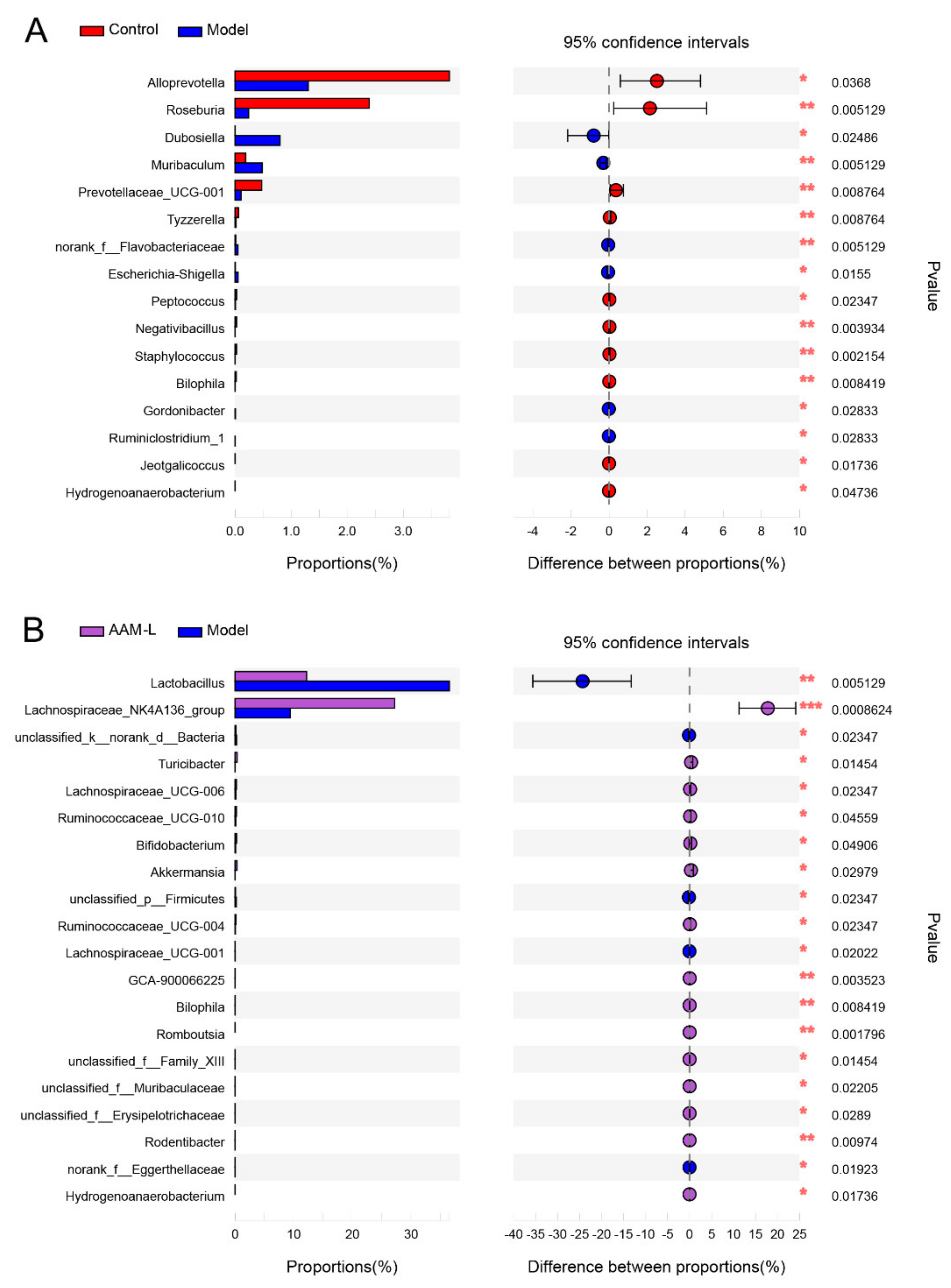
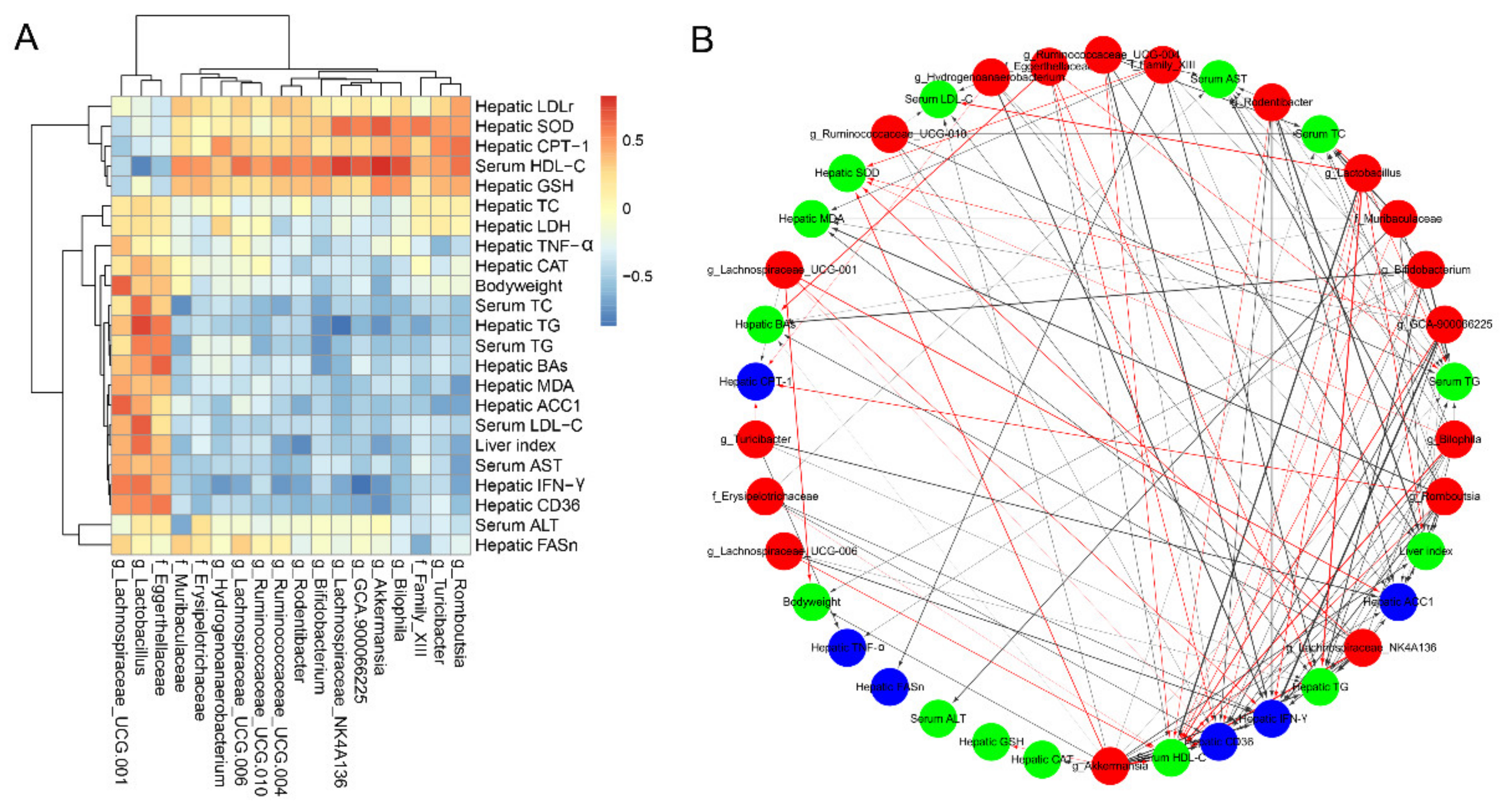
3.6. AAM Modulated Intestinal Microbiota in Mice Exposed to Alcohol Intake
3.7. Effects of AAM on the mRNA Levels of Lipid Metabolism and Inflammatory Response Related Genes in Liver
3.8. Correlations of Microbial Phylotypes with Metabolic Parameters and Liver Gene Transcription Profile
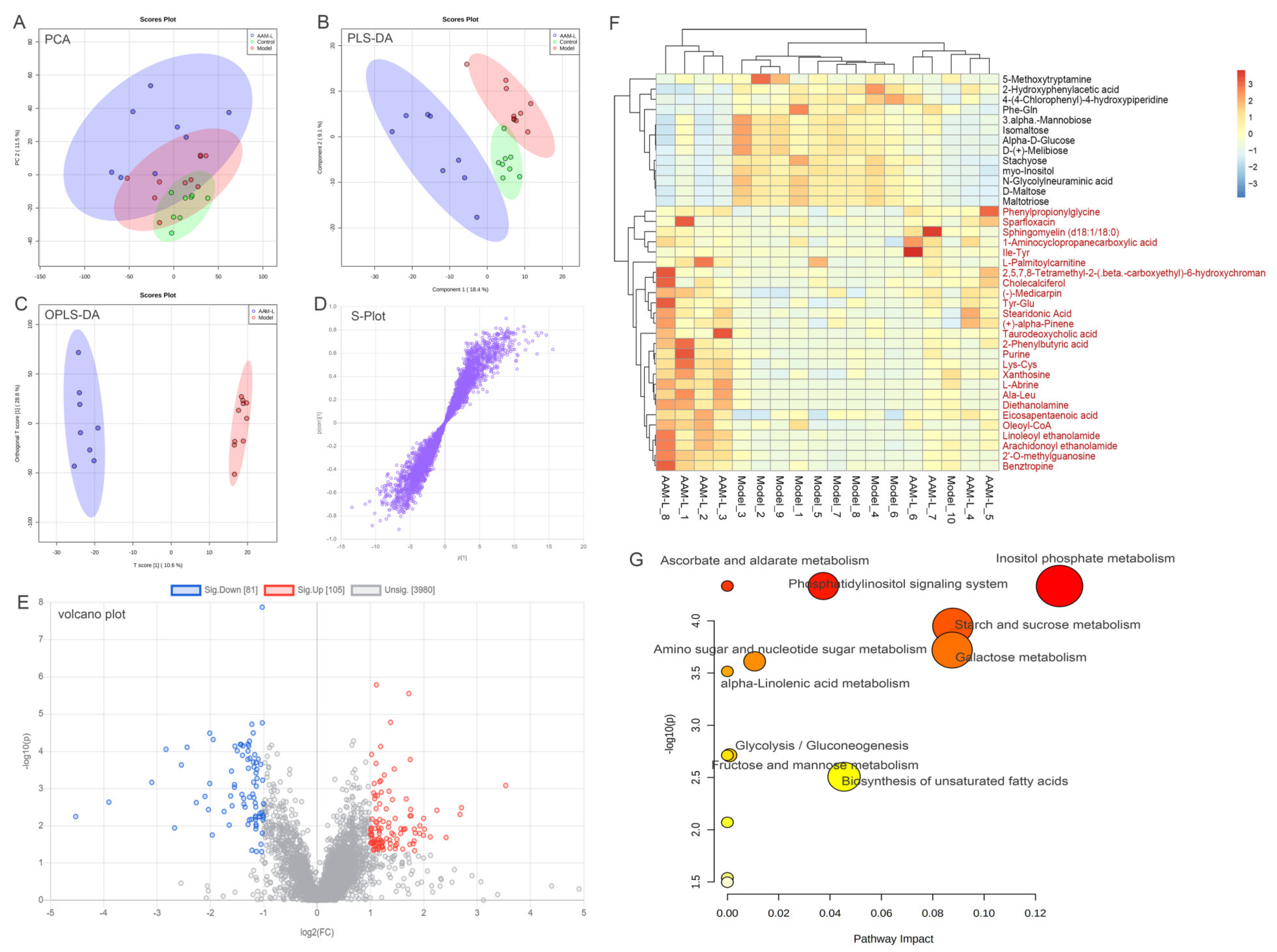
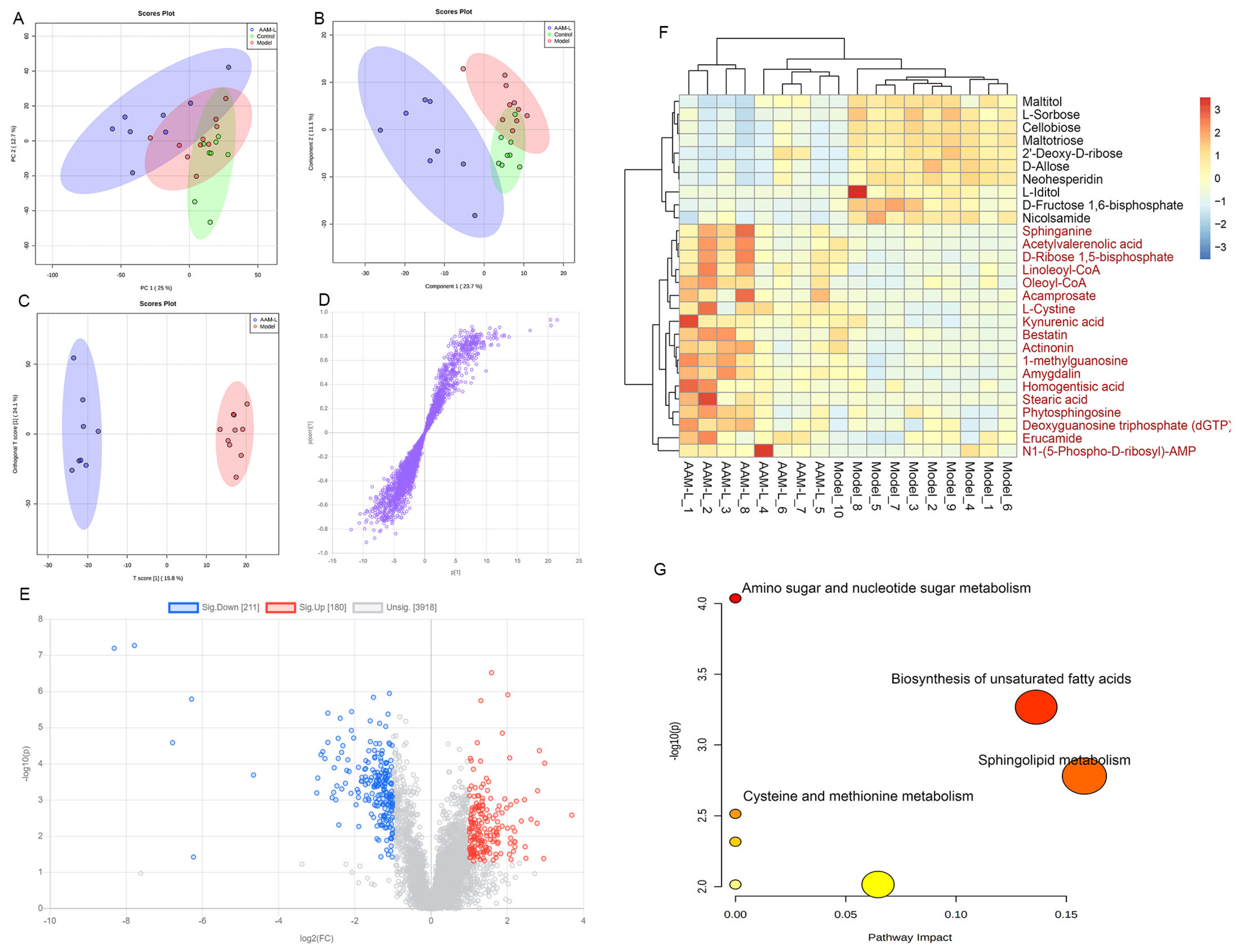
3.9. Effects of AAM on the Liver Metabolomic Profiling in Model Mice
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Nagy, L.E.; Ding, W.X.; Cresci, G.; Saikia, P.; Shah, V.H. Linking Pathogenic Mechanisms of Alcoholic Liver Disease with Clinical Phenotypes. Gastroenterology 2016, 150, 1756–1768. [Google Scholar] [CrossRef]
- Dunn, W.; Shah, V.H. Pathogenesis of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Samokhvalov, A.V.; Shield, K.D. Global Burden of Alcoholic Liver Diseases. J. Hepatol. 2013, 59, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Pak, S.; Chen, F.; Ma, L.; Hu, X.; Ji, J. Functional Perspective of Black Fungi (Auricularia auricula): Major Bioactive Components, Health Benefits and Potential Mechanisms. Trends Food Sci. Technol. 2021, 114, 245–261. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Wang, D.; Hu, Y.; Fan, Y.; Wang, J.; Abula, S.; Guo, L.; Zhang, J.; Khakame, S.K.; Dang, B.K. Immuno-Enhancing Activity of Sulfated Auricularia auricula Polysaccharides. Carbohydr. Polym. 2012, 89, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Y.; Jiang, K. Antioxidant Activity in Vitro and in Vivo of the Polysaccharides from Different Varieties of Auricularia auricula. Food Funct. 2016, 7, 3868–3879. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Wang, J.; Zhang, L.; Zhang, Y.; Ding, K. Evaluation of Water Soluble Β-D-Glucan from Auricularia auricular-Judae as Potential Anti-Tumor Agent. Carbohydr. Polym. 2010, 80, 977–983. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, M.; Yang, H.; Zhou, H.; Yang, H. Production, Physico-Chemical Characterization and Antioxidant Activity of Natural Melanin From Submerged Cultures of the Mushroom Auricularia auricula. Food Biosci. 2018, 26, 49–56. [Google Scholar] [CrossRef]
- Golyshkin, D.V.; Falalyeyeva, T.M.; Neporada, K.S.; Beregova, T.V. The Influence of Melanin on the Gastric Mucosa and Hypothalamic-Pituitary-Adrenocortical Axis Under Acute Stress Conditions. Fiziol. Zh. 2015, 61, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Belemets, N.; Kobyliak, N.; Virchenko, O.; Falalyeyeva, T.; Olena, T.; Bodnar, P.; Savchuk, O.; Galenova, T.; Caprnda, M.; Rodrigo, L.; et al. Effects of Polyphenol Compounds Melanin on NAFLD/NASH Prevention. Biomed. Pharmacother. 2017, 88, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Hu, W.; Ma, K.; Tian, M. Physicochemical Properties and Antioxidant Activities of Melanin and Fractions from Auricularia auricula Fruiting Bodies. Food Sci. Biotechnol. 2015, 24, 15–21. [Google Scholar] [CrossRef]
- Kamei, H.; Koide, T.; Hashimoto, Y.; Kojima, T.; Hasegawa, M.; Umeda, T. Effect of Allomelanin on Tumor Growth Suppression in Vivo and On the Cell Cycle Phase. Cancer Biother. Radiopharm. 1997, 12, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Adhikary, B.; Jayakumar, S.; Barik, A.; Chattopadhyay, S.; Raghukumar, S.; Priyadarsini, K.I. Melanin, a Promising Radioprotector: Mechanisms of Actions in a Mice Model. Toxicol. Appl. Pharm. 2012, 264, 202–211. [Google Scholar] [CrossRef]
- Oh, J.J.; Kim, J.Y.; Kim, Y.J.; Kim, S.; Kim, G.H. Utilization of Extracellular Fungal Melanin as an Eco-Friendly Biosorbent for Treatment of Metal-Contaminated Effluents. Chemosphere 2021, 272, 129884. [Google Scholar] [CrossRef]
- Hou, R.; Liu, X.; Yan, J.; Xiang, K.; Wu, X.; Lin, W.; Chen, G.; Zheng, M.; Fu, J. Characterization of Natural Melanin From Auricularia auricula and its Hepatoprotective Effect on Acute Alcohol Liver Injury in Mice. Food Funct. 2019, 10, 1017–1027. [Google Scholar] [CrossRef]
- Petrof, E.O. Probiotics and Gastrointestinal Disease: Clinical Evidence and Basic Science. Antiinflamm. Antiallergy Agents Med. Chem. 2009, 8, 260–269. [Google Scholar] [CrossRef][Green Version]
- Vassallo, G.; Mirijello, A.; Ferrulli, A.; Antonelli, M.; Landolfi, R.; Gasbarrini, A.; Addolorato, G. Review Article: Alcohol and Gut Microbiota—The Possible Role of Gut Microbiota Modulation in the Treatment of Alcoholic Liver Disease. Aliment. Pharmacol Ther. 2015, 41, 917–927. [Google Scholar] [CrossRef]
- Li, H.; Shi, J.; Zhao, L.; Guan, J.; Liu, F.; Huo, G.; Li, B. Lactobacillus Plantarum KLDS1.0344 and Lactobacillus Acidophilus KLDS1.0901 Mixture Prevents Chronic Alcoholic Liver Injury in Mice by Protecting the Intestinal Barrier and Regulating Gut Microbiota and Liver-Related Pathways. J. Agric. Food Chem. 2021, 69, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, S.; Matamoros, S.; Cani, P.D.; Neyrinck, A.M.; Jamar, F.; Starkel, P.; Windey, K.; Tremaroli, V.; Backhed, F.; Verbeke, K.; et al. Intestinal Permeability, Gut-Bacterial Dysbiosis, and Behavioral Markers of Alcohol-Dependence Severity. Proc. Natl. Acad. Sci. USA 2014, 111, E4485–E4493. [Google Scholar] [CrossRef]
- Schnabl, B.; Brenner, D.A. Interactions between the Intestinal Microbiome and Liver Diseases. Gastroenterology 2014, 146, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wang, L.; Yi, H.; Wu, X. Beneficial Effects of LRP6-CRISPR on Prevention of Alcohol-Related Liver Injury Surpassed Fecal Microbiota Transplant in a Rat Model. Gut Microbes 2020, 11, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, M.; Fu, X.; Zhang, Z.; Zhu, L.; Zheng, X.; Liu, J. Astaxanthin Prevents Alcoholic Fatty Liver Disease by Modulating Mouse Gut Microbiota. Nutrients 2018, 10, 1298. [Google Scholar] [CrossRef]
- Dubinkina, V.B.; Tyakht, A.V.; Odintsova, V.Y.; Yarygin, K.S.; Kovarsky, B.A.; Pavlenko, A.V.; Ischenko, D.S.; Popenko, A.S.; Alexeev, D.G.; Taraskina, A.Y.; et al. Links of Gut Microbiota Composition with Alcohol Dependence Syndrome and Alcoholic Liver Disease. Microbiome 2017, 5, 141. [Google Scholar] [CrossRef]
- Hou, R.; Liu, X.; Wu, X.; Zheng, M.; Fu, J. Therapeutic Effect of Natural Melanin from Edible Fungus Auricularia auricula on Alcohol-Induced Liver Damage in Vitro and in Vivo. Food Sci. Hum. Wellness 2021, 10, 514–522. [Google Scholar] [CrossRef]
- Guo, W.L.; Deng, J.C.; Pan, Y.Y.; Xu, J.X.; Hong, J.L.; Shi, F.F.; Liu, G.L.; Qian, M.; Bai, W.D.; Zhang, W.; et al. Hypoglycemic and Hypolipidemic Activities of Grifola Frondosa Polysaccharides and their Relationships with the Modulation of Intestinal Microflora in Diabetic Mice Induced by High-Fat Diet and Streptozotocin. Int. J. Biol. Macromol. 2020, 153, 1231–1240. [Google Scholar] [CrossRef]
- Zhang, M.; Xiao, G.; Thring, R.W.; Chen, W.; Zhou, H.; Yang, H. Production and Characterization of Melanin by Submerged Culture of Culinary and Medicinal Fungi Auricularia auricula. Appl. Biochem. Biotechnol. 2015, 176, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, X.; Sun, S.; Zhang, L.; Shan, S.; Zhu, H. Production of Natural Melanin by Auricularia auricula and Study on its Molecular Structure. Food Chem. 2016, 190, 801–807. [Google Scholar] [CrossRef]
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic Liver Disease: Pathogenesis, Management, and Novel Targets for Therapy. J. Gastroenterol. Hepatol. 2013, 28, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Vettraino, M.; Manerba, M.; Govoni, M.; di Stefano, G. Galloflavin Suppresses Lactate Dehydrogenase Activity and Causes MYC Downregulation in Burkitt Lymphoma Cells through NAD/NADH-dependent Inhibition of Sirtuin-1. Anticancer Drugs 2013, 24, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Jones, D.; Day, C.P. Alcoholic Liver Disease: New Insights Into Mechanisms and Preventative Strategies. Trends Mol. Med. 2001, 7, 408–413. [Google Scholar] [CrossRef]
- Sarin, S.K.; Pande, A.; Schnabl, B. Microbiome as a Therapeutic Target in Alcohol-Related Liver Disease. J. Hepatol. 2019, 70, 260–272. [Google Scholar] [CrossRef]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage Targeting of Gut Bacterium Attenuates Alcoholic Liver Disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Martin-Mateos, R.M.; Shah, V.H.; Axis, G. Cirrhosis and Portal Hypertension: The Chicken and the Egg. Hepatol. Int. 2018, 12, 24–33. [Google Scholar] [CrossRef]
- Raman, M.; Ahmed, I.; Gillevet, P.M.; Probert, C.S.; Ratcliffe, N.M.; Smith, S.; Greenwood, R.; Sikaroodi, M.; Lam, V.; Crotty, P.; et al. Fecal Microbiome and Volatile Organic Compound Metabolome in Obese Humans with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 868–875. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; de Vos, W.M. Akkermansia Muciniphila and its Role in Regulating Host Functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Lowe, P.P.; Gyongyosi, B.; Satishchandran, A.; Iracheta-Vellve, A.; Ambade, A.; Kodys, K.; Catalano, D.; Ward, D.V.; Szabo, G. Alcohol-Related Changes in the Intestinal Microbiome Influence Neutrophil Infiltration, Inflammation and Steatosis in Early Alcoholic Hepatitis in Mice. PLoS ONE 2017, 12, e174544. [Google Scholar]
- Wu, W.; Lv, L.; Shi, D.; Ye, J.; Fang, D.; Guo, F.; Li, Y.; He, X.; Li, L. Protective Effect of Akkermansia Muciniphila Against Immune-Mediated Liver Injury in a Mouse Model. Front. Microbiol. 2017, 8, 1804. [Google Scholar] [CrossRef]
- Xue, L.; He, J.; Gao, N.; Lu, X.; Li, M.; Wu, X.; Liu, Z.; Jin, Y.; Liu, J.; Xu, J.; et al. Probiotics May Delay the Progression of Nonalcoholic Fatty Liver Disease by Restoring the Gut Microbiota Structure and Improving Intestinal Endotoxemia. Sci. Rep. 2017, 7, 45176. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, D.; Han, S.; Zhou, S.; Jia, G. Hepatotoxicity and the Role of the Gut-Liver Axis in Rats After Oral Administration of Titanium Dioxide Nanoparticles. Part. Fibre. Toxicol. 2019, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, M.A.; Lozupone, C.A.; Wagner, B.D.; Eggesbo, M.; Sontag, M.K.; Nusbacher, N.M.; Martinez, M.; Dabelea, D. Gut Microbiota in Adolescents and the Association with Fatty Liver: The EPOCH Study. Pediatric Res. 2018, 84, 219–227. [Google Scholar] [CrossRef]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are Short Chain Fatty Acids in Gut Microbiota Defensive Players for Inflammation and Atherosclerosis? J. Atheroscler. Thromb. 2017, 2, 660–672. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Nunez, G. Gut Microbiota: Role in Pathogen Colonization, Immune Responses, and Inflammatory Disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Tsai, M.C.; Liu, Y.Y.; Lin, C.C.; Wang, C.C.; Wu, Y.J.; Yong, C.C.; Chen, K.D.; Chuah, S.K.; Yao, C.C.; Huang, P.Y.; et al. Gut Microbiota Dysbiosis in Patients with Biopsy-Proven Nonalcoholic Fatty Liver Disease: A Cross-Sectional Study in Taiwan. Nutrients 2020, 12, 820. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Yang, X.; Zhao, Y. Gut Microbiota and Metabolome Response of Decaisnea Insignis Seed Oil On Metabolism Disorder Induced by Excess Alcohol Consumption. J. Agric. Food Chem. 2019, 67, 10667–10677. [Google Scholar] [CrossRef]
- Dong, L.; Han, X.; Tao, X.; Xu, L.; Xu, Y.; Fang, L.; Yin, L.; Qi, Y.; Li, H.; Peng, J. Protection by the Total Flavonoids From Rosa Laevigata Michx Fruit against Lipopolysaccharide-Induced Liver Injury in Mice Via Modulation of FXR Signaling. Foods 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, J.; Sun, P.; Yi, R.; Han, X.; Zhao, X. Raw Bowl Tea (Tuocha) Polyphenol Prevention of Nonalcoholic Fatty Liver Disease by Regulating Intestinal Function in Mice. Biomolecules 2019, 9, 435. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Ahmad, O.; Cao, Y.; Wang, B.; He, Q.; Li, J.; Yin, H.; Zhang, Y.; He, J.; et al. Lipid-Modulate Activity of Cichorium Glandulosum Boiss. Et Huet Polysaccharide in Nonalcoholic Fatty Liver Disease Larval Zebrafish Model. J. Pharmacol. Sci. 2018, 138, 257–262. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yuan, M.H.; Zhang, C.Y.; Liu, H.M.; Liu, J.R.; Wei, A.L.; Ye, Q.; Zeng, B.; Li, M.F.; Guo, Y.P.; et al. Puerariae Lobatae Radix Flavonoids and Puerarin Alleviate Alcoholic Liver Injury in Zebrafish by Regulating Alcohol and Lipid Metabolism. Biomed. Pharmacother. 2021, 134, 111121. [Google Scholar] [CrossRef]
- Zhong, S.; Zhao, L.; Wang, Y.; Zhang, C.; Liu, J.; Wang, P.; Zhou, W.; Yang, P.; Varghese, Z.; Moorhead, J.F.; et al. Cluster of Differentiation 36 Deficiency Aggravates Macrophage Infiltration and Hepatic Inflammation by Upregulating Monocyte Chemotactic Protein-1 Expression of Hepatocytes through Histone Deacetylase 2-Dependent Pathway. Antioxid. Redox Signal. 2017, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, P.; Zhao, L.; Chen, Y.; Zhang, X.; Zeng, S.; Wei, L.; Varghese, Z.; Moorhead, J.F.; Chen, Y.; et al. CD36 Plays a Negative Role in the Regulation of Lipophagy in Hepatocytes through an AMPK-dependent Pathway. J. Lipid. Res. 2019, 60, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, P.F.; Byun, J.H.; Platko, K.; Al-Hashimi, A.A.; Lhotak, S.; MacDonald, M.E.; Mejia-Benitez, A.; Prat, A.; Igdoura, S.A.; Trigatti, B.; et al. Pcsk9 Knockout Exacerbates Diet-Induced Non-Alcoholic Steatohepatitis, Fibrosis and Liver Injury in Mice. JHEP Rep. 2019, 1, 418–429. [Google Scholar] [CrossRef]
- Xiang, X.; Feng, D.; Hwang, S.; Ren, T.; Wang, X.; Trojnar, E.; Matyas, C.; Mo, R.; Shang, D.; He, Y.; et al. Interleukin-22 Ameliorates Acute-On-Chronic Liver Failure by Reprogramming Impaired Regeneration Pathways in Mice. J. Hepatol. 2020, 72, 736–745. [Google Scholar] [CrossRef]
- Zhou, Y.; Tan, F.; Li, C.; Li, W.; Liao, W.; Li, Q.; Qin, G.; Liu, W.; Zhao, X. White Peony (Fermented Camellia Sinensis) Polyphenols Help Prevent Alcoholic Liver Injury Via Antioxidation. Antioxidants (Basel) 2019, 8, 524. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL Receptor. Arter. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Manna, S.K.; Patterson, A.D.; Yang, Q.; Krausz, K.W.; Li, H.; Idle, J.R.; Fornace, A.J.; Gonzalez, F.J. Identification of Noninvasive Biomarkers for Alcohol-Induced Liver Disease Using Urinary Metabolomics and the Ppara-null Mouse. J. Proteome. Res. 2010, 9, 4176–4188. [Google Scholar] [CrossRef]
- Asakawa, H.; Sasabe, M.; Miyazaki, R.; Matsuda, H.; Fukai, F.; Hanada, K.; Hirano, H.; Takasaki, S. The Analysis of N-glycolylneuraminic acid(NeuGc) of Hepatoma Tissue and K562 Cell Ferritins Using HPLC and Mass Spectrometry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2006, 82, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Pi, Z.; Yue, H.; Wang, Y.; Yu, Q.; Liu, S. Effect of Ginseng Polysaccharide On the Urinary Excretion of Type 2 Diabetic Rats Studied by Liquid Chromatography-Mass Spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 907, 7–12. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, C.; Cheng, J.; Krausz, K.W.; Li, T.; Ferrell, J.M.; Gonzalez, F.J.; Chiang, J.Y. Bile Acid Signaling in Lipid Metabolism: Metabolomic and Lipidomic Analysis of Lipid and Bile Acid Markers Linked to Anti-Obesity and Anti-Diabetes in Mice. Biochim. Biophys. Acta 2015, 1851, 19–29. [Google Scholar] [CrossRef]
- Tagliaferri, S.; Porri, D.; de Giuseppe, R.; Manuelli, M.; Alessio, F.; Cena, H. The Controversial Role of Vitamin D as an Antioxidant: Results From Randomised Controlled Trials. Nutr. Res. Rev. 2019, 32, 99–105. [Google Scholar] [CrossRef]
- Sepidarkish, M.; Farsi, F.; Akbari-Fakhrabadi, M.; Namazi, N.; Almasi-Hashiani, A.; Maleki, H.A.; Heshmati, J. The Effect of Vitamin D Supplementation On Oxidative Stress Parameters: A Systematic Review and Meta-Analysis of Clinical Trials. Pharmacol. Res. 2019, 139, 141–152. [Google Scholar] [CrossRef]
- Boontanrart, M.; Hall, S.D.; Spanier, J.A.; Hayes, C.E.; Olson, J.K. Vitamin D3 Alters Microglia Immune Activation by an IL-10 Dependent SOCS3 Mechanism. J. Neuroimmunol. 2016, 292, 126–136. [Google Scholar] [CrossRef]
- Turcotte, C.; Chouinard, F.; Lefebvre, J.S.; Flamand, N. Regulation of Inflammation by Cannabinoids, the Endocannabinoids 2-Arachidonoyl-Glycerol and Arachidonoyl-Ethanolamide, and their Metabolites. J. Leukoc. Biol. 2015, 97, 1049–1070. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.J.; Yang, Y.; Xiao, Z.; Wan, J.B. N-3 Polyunsaturated Fatty Acids for the Management of Alcoholic Liver Disease: A Critical Review. Crit. Rev. Food Sci. Nutr. 2019, 59, S116–S129. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, L.; Zhu, B.; Liu, F.; Wang, Y.; Gu, X.; Yan, C. Expanded Metabolomics Approach to Profiling Endogenous Carbohydrates in the Serum of Ovarian Cancer Patients. J. Sep. Sci. 2016, 39, 316–323. [Google Scholar] [CrossRef]
- Koh, G.; Lee, D.H.; Woo, J.T. 2-Deoxy-D-ribose Induces Cellular Damage by Increasing Oxidative Stress and Protein Glycation in a Pancreatic Beta-Cell Line. Metab. Clin. Exp. 2010, 59, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, J.; Zhong, Q.; Zhao, M.; Hu, T.; Chen, T.; Qiu, L.; Wen, L. Curcumin Improves Alcoholic Fatty Liver by Inhibiting Fatty Acid Biosynthesis. Toxicol. Appl. Pharm. 2017, 328, 1–9. [Google Scholar] [CrossRef]
- Chang, H.; Meng, H.Y.; Liu, S.M.; Wang, Y.; Yang, X.X.; Lu, F.; Wang, H.Y. Identification of Key Metabolic Changes During Liver Fibrosis Progression in Rats Using a Urine and Serum Metabolomics Approach. Sci. Rep. 2017, 7, 11433. [Google Scholar] [CrossRef] [PubMed]
- Witkiewitz, K.; Saville, K.; Hamreus, K. Acamprosate for Treatment of Alcohol Dependence: Mechanisms, Efficacy, and Clinical Utility. Ther. Clin. Risk Manag. 2012, 8, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Staner, L.; Boeijinga, P.; Danel, T.; Gendre, I.; Muzet, M.; Landron, F.; Luthringer, R. Effects of Acamprosate On Sleep During Alcohol Withdrawal: A Double-Blind Placebo-Controlled Polysomnographic Study in Alcohol-Dependent Subjects. Alcohol. Clin. Exp. Res. 2006, 30, 1492–1499. [Google Scholar]
- Marciniak, S.; Wnorowski, A.; Smolinska, K.; Walczyna, B.; Turski, W.; Kocki, T.; Paluszkiewicz, P.; Parada-Turska, J. Kynurenic Acid Protects Against Thioacetamide-Induced Liver Injury in Rats. Anal. Cell. Pathol. (Amst.) 2018, 2018, 1270483. [Google Scholar] [CrossRef]
- Erces, D.; Varga, G.; Fazekas, B.; Kovacs, T.; Tokes, T.; Tiszlavicz, L.; Fulop, F.; Vecsei, L.; Boros, M.; Kaszaki, J. N-methyl-D-aspartate Receptor Antagonist Therapy Suppresses Colon Motility and Inflammatory Activation Six Days after the Onset of Experimental Colitis in Rats. Eur. J. Pharmacol. 2012, 691, 225–234. [Google Scholar] [CrossRef]
- Koen, Y.M.; Sarma, D.; Hajovsky, H.; Galeva, N.A.; Williams, T.D.; Staudinger, J.L.; Hanzlik, R.P. Protein Targets of Thioacetamide Metabolites in Rat Hepatocytes. Chem. Res. Toxicol. 2013, 26, 564–574. [Google Scholar] [CrossRef]
- Tang, F.; Fan, K.; Wang, K.; Bian, C. Amygdalin Attenuates Acute Liver Injury Induced by D-galactosamine and Lipopolysaccharide by Regulating the NLRP3, NF-kappaB and Nrf2/NQO1 Signalling Pathways. Biomed. Pharm. 2019, 111, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, A.; Farahabadi, M.; Chavoshzadeh, S.A.; Barati, A.; Ababzadeh, S.; Mohammadbeigi, A. The Effect of Amygdalin on Endoplasmic Reticulum (ER) Stress Induced Hepatic Steatosis in Mice. Malays. J. Med. Sci. 2018, 25, 16–23. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward Primer (5′−3′) | Reverse Primer (5′−3′) |
|---|---|---|
| TNF-α | AAGCCTGTAGCCCACGTCGTA | AGGTACAACCCATCGGCTGG |
| LDLr | ATGCTGGAGATAGAGTGGAGTT | CCGCCAAGATCAAGAAAG |
| ACC1 | GCCATCCGGTTTGTTGTCA | GGATACCTGCAGTTTGAGCCA |
| CPT-1 | TCCATGCATACCAAAGTGGA | TGGTAGGAGAGCAGCACCTT |
| FASn | CTG CCA CAA CTC TGA GGA CA | TTC GTA CCT CCT TGG CAA AC |
| IFN-γ | ACAGCAAGGCGAAAAAGGATG | TGGTGGACCACTCGGATGA |
| CD36 | ACTTGGGATTGGAGTGGTGATGT | GGATACCTGCAGTTTGAGCCA |
| 18S | AGTCCCTGCCCTTTGTACACA | CGATCCCAGGGCCTCACTA |
| Element Composition (%) | |||||
|---|---|---|---|---|---|
| Source | C (%) | H (%) | O (%) * | N (%) | S (%) |
| AAM | 47.41 | 7.30 | 39.24 | 6.05 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Chen, H.; Cao, Y.; Zhang, Y.; Li, W.; Guo, W.; Lv, X.; Rao, P.; Ni, L.; Liu, P. Auricularia auricula Melanin Protects against Alcoholic Liver Injury and Modulates Intestinal Microbiota Composition in Mice Exposed to Alcohol Intake. Foods 2021, 10, 2436. https://doi.org/10.3390/foods10102436
Lin Y, Chen H, Cao Y, Zhang Y, Li W, Guo W, Lv X, Rao P, Ni L, Liu P. Auricularia auricula Melanin Protects against Alcoholic Liver Injury and Modulates Intestinal Microbiota Composition in Mice Exposed to Alcohol Intake. Foods. 2021; 10(10):2436. https://doi.org/10.3390/foods10102436
Chicago/Turabian StyleLin, Yichen, Hua Chen, Yingjia Cao, Yuanhui Zhang, Wenfeng Li, Weiling Guo, Xucong Lv, Pingfan Rao, Li Ni, and Penghu Liu. 2021. "Auricularia auricula Melanin Protects against Alcoholic Liver Injury and Modulates Intestinal Microbiota Composition in Mice Exposed to Alcohol Intake" Foods 10, no. 10: 2436. https://doi.org/10.3390/foods10102436
APA StyleLin, Y., Chen, H., Cao, Y., Zhang, Y., Li, W., Guo, W., Lv, X., Rao, P., Ni, L., & Liu, P. (2021). Auricularia auricula Melanin Protects against Alcoholic Liver Injury and Modulates Intestinal Microbiota Composition in Mice Exposed to Alcohol Intake. Foods, 10(10), 2436. https://doi.org/10.3390/foods10102436