Effect of High Hydrostatic Pressure on the Extractability and Bioaccessibility of Carotenoids and Their Esters from Papaya (Carica papaya L.) and Its Impact on Tissue Microstructure
Abstract
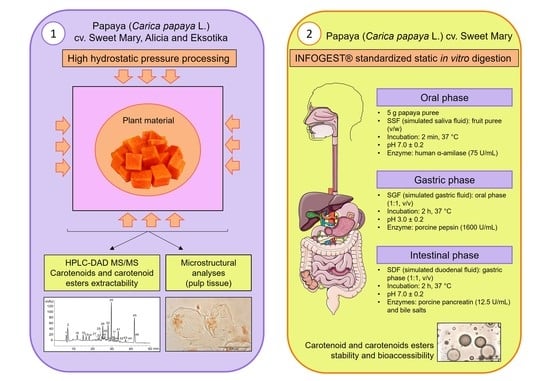
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals, Standards and Reagents
2.3. High Hydrostatic Pressure Treatments
2.4. In Vitro Gastrointestinal Digestion Assay
2.5. Carotenoid Analysis
2.5.1. Carotenoid Extraction from Fresh and Freeze-Dried Papaya
2.5.2. Carotenoid Extraction from In Vitro Digestion Phases
2.6. Carotenoid Analysis by HPLC-DAD
2.7. Liquid Chromatography Mass Spectrometry (LC-MS/MS (APCI+))
2.8. Optical Microscopy
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Carotenoid and Carotenoid Ester Profile
3.1.1. Individual Carotenoid Profile in Papaya Tissues
3.1.2. Individual Carotenoid and Carotenoid Ester Content in Papaya Pulp Processed by HHP
3.2. Stability of Carotenoids and Carotenoid Esters in Papaya during In Vitro Digestion
3.3. Bioccessibility of Carotenoid and Carotenoid Esters in Papaya Submitted to HHP Treatments
3.4. Microstructure of Papaya Fruit and Digesta during In Vitro Digestion
3.4.1. Effect of HHP on Cell Wall and Morphology of Papaya Pulp
3.4.2. Carotenoid Deposition and Factors Affecting Their Stability during In Vitro Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Chen, W.D.; Wang, Y.D. The relationship between gut microbiota and inflammatory diseases: The role of macrophages. Front. Microbiol. 2020, 11, 1065. [Google Scholar] [CrossRef] [PubMed]
- Bleich, S.N.; Jones-Smith, J.; Wolfson, J.A.; Zhu, X.; Story, M. The complex relationship between diet and health. Health Aff. 2015, 34, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Bazzano, L.A.; Joint FAO; >World Health Organization. Dietary Intake of Fruit and Vegetables and Risk of Diabetes Mellitus and Cardiovascular Diseases; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- WHO (World Health Organization). Diet, Nutrition and the Prevention of Chronic Diseases. WHO technical report series, 916; WHO Publication: Geneva, Switzerland, 2003. [Google Scholar]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruits, vegetables and coronary heart disease. Nat. Rev. Cardiol. 2009, 6, 599–608. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules. 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Jadeja, R.N. Beneficial role of phytochemicals on oxidative stress and age-related diseases. Biomed. Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Engwa, G.A. Free radicals and the role of plant phytochemicals as antioxidants against oxidative stress-related diseases. Phytochemicals: Source of Antioxidants and Role in Disease Prevention. BoD–Books on Demand. 2018, 7, 49–74. [Google Scholar] [CrossRef]
- Zakaria, L. Diversity of Colletotrichum Species Associated with Anthracnose Disease in Tropical Fruit Crops—A Review. Agriculture 2021, 11, 297. [Google Scholar] [CrossRef]
- Altendorf, S. Major Tropical Fruits Market Rreview; License: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2017; p. 10. [Google Scholar]
- Lara-Abia, S.; Lobo-Rodrigo, G.; Welti-Chanes, J.; Cano, M.P. Carotenoid and carotenoid ester profile and their deposition in plastids in fruits of new papaya (Carica papaya L.) varieties from the Canary Islands. Foods 2021, 10, 434. [Google Scholar] [CrossRef]
- Laurora, A.; Bingham, J.P.; Poojary, M.M.; Wall, M.M.; Ho, K.K. Carotenoid composition and bioaccessibility of papaya cultivars from Hawaii. J. Food Compos. Anal. 2021, 101, 103984. [Google Scholar] [CrossRef]
- Shen, Y.H.; Yang, F.Y.; Lu, B.G.; Zhao, W.W.; Jiang, T.; Feng, L.; Ming, R. Exploring the differential mechanisms of carotenoid biosynthesis in the yellow peel and red flesh of papaya. BMC Genom. 2019, 20, 49. [Google Scholar] [CrossRef] [Green Version]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef]
- Bohn, T.; Bonet, M.; Borel, P.; Keijer, J.; Landrier, J.; Milisav, I.; Dulińska-Litewka, J. Mechanistic aspects of carotenoid health benefits—Where are we now? Nutr. Res. Rev. 2021, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Kadonosono, K. Health benefits of polyphenols and carotenoids in age-related eye diseases. Oxid. Med. Cell Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Parra, P.A.; García-Salinas, C.; Rodríguez-López, C.E.; García, N.; García-Rivas, G.; Hernández-Brenes, C.; de la Garza, R.I.D. High hydrostatic pressure treatments trigger de novo carotenoid biosynthesis in papaya fruit (Carica papaya cv. Maradol). Food Chem. 2019, 277, 362–372. [Google Scholar] [CrossRef]
- Hernandez-Brenes, C.; Ramos-Parra, P.A.; Jacobo-Velázquez, D.A.; Villarreal-Lara, R.; Díaz-De la Garza, R.I. High hydrostatic pressure processing as a strategy to increase carotenoid contents of tropical fruits. In Tropical and Subtropical Fruits: Flavors, Color, and Health Benefits; American Chemical Society: Washington, DC, USA, 2013; pp. 29–42. [Google Scholar] [CrossRef]
- Cano, M.P.; Gomez-Maqueo, A.; Fernandez-Lopez, R.; Welti-Chanes, J.; Garcia-Cayuela, T. Impact of high hydrostatic pressure and thermal treatment on the stability and bioaccessibility of carotenoid and carotenoid esters in astringent persimmon (Diospyros kaki Thunb, var. Rojo Brillante). Food Res. Int. 2019, 123, 538–549. [Google Scholar] [CrossRef]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 1002. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mariutti, L.R.B.; Mercadante, A.Z. An in vitro digestion method adapted for carotenoids and carotenoid esters: Moving forward towards standardization. Food Funct. 2016, 7, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Kean, E.G.; Hamaker, B.R.; Ferruzzi, M.G. Carotenoid bioaccessibility from whole grain and degermed maize meal products. J. Agric. Food Chem. 2008, 56, 9918–9926. [Google Scholar] [CrossRef]
- Schweiggert, R.M.; Mezger, D.; Schimpf, F.; Steingass, C.B.; Carle, R. Influence of chromoplast morphology on carotenoid bioaccessibility of carrot, mango, papaya, and tomato. Food Chem. 2012, 135, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Rodrigo, D.; Martinez, A.; Barbosa-Cánovas, G.V.; Rodrigo, M. Effect of PEF and heat pasteurization on the physical–chemical characteristics of blended orange and carrot juice. LWT Food Sci. Technol. 2006, 39, 1163–1170. [Google Scholar] [CrossRef]
- Calligaris, S.; Foschia, M.; Bartolomeoli, I.; Maifreni, M.; Manzocco, L. Study on the applicability of high-pressure homogenization for the production of banana juices. LWT Food Sci. Technol. 2012, 45, 117–121. [Google Scholar] [CrossRef]
- San Martin, M.F.; Barbosa-Cánovas, G.V.; Swanson, B.G. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 2002, 42, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Li, R.; Bi, X.; Liao, X. Effects of high hydrostatic pressure and high temperature short time on antioxidant activity, antioxidant compounds and color of mango nectars. Innov. Food Sci. Emerg Technol. 2014, 21, 35–43. [Google Scholar] [CrossRef]
- Ramos-Parra, P.A.; García-Salinas, C.; Hernández-Brenes, C.; Díaz de la Garza, R.I. Folate levels and polyglutamylation profiles of papaya (Carica papaya cv. Maradol) during fruit development and ripening. J. Agric. Food Chem. 2013, 61, 3949–3956. [Google Scholar] [CrossRef] [PubMed]
- Plaza, L.; Colina, C.; de Ancos, B.; Sánchez-Moreno, C.; Cano, M.P. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem. 2012, 130, 591–597. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, C.; Bourlieu, C.; Brodkorb, A. A standardized static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, J.N.; Luu, A.Y.; Dragsted, L.O.; Arrigoni, E. Adaption of an in vitro digestion method to screen carotenoid liberation and in vitro accessibility from differently processed spinach preparations. Food Chem. 2017, 224, 407–413. [Google Scholar] [CrossRef]
- Cano, M.P.; Gomez-Maqueo, A.; Garcia-Cayuela, T.; Welti-Chanes, J. Characterization of carotenoid profile of Spanish Sanguinos and Verdal prickly pear (Opuntia ficus-indica, spp.) tissues. Food Chem. 2017, 237, 612–622. [Google Scholar] [CrossRef]
- Petry, F.C.; Mercadante, A.Z. Impact of in vitro digestion phases on the stability and bioaccessibility of carotenoids and their esters in mandarin pulps. Food Funct. 2017, 8, 3951–3963. [Google Scholar] [CrossRef]
- Breithaupt, D.E.; Wirt, U.; Bamedi, A. Differentiation between lutein monoester regioisomers and detection of lutein diesters from marigold flowers (Tagetes erecta L.) and several fruits by liquid chromatography- mass spectrometry. J. Agric. Food Chem. 2002, 50, 66–70. [Google Scholar] [CrossRef]
- De Faria, A.F.; De Rosso, V.V.; Mercadante, A.Z. Carotenoid composition of jackfruit (Artocarpus heterophyllus), determined by HPLC-PDA MS/MS. Plant. Foods Hum. Nutr. 2009, 64, 108–115. [Google Scholar] [CrossRef] [PubMed]
- De Rosso, V.V.; Mercadante, A.Z. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 2007, 55, 5062–5072. [Google Scholar] [CrossRef]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold carotenoids: Much more than lutein esters. Food Res. Int. 2019, 119, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Dong, L.; Pajkovic, N.D. Atmospheric pressure chemical ionization tandem mass spectrometry of carotenoids. Int. J. Mass Spectrom. 2012, 312, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Institute of Medicine (US). Panel on Micronutrients—Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef] [Green Version]
- Breithaupt, D.; Schwack, W. Determination of free and bound carotenoids in paprika (Capsicum annuum L.) by LC/MS. Eur. Food Res. Technol. 2000, 211, 52–55. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Farkas, D.; Turek, E.J. Preserving foods through high-pressure processing. Food Technol. 2008, 62, 32–38. [Google Scholar]
- Yi, J.; Feng, H.; Bi, J.; Zhou, L.; Zhou, M.; Cao, J.; Li, J. High hydrostatic pressure induced physiological changes and physical damages in asparagus spears. Postharvest Biol. Technol. 2016, 118, 111381. [Google Scholar] [CrossRef]
- Vazquez-Gutiérrez, J.L.; Quiles, A.; Hernando, I.; Pérez-Munuera, I. Changes in the microstructure and location of some bioactive compounds in persimmons treated by high hydrostatic pressure. Postharvest Biol. Technol. 2011, 61, 137–144. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Hernández-Brenes, C. Stability of avocado paste carotenoids as affected by high hydrostatic pressure processing and storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 121–128. [Google Scholar] [CrossRef]
- Hernández-Carrión, M.; Hernando, I.; Quiles, A. High hydrostatic pressure treatment as an alternative to pasteurization to maintain bioactive compound content and texture in red sweet pepper. Innov. Food Sci. Emerg. Technol. 2014, 26, 76–85. [Google Scholar] [CrossRef]
- Chen, D.; Pang, X.; Zhao, J.; Gao, L.; Liao, X.; Wu, J.; Li, Q. Comparing the effects of high hydrostatic pressure and high temperature short time on papaya beverage. Innov. Food Sci. Emerg. Technol. 2015, 32, 16–28. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Esteve, M.J.; Frigola, A. Effect of Stevia rebaudiana addition on bioaccessibility of bioactive compounds and antioxidant activity of beverages based on exotic fruits mixed with oat following simulated human digestion. Food Chem. 2015, 184, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.K.; Zerdin, K.; Howe, E.; Goicoechea, D.; Paramanandhan, P.; Stockman, R.; Stewart, C.M. The effect of high pressure processing on the microbial, physical and chemical properties of Valencia and Navel orange juice. Innov. Food Sci. Emerg. Technol. 2004, 5, 135–149. [Google Scholar] [CrossRef]
- Vargas-Murga, L.; de Rosso, V.V.; Mercadante, A.Z.; Olmedilla-Alonso, B. Fruits and vegetables in the Brazilian Household Budget Survey (2008–2009): Carotenoid content and assessment of individual carotenoid intake. J. Food Compos. Anal. 2016, 50, 88–96. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Maqueo, A.; Bandino, E.; Hormaza, J.I.; Cano, M.P. Characterization and the impact of in vitro simulated digestion on the stability and bioaccessibility of carotenoids and their esters in two Pouteria lucuma varieties. Food Chem. 2020, 316, 126369. [Google Scholar] [CrossRef]
- Guimaraes, G.C.; Coelho Júnior, M.C.; Garcia Rojas, E.E. Density and kinematic viscosity of pectin aqueous solution. J. Chem. Eng. Data 2009, 54, 662–667. [Google Scholar] [CrossRef]
- Reboul, E.; Richelle, M.; Perrot, E.; Desmoulins-Malezet, C.; Pirisi, V.; Borel, P. Bioaccessibility of carotenoids and vitamin E from their main dietary sources. J. Agric. Food Chem. 2006, 54, 8749–8755. [Google Scholar] [CrossRef]
- Failla, M.L.; Chitchumronchokchai, C.; Ferruzzi, M.G.; Goltz, S.R.; Campbell, W.W. Unsaturated fatty acids promote bioaccessibility and basolateral secretion of carotenoids and α-tocopherol by Caco-2 cells. Food Funct. 2014, 5, 1101–1112. [Google Scholar] [CrossRef] [Green Version]
- Rich, G.T.; Faulks, R.M.; Wickham, M.S.; Fillery-Travis, A. Solubilization of carotenoids from carrot juice and spinach in lipid phases: II. Modeling the duodenal environment. Lipids 2003, 38, 947–956. [Google Scholar] [CrossRef]
- Velderrain-Rodriguez, G.; Quiros-Sauceda, A.; Mercado-Mercado, G.; Ayala-Zavala, J.F.; Astiazaran-Garcia, H.; Robles-Sánchez, R.M.; Gonzalez-Aguilar, G.A. Effect of dietary fiber on the bioaccessibility of phenolic compounds of mango, papaya and pineapple fruits by an in vitro digestion model. Food Sci. Technol. 2016, 36, 188–194. [Google Scholar] [CrossRef] [Green Version]
- Schweiggert, R.M.; Steingass, C.B.; Heller, A.; Esquivel, P.; Carle, R. Characterization of chromoplasts and carotenoids of red-and yellow-fleshed papaya (Carica papaya L.). Planta 2011, 234, 1031. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Maqueo, A.; García-Cayuela, T.; Welti-Chanes, J.; Cano, M.P. Enhancement of anti-inflammatory and antioxidant activities of prickly pear fruits by high hydrostatic pressure: A chemical and microstructural approach. Innov. Food Sci. Emerg. Technol. 2019, 54, 132–142. [Google Scholar] [CrossRef]




| cv. Sweet Mary | ||||||||
|---|---|---|---|---|---|---|---|---|
| CUT | 5 min | |||||||
| No | Carotenoid Compound | Control | 100 MPa | 350 MPa | 600 MPa | 100 MPa | 350 MPa | 600 MPa |
| 1 | (13Z)-violaxanthin | 1 ± 0 a | 64 ± 4 b | 36 ± 2 ab | 66 ± 2 b | 6 ± 0 a | 164 ± 29 c | 17 ± 1 a |
| 2 | (all-E)-violaxanthin | 3 ± 0 a | 85 ± 2 d | 65 ± 0 c | 50 ± 2 b | 15 ± 1 a | 122 ± 8 e | 13 ± 2 a |
| 3 | (9Z)-neoxanthin | n.d. a | 33 ± 1 c | 52 ± 3 d | 63 ± 0 e | 17 ± 1 b | 74 ± 1 f | 15 ± 0 b |
| 4 | (all-E)-neoxanthin | n.d. a | 23 ± 0 b | n.d. a | 18 ± 0 b | 14 ± 0 b | 87 ± 9 c | n.d. a |
| 5 | (all-E)-lutein | n.d. a | 50 ± 3 b | n.d. a | 42 ± 3 b | n.d. a | 270 ± 11 c | n.d. a |
| 6 | (all-E)-zeaxanthin | n.d. a | 33 ± 1 b | n.d. a | 97 ± 3 c | 28 ± 0 b | 225 ± 2 d | n.d. a |
| 7 | Lutein-5,6-epoxide | n.d. a | 87 ± 3 c | n.d. a | 47 ± 3 b | n.d. a | 143 ± 1 d | n.d. a |
| 8 | (all-E)-antheraxanthin | 15 ± 1 b | 38 ± 0 c | n.d. a | 15 ± 1 b | n.d. a | 77 ± 2 d | n.d. a |
| 9 | (9Z)-violaxanthin | 6 ± 0 b | 50 ± 3 c | n.d. a | 11 ± 1 b | 7 ± 1 b | 49 ± 2 c | n.d. a |
| 10 | β-cryptoxanthin-5,6-epoxide | n.d. a | 46 ± 1 c | n.d. a | 17 ± 1 b | n.d. a | n.d. a | n.d. a |
| 11 | (9Z)-α-cryptoxanthin | n.d. a | 25 ± 1 b | n.d. a | 35 ± 0 c | n.d. a | n.d. a | n.d. a |
| 12 | (all-E)-α-cryptoxanthin | 3 ± 0 c | 4 ± 0 c | n.d. a | 6 ± 1 d | 2 ± 0 b | n.d. a | n.d. a |
| 13 | (all-E)-β-cryptoxanthin | 43 ± 1 a | 388 ± 3 e | 92 ± 3 c | 305 ± 0 d | 79 ± 1 b | 73 ± 2 b | 29 ± 2 a |
| 14 | α-carotene-5,6-epoxide | 3 ± 0 b | 24 ± 0 c | n.d. a | n.d. a | 21 ± 1 c | 40 ± 0 d | n.d. a |
| 15 | (all-E)-luteoxanthin | n.d. a | 39 ± 0 b | n.d. a | n.d. a | n.d. a | n.d. a | n.d. a |
| 16 | (13Z)-α-carotene | 14 ± 0 c | 14 ± 0 c | n.d. a | 22 ± 1 f | 19 ± 1 d | 77 ± 1 e | 5 ± 0 b |
| 17 | (13Z)-β-carotene | 4 ± 0 b | 41 ± 0 e | 32 ± 0 d | n.d. a | 35 ± 1 d | 10 ± 1 b | n.d. a |
| 18 | (all-E)-violaxanthin laurate | 11 ± 1 b | 10 ± 0 b | n.d. a | 21 ± 1 c | 25 ± 1 c | 34 ± 0 d | 41 ± 3 e |
| 19 | α-cryptoxanthin-5,8-epoxide | 8 ± 1 d | 8 ± 0 d | 7 ± 0 c | n.d. a | 6 ± 0 b | 11 ± 0 e | 5 ± 1 b |
| 20 | (all-E)-ζ-carotene | 4 ± 0 b | 13 ± 0 c | n.d. a | 21 ± 1 d | 33 ± 2 e | 18 ± 1 d | 12 ± 1 c |
| 21 | α-cryptoxanthin-5,8′-epoxide | 29 ± 0 b | 13 ± 0 a | 12 ± 0 a | 15 ± 1 a | 30 ± 2 b | 13 ± 1 a | 15 ± 1 a |
| 22 | (all-E)-α-carotene | 75 ± 0 c | 77 ± 0 c | 81 ± 0 c | 59 ± 4 b | 76 ± 5 c | 85 ± 0 c | 40 ± 2 a |
| 23 | (9Z)-α-carotene | 3 ± 0 b | n.d. a | n.d. a | n.d. a | n.d. a | 8 ± 0 c | n.d. a |
| 24 | (9Z)-violaxanthin laurate | 51 ± 3 e | 16 ± 1 c | n.d. a | 23 ± 1 d | 103 ± 2 g | 11 ± 1 b | 92 ± 3 f |
| 25 | (all-E)-lutein-3-O-myristate | 169 ± 0 b | 31 ± 1 a | n.d. a | 144 ± 1 b | 247 ± 13 c | 218 ± 19 c | 165 ± 16 b |
| 26 | (all-E)-β-carotene | 165 ± 7 b | 203 ± 12 b | 649 ± 17 f | 250 ± 13 c | 315 ± 2 d | 359 ± 2 e | 140 ± 7 a |
| 27 | (9Z)-β-carotene | 6 ± 0 a | n.d. a | 49 ± 4 d | 20 ± 1 b | n.d. a | 33 ± 1 c | 7 ± 1 a |
| 28 | (all-E)-violaxanthin dimyristate | 36 ± 7 b | n.d. a | 69 ± 5 d | 46 ± 1 c | n.d. a | 54 ± 1 c | 48 ± 3 c |
| 29 | (all-E)-antheraxanthin myristate palmitate | 43 ± 2 b | 33 ± 1 a | 126 ± 3 f | 69 ± 1 c | 27 ± 0 a | 94 ± 1 e | 81 ± 5 d |
| 30 | (all-E)-violaxanthin palmitate | 7 ± 1 b | 13 ± 0 c | 47 ± 1 e | 23 ± 0 d | n.d. a | 27 ± 1 d | 16 ± 2 c |
| 31 | (9Z)-neoxanthin dibutyrate | 8 ± 0 b | n.d. a | 43 ± 1 e | 18 ± 0 c | 81 ± 2 f | 26 ± 0 d | 2 ± 2 b |
| 32 | (all-E)-β-cryptoxanthin caprate | 82 ± 2 b | 32 ± 1 a | 236 ± 11 f | 111 ± 3 c | 135 ± 0 d | 170 ± 5 e | 72 ± 5 b |
| 33 | (all-E)-violaxanthin myristate palmitate | n.d. a | n.d. a | 14 ± 1 b | n.d. a | n.d. a | n.d. a | n.d. a |
| 34 | (all-E)-lutein dimyristate | 60 ± 4 c | 7 ± 0 a | 162 ± 3 f | 40 ± 3 b | 110 ± 0 e | 65 ± 4 c | 87 ± 4 d |
| 35 | (all-E)-β-cryptoxanthin laurate | 175 ± 10 b | 59 ± 1 a | 365 ± 13 e | 212 ± 2 c | 209 ± 0 c | 338 ± 9 d | 146 ± 9 b |
| 36 | (all-E)-antheraxanthin-3-O palmitate | n.d. a | n.d. a | 86 ± 4 c | n.d. a | n.d. a | 54 ± 0 b | n.d. a |
| 37 | (all-E)-antheraxanthin laurate myristate | 22 ± 2 b | 12 ± 0 a | 28 ± 1 c | 33 ± 2 d | 33 ± 1 d | 18 ± 1 b | 21 ± 2 b |
| 38 | (all-E)-β-cryptoxanthin myristate | 19 ± 1 b | 5 ± 0 a | 62 ± 0 f | 25 ± 1 d | 22 ± 0 c | 42 ± 1 e | 16 ± 1 b |
| 39 | (Z)-lycopene isomer 1 | 14 ± 0 b | n.d. a | 23 ± 1 d | n.d. a | 6 ± 0 b | 20 ± 1 c | n.d. a |
| 40 | (all-E)-β-cryptoxanthin palmitate | 14 ± 0 c | n.d. a | 25 ± 2 e | 5 ± 1 bc | 7 ± 0 c | 16 ± 0 d | 3 ± 0 ab |
| 41 | (13Z)-lycopene isomer 2 | 113 ± 0 f | 78 ± 2 d | 63 ± 1 c | 47 ± 2 b | 108 ± 0 e | 114 ± 3 f | 40 ± 2 a |
| 42 | (13′Z)-lycopene isomer 3 | 22 ± 0 d | 16 ± 0 c | 12 ± 0 b | n.d. a | 21 ± 1 d | 21 ± 0 d | n.d. a |
| 43 | (9Z)-lycopene isomer 4 | 26 ± 0 d | 9 ± 0 b | 15 ± 0 c | n.d. a | 13 ± 0 bc | 9 ± 0 b | 32 ± 3 d |
| 44 | (9′Z)-lycopene isomer 5 | 12 ± 0 c | 7 ± 0 b | 19 ± 1 d | n.d. a | 10 ± 0 c | n.d. a | n.d. a |
| 45 | (all-E)-lycopene | 378 ± 5 c | 120 ± 4 a | 374 ± 15 c | 359 ± 15 c | 231 ± 4 b | 1302 ± 52 d | 316 ± 19 c |
| 46 | (Z)-lycopene isomer 6 | 23 ± 1 b | n.d. a | n.d. a | n.d. a | n.d. a | n.d. a | n.d. a |
| Total free xanthophylls | 109 ± 4 a | 985 ± 4 d | 264 ± 9 b | 787 ± 7 c | 204 ± 3 b | 1308 ± 66 e | 95 ± 6 a | |
| Total hydrocarbon carotenoids | 696 ± 30 a | 602 ± 11 a | 1314 ± 37 c | 778 ± 29 b | 888 ± 1 b | 2096 ± 52 d | 590 ± 37 a | |
| Total xanthophyll esters | 859 ± 15 b | 219 ± 4 a | 1263 ± 43 e | 770 ± 3 b | 997 ± 18 c | 1166 ± 5 d | 811 ± 54 b | |
| Total carotenoids | 1664 ± 48 a | 1807 ± 11 b | 2841 ± 89 d | 2336 ± 33 c | 2089 ± 14 b | 4469 ± 124 e | 1496 ± 99 a | |
| RAE | 23 ± 1 a | 44 ± 1 b | 93 ± 3 e | 52 ± 1 c | 51 ± 0 c | 67 ± 1 d | 25 ± 2 a | |
| Compound | Phase (Digesta) | Non-Treated | 100 MPa/CUT | 100 MPa/5 min | 350 MPa/CUT | 350 MPa/5 min | 600 MPa/CUT | 600 MPa/5 min |
|---|---|---|---|---|---|---|---|---|
| Free xanthophylls | ||||||||
| (all-E)-violaxanthin | Oral | 27.4 ± 1.8 Ab | 61.7 ± 1.1 Af | 65.5 ± 0.1 Af | 22.2 ± 0.2 Aa | 39.3 ± 0.8 Ac | 57.1 ± 0.8 Ae | 46.4 ± 0.8 Ad |
| Gastric | 175.4 ±1.8 Bf | 73.0 ± 0.3 Bc | 149.1 ± 1.7 Ce | 39.6 ± 0.4 Ba | 181.9 ± 0.8 Cg | 66.4 ± 0.3 Bb | 91.6 ± 2.6 Cd | |
| Intestinal | 150.8 ± 3.9 Be | 110.9 ± 0.1 Cd | 114.8 ± 0.1 Bd | 63.4 ± 1.3 Ca | 91.0 ± 2.3 Bc | 81.5 ± 0.7 Cb | 61.0 ± 0.4 Ba | |
| (all-E)-zeaxanthin | Oral | 15.8 ± 0.9 Aa | 33.1 ± 0.4 Ab | 39.6 ± 0.7 Bc | 51.4 ± 0.2 Ce | 43.1 ± 1.0 Ad | 51.0 ± 0.4 Be | 54.9 ± 0.7 Bf |
| Gastric | 65.1 ± 3.2 Ce | 71.0 ± 0.4 Cf | 43.3 ± 0.9 Cd | 34.4 ± 0.1 Bb | 51.1 ± 0.1 Bd | 19.2 ± 0.6 Aa | 44.9 ± 0.5 Ab | |
| Intestinal | 47.6 ± 1.6 Bb | 59.3 ± 0.2 Bd | 22.0 ± 0.1 Aa | 22.3 ± 0.3 Aa | 93.3 ± 0.5 Cf | 89.3± 1.4 Ce | 54.1 ± 0.7 Bc | |
| (all-E)-antheraxanthin | Oral | 210.7± 0.4 Cc | 137.6 ± 0.4 Ab | n.d. Aa | n.d. Aa | n.d. Aa | n.d. Aa | 138.9 ± 0.8 Cb |
| Gastric | 75.5 ± 1.7 Ab | 147.8 ± 1.2 Bd | n.d. Aa | n.d. Aa | n.d. Aa | n.d. Aa | 119.2 ± 1.8 Bc | |
| Intestinal | 129.4 ± 3.6 Bc | 262.7± 5.7 Cd | n.d. Aa | n.d. Aa | n.d. Aa | n.d. Aa | 76.2 ± 0.5 Ab | |
| (all-E)-β-cryptoxanthin | Oral | 201.1 ± 0.3 Bg | 59.4 ± 1.2 Bd | 31.2 ± 0.7 Bb | 38.8 ± 0.9 Cc | 67.5 ± 0.2 Be | 179.0 ± 1.9 Cf | 13.7 ± 0.4 Ba |
| Gastric | 181.0 ± 2.0 Ae | 27.7 ± 0.7 Ab | 12.5 ± 0.3 Aa | 23.3 ± 0.1 Ab | 62.5 ± 0.1 Ac | 144.9 ± 2.8 Bd | 6.9 ± 0.2 Aa | |
| Intestinal | 232.2 ± 2.6 Cf | 185.1 ± 4.2 Ce | 180.6 ± 1.0 Ce | 30.5 ± 0.8 Bb | 90.9 ± 0.1 Cc | 128.6 ± 0.1 Ad | 15.4 ± 0.2 Ba | |
| Total free xanthophylls recovery | ||||||||
| Oral phase | 93.5 ± 1.0 Ad | 173.0 ± 3.5 Ce | 47.4 ± 4.1 Ab | 43.2 ± 1.2 Cab | 46.7 ± 0.4 Ab | 83.0 ± 1.1 Bc | 34.3 ± 0.2 Ba | |
| Gastric phase | 160.5 ± 8.6 Cd | 47.6 ± 0.2 Aab | 51.9 ± 1.9 Aab | 34.6 ± 0.6 Aa | 92.2 ± 1.1 Ce | 53.5 ± 0.8 Ab | 37.1 ± 0.2 Cab | |
| Intestinal phase | 132.8 ± 0.1 Bg | 107.0 ± 1.3 Bf | 83.9 ± 1.7 Be | 37.9 ± 0.1 Bb | 72.1 ± 0.7 Bc | 77.4 ± 1.2 Bd | 27.6 ± 0.4 Aa | |
| Xanthophyll esters | ||||||||
| (all-E)-lutein-3-O-myristate | Oral | n.d. Aa | 69.2 ± 0.7 Cd | n.d. Aa | n.d. Aa | 3.6 ± 0.1 Ba | n.d. Aa | 75.1 ± 2.4 Cc |
| Gastric | n.d. Aa | 32.7 ± 0.6 Bd | n.d. Aa | n.d. Aa | 2.1 ± 0.0 Ab | n.d. Aa | 29.8 ± 0.7 Ac | |
| Intestinal | n.d. Aa | 8.2 ± 0.1 Ab | n.d. Aa | n.d. Aa | 41.3 ± 0.3 Cc | n.d. Aa | 40.6 ± 0.9 Bc | |
| (9Z)-violaxanthin dimyristate | Oral | 41.1 ± 1.0 Ab | 95.2 ± 0.2 Cf | n.d. Aa | 89.1 ± 0.7 Ce | 55.0 ± 0.2 Ac | 74.4 ± 1.9 Cd | 108.3 ± 2.3 Cg |
| Gastric | 91.6 ± 5.2 Bc | 59.9 ± 1.7 Bb | n.d. Aa | 58.2 ± 0.6 Ab | 133.5 ± 0.9 Bd | 51.5 ± 1.4 Ab | 85.6 ± 1.0 Bc | |
| Intestinal | 52.7 ± 1.7 Ac | n.d. Aa | n.d. Aa | 69.9 ± 0.3 Be | 58.0 ± 0.8 Ad | 61.5 ± 1.0 Bd | 40.1 ± 1.6 Ab | |
| (all-E)-antheraxanthin myristate palmitate | Oral | 103.1 ± 1.9 Bc | 77.2 ± 5.3 Cb | n.d. Aa | 103.9 ± 3.8 Cc | 171.4 ± 7.7 Cd | 219.5 ± 2.2 Ce | 16.6 ± 0.7 Ba |
| Gastric | 121.7 ± 0.2 Cb | 52.5 ± 2.1 Bd | n.d. Aa | 77.6 ± 0.5 Be | 120.2 ± 0.3 Bg | 97.5 ± 0.8 Bf | 42.6 ± 1.1 Cc | |
| Intestinal | 94.3 ± 0.8 Af | n.d. Aa | n.d. Aa | 40.4 ± 0.2 Ac | 80.1 ± 1.0 Ae | 47.0 ± 0.4 Ad | 11.0 ± 0.0 Ab | |
| (all-E)-lutein dimyristate | Oral | 10.0 ± 0.2 Ab | 86.3 ± 1.7 Cf | 0.7 ± 0.0 Aa | 39.7 ± 0.1 Bd | 19.9 ± 0.6 Bc | 72.0 ± 2.6 Ae | 192.8 ± 0.3 Cg |
| Gastric | 18.7 ± 1.9 Bb | 51.5 ± 0.3 Bd | 7.4 ± 0.0 Ba | 29.8 ± 0.9 Ac | 12.4 ± 0.7 Aa | 96.8 ± 1.9 Bf | 91.4 ± 0.7 Ae | |
| Intestinal | 12.2 ± 0.4 Ab | 25.5 ± 0.2 Ac | 1.4 ± 0.0 Aa | 29.8 ± 0.2 Ad | 63.3 ± 1.0 Ce | 115.5 ± 0.5 Cg | 100.9 ± 0.4 Bf | |
| (all-E)-β-cryptoxanthin caprate | Oral | 75.0 ± 2.5 Ac | 59.0± 0.9 Cb | 36.8 ± 0.4 Ba | 110.9 ± 2.2 Cd | 161.0 ± 1.9 Cf | 146.4 ± 3.1 Ce | 31.5 ± 0.2 Ca |
| Gastric | 144.9 ± 4.9 Ce | 50.8 ± 1.1 Bb | 37.4 ± 0.2 Ba | 79.1 ± 0.8 Bc | 128.6 ± 2.1 Bd | 119.7 ± 1.9 Bd | 27.9 ± 0.1 Ba | |
| Intestinal | 96.2 ± 1.9 Bf | 22.9 ± 0.1 Ab | n.d. Aa | 56.3 ± 0.7 Ad | 63.1 ± 0.9 Ae | 49.1 ± 0.5 Ac | 23.9 ± 0.2 Ab | |
| (all-E)-β-cryptoxanthin laurate | Oral | 37.5 ± 0.0 Ab | 65.0 ± 1.3 Cd | 38.4 ± 0.1 Ab | 56.3 ± 0.5 Cc | 112.2 ± 0.3 Cf | 104.3 ± 1.1 Ce | 33.9 ± 0.0 Ca |
| Gastric | 96.4 ± 4.5 Cd | 45.9± 1.7 Bb | 37.5 ± 0.0 Ab | 42.0 ± 0.9 Bb | 70.0 ± 0.8 Bc | 77.5 ± 0.5 Bc | 23.7 ± 0.0 Aa | |
| Intestinal | 72.0 ± 0.2 Bf | 23.3 ± 0.4 Aa | 76.9 ± 0.4 Bg | 36.9 ± 0.1 Ac | 55.0 ± 0.6 Ae | 40.7 ± 0.4 Ad | 26.0 ± 0.3 Bb | |
| (all-E)-β-cryptoxanthin myristate | Oral | 13.5 ± 1.3 Aa | 49.1 ± 0.6 Cc | 28.4 ± 0.4 Ab | 65.1 ± 0.4 Be | 102.2 ± 0.6 Cf | 129.1 ± 1.6 Cg | 54.3 ± 0.1 Cd |
| Gastric | 48.0 ± 1.6 Cb | 42.0 ± 0.6 Bb | 84.6 ± 0.5 Cd | 94.3 ± 0.4 Ce | 72.5 ± 3.0 Bc | 68.7 ± 0.5 Bc | 25.3 ± 0.1 Aa | |
| Intestinal | 34.9 ± 3.0 Bb | 9.9 ± 0.3 Aa | 62.2 ± 0.3 Bd | 52.2 ± 0.3 Ac | 60.8 ± 0.9 Ad | 35.0 ± 0.4 Ab | 38.6 ± 0.7 Bb | |
| (all-E)-β-cryptoxanthin palmitate | Oral | n.d. Aa | 101.7 ± 0.7 Ce | 66.9 ± 0.4 Bb | 94.2 ± 0.0 Cd | 78.2 ± 0.6 Cc | 102.3 ± 0.9 Ce | n.d. Aa |
| Gastric | n.d. Aa | 65.5 ± 1.0 Bd | 157.5 ± 1.3 Ce | 21.2± 0.8 Bb | 60.0 ± 0.1 Bc | 61.0 ± 0.2 Bc | n.d. Aa | |
| Intestinal | n.d. Aa | 13.0 ± 0.3 Ab | n.d. Aa | 12.1 ± 0.3 Ab | n.d. Aa | 41.7 ± 0.7 Ac | n.d. Aa | |
| Total xanthophyll ester recovery | ||||||||
| Oral phase | 42.3 ± 0.3 Ac | 73.0 ± 0.1 Cfc | 4.8 ± 0.0 Aa | 38.2 ± 0.1 Bb | 44.0 ± 0.3 Ac | 71.2 ± 0.0 Be | 67.4 ± 0.9 Cd | |
| Gastric phase | 78.8 ± 1.5 Ce | 43.2 ± 0.5 Bc | 15.1 ± 0.1 Ca | 27.7 ± 0.1 Ab | 50.2 ± 1.5 Bd | 50.8 ± 0.2 Ad | 41.6 ± 0.1 Bc | |
| Intestinal phase | 58.0 ± 0.7 Be | 15.9 ± 0.2 Ab | 6.9 ± 0.0 Ba | 49.7 ± 0.1 Cd | 51.9 ± 1.0 Bd | 69.0 ± 0.5 Be | 37.6 ± 0.5 Ac | |
| Hydrocarbon carotenoids | ||||||||
| (all-E)-α-carotene | Oral | 218.5 ± 7.2 Be | 43.4 ± 0.5 Ab | 43.7 ± 0.4 Bb | 49.9 ± 0.8 Ab | 86.5 ± 0.1 Cc | 189.7 ± 3.0 Cd | 23.9 ± 0.0 Ca |
| Gastric | 245.7± 11.0 Bd | 85.2 ± 0.9 Bc | 22.5 ± 0.7 Aa | 83.6 ± 0.2 Cc | 63.8 ± 0.4 Bb | 94.6 ± 1.8 Bc | 21.1 ± 0.6 Ba | |
| Intestinal | 69.6 ± 1.1 Ad | 213.2 ± 1.4 Cf | 201.6 ± 1.2 Ce | 65.7± 0.7 Bd | 25.4 ± 0.1 Ab | 56.1 ± 0.7 Ac | 13.2 ± 0.4 Aa | |
| (all-E)-β-carotene | Oral | 50.5 ± 0.5 Ac | 57.7± 0.9 Cd | 43.6 ± 0.6 Bb | 74.4 ± 1.0 Ce | 138.4 ± 1.4 Cf | 215.7 ± 3.3 Cg | 29.6 ± 0.6 Ca |
| Gastric | 70.0 ± 1.3 Bf | 32.5 ± 0.9 Ac | 26.1 ± 0.1 Ab | 46.4 ± 0.3 Bd | 51.1 ± 0.4 Ae | 109.5 ± 1.0 Bg | 21.1 ± 0.4 Aa | |
| Intestinal | 76.9 ± 0.7 Cg | 37.2 ± 0.3 Bb | 56.8 ± 0.9 Cd | 41.7 ± 0.2 Ac | 66.9 ± 0.7 Bf | 62.3 ± 1.0 Ae | 25.1 ± 0.5 Ba | |
| (13Z)-lycopene isomer 2 | Oral | 36.3 ± 1.4 Aa | 77.2 ± 0.0 Bcd | 33.2 ± 0.0 Aa | 74.0 ± 2.0 Bc | 54.2 ± 0.5 Ab | 106.3 ± 2.3 Ce | 80.9 ± 0.3 Cd |
| Gastric | 60.9 ± 0.1 Cb | 76.9 ± 0.8 Be | 65.2 ± 0.2 Cc | 35.5 ± 0.9 Aa | 81.2 ± 1.2 Bf | 87.0 ± 1.0 Bg | 70.3 ± 1.1 Bd | |
| Intestinal | 54.4 ± 1.5 Bc | 27.4 ± 0.5 Aa | 41.6 ± 0.7 Bb | 85.8 ± 0.9 Ce | 94.9 ± 0.5 Cf | 70.5 ± 0.6 Ad | 46.0 ± 1.8 Ab | |
| (9Z)-lycopene isomer 4 | Oral | 86.9 ± 0.3 Ab | 97.8 ± 0.0 Bc | 177.3 ± 0.0 Bf | 126.6 ± 2.2 Ae | 87.5 ± 0.8 Ab | 63.1 ± 0.4 Aa | 111.6 ± 0.4 Ad |
| Gastric | 93.0 ± 8.0 Aa | 161.2 ± 1.9 Cc | 229.2 ± 1.2 Cd | 141.6± 1.2 Bb | 217.4 ± 2.2 Cd | 105.9 ± 1.1 Ba | 157.0 ± 1.6 Bc | |
| Intestinal | 89.5 ± 1.3 Ac | 57.5 ± 0.3 Ab | 41.8 ± 0.9 Aa | 171.8 ± 0.6 Cf | 144.0 ± 0.4 Bd | 151.7 ± 0.1 Ce | 236.7 ± 0.1 Cg | |
| (all-E)-lycopene | Oral | 1.4 ± 0.0 Aa | 29.3 ± 0.9 Ab | 29.9 ± 0.1 Ab | 41.6 ± 0.8 Cc | 69.3 ± 0.9 Bd | 79.5 ± 0.2 Ce | 97.6 ± 1.4 Cf |
| Gastric | 29.4 ± 0.1 Bc | 34.8 ± 0.4 Bd | 36.0 ± 0.0 Bd | 38.7 ± 0.0 Be | 20.1 ± 0.4 Aa | 36.8 ± 0.2 Bde | 26.8 ± 1.0 Ab | |
| Intestinal | 31.4 ± 0.8 Bb | 35.0 ± 0.4 Bb | 69.2 ± 1.1 Cc | 30.4 ± 0.0 Ab | 71.1 ± 2.2 Bc | 10.0 ± 0.1 Aa | 70.9 ± 0.2 Bc | |
| (Z)-lycopene isomer 6 | Oral | 68.9 ± 0.5 Cc | 176.4 ± 2.5 Cf | 76.2 ± 0.3 Bd | 114.7 ± 3.4 Ce | 42.1 ± 0.3 Ab | n.d. Aa | 36.2 ± 0.3 Bb |
| Gastric | 42.7 ± 0.0 Bc | 108.5 ± 0.0 Bf | 123.6 ± 0.5 Cg | 35.8 ± 0.7 Ab | 74.5 ± 0.3 Be | n.d. Aa | 56.4 ± 0.1 Cd | |
| Intestinal | 18.8 ± 0.5 Ab | 72.0 ± 0.3 Ae | 49.8 ± 0.2 Ac | 65.4 ± 0.7 Bd | 128.1 ± 1.0 Cf | n.d. Aa | n.d. Aa | |
| Total hydrocarbon carotenoid recovery | ||||||||
| Oral phase | 34.2 ± 0.2 Aa | 39.6 ± 0.1 Bb | 33.3 ± 0.1 Aa | 46.4 ± 0.7 Cc | 72.5 ± 0.2 Bd | 89.2 ± 0.3 Cf | 83.1 ± 0.7 Ce | |
| Gastric phase | 37.3 ± 0.3 Bb | 39.6 ± 0.3 Bc | 43.3 ± 0.0 Bd | 39.4 ± 0.1 Bc | 29.8 ± 0.7 Aa | 43.9 ± 0.2 Bd | 29.0 ± 0.3 Aa | |
| Intestinal phase | 37.0 ± 0.0 Bc | 34.3 ± 0.0 Ab | 68.0 ± 0.3 Ce | 36.5 ± 0.0 Ac | 71.5 ± 0.4 Bf | 17.7 ± 0.2 Aa | 60.4 ± 0.2 Bd | |
| Total carotenoids | ||||||||
| Oral phase | 23.4 ± 0.1 Aa | 49.0 ± 0.1 Cc | 22.4 ± 0.2 Aa | 44.2 ± 0.5 Cb | 64.8 ± 0.0 Bd | 84.6 ± 0.1 Cf | 76.4 ± 0.3 Ce | |
| Gastric phase | 51.1 ± 0.9 Ce | 40.5 ± 0.3 Bc | 32.5 ± 0.0 Ba | 36.2 ± 0.0 Ab | 36.5 ± 0.8 Ab | 45.6 ± 0.1 Bd | 32.8 ± 0.1 Aa | |
| Intestinal phase | 45.1 ± 0.1 Bd | 32.0 ± 0.1 Aa | 44.6 ± 0.0 Cc | 39.9 ± 0.0 Bb | 66.6 ± 0.0 Cf | 31.8 ± 0.1 Aa | 52.6 ± 0.1 Be | |
| Compound | Non-Treated | 100 MPa/CUT | 100 MPa/5 min | 350 MPa/CUT | 350 MPa/5 min | 600 MPa/CUT | 600 MPa/5 min |
|---|---|---|---|---|---|---|---|
| Free xanthophylls | |||||||
| (all-E)-violaxanthin | 0.6 ± 0.0 a | 1.5 ± 0.0 b | 1.4 ± 0.0 b | 3.2 ± 0.1 e | 2.6 ± 0.1 d | 2.4 ± 0.1 d | 2.0 ± 0.0 c |
| (all-E)-zeaxanthin | 1.5 ± 0.0 a | 2.6 ± 0.1 d | 1.7 ± 0.2 ab | 2.1 ± 0.0 bc | 2.4 ± 0.1 cd | 1.3 ± 0.0 a | 1.7 ± 0.0 ab |
| (all-E)-β-cryptoxanthin | 3.4 ± 0.1 f | 1.9 ± 0.1 c | n.d. a | 1.4 ± 0.0 b | 2.7 ± 0.1 e | 2.4 ± 0.0 d | 1.7 ± 0.0 c |
| Xanthophyll esters | |||||||
| (all-E)-β-cryptoxanthin laurate | 0.3 ± 0.0 d | n.d. a | n.d. a | 0.4 ± 0.0 e | 0.3 ± 0.0 d | 0.2 ± 0.0 c | 0.1 ± 0.0 b |
| Hydrocarbon carotenoids | |||||||
| (all-E)-α-carotene | n.d. a | n.d. a | n.d. a | 2.9 ± 0.0 b | n.d. a | n.d. a | n.d. a |
| (all-E)-β-carotene | 0.6 ± 0.0 c | n.d. a | n.d. a | n.d. a | 0.9 ± 0.0 e | 0.8 ± 0.0 d | 0.2 ± 0.0 b |
| (13Z)-lycopene isomer 2 | n.d. a | n.d. a | n.d. a | 0.9 ± 0.0 c | 0.4 ± 0.0 b | n.d. a | n.d. a |
| (all-E)-lycopene | 0.3 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.5 ± 0.0 b | 0.8 ± 0.1 c | 0.2 ± 0.0 a | 0.1 ± 0.0 a |
| (Z)-lycopene isomer 6 | n.d. a | 0.4 ± 0.0 c | 0.3 ± 0.0 b | 1.6 ± 0.0 d | n.d. a | n.d. a | n.d. a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Abia, S.; Welti-Chanes, J.; Cano, M.P. Effect of High Hydrostatic Pressure on the Extractability and Bioaccessibility of Carotenoids and Their Esters from Papaya (Carica papaya L.) and Its Impact on Tissue Microstructure. Foods 2021, 10, 2435. https://doi.org/10.3390/foods10102435
Lara-Abia S, Welti-Chanes J, Cano MP. Effect of High Hydrostatic Pressure on the Extractability and Bioaccessibility of Carotenoids and Their Esters from Papaya (Carica papaya L.) and Its Impact on Tissue Microstructure. Foods. 2021; 10(10):2435. https://doi.org/10.3390/foods10102435
Chicago/Turabian StyleLara-Abia, Sara, Jorge Welti-Chanes, and M. Pilar Cano. 2021. "Effect of High Hydrostatic Pressure on the Extractability and Bioaccessibility of Carotenoids and Their Esters from Papaya (Carica papaya L.) and Its Impact on Tissue Microstructure" Foods 10, no. 10: 2435. https://doi.org/10.3390/foods10102435
APA StyleLara-Abia, S., Welti-Chanes, J., & Cano, M. P. (2021). Effect of High Hydrostatic Pressure on the Extractability and Bioaccessibility of Carotenoids and Their Esters from Papaya (Carica papaya L.) and Its Impact on Tissue Microstructure. Foods, 10(10), 2435. https://doi.org/10.3390/foods10102435