Development of a New Monoclonal Antibody against Brevetoxins in Oyster Samples Based on the Indirect Competitive Enzyme-Linked Immunosorbent Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
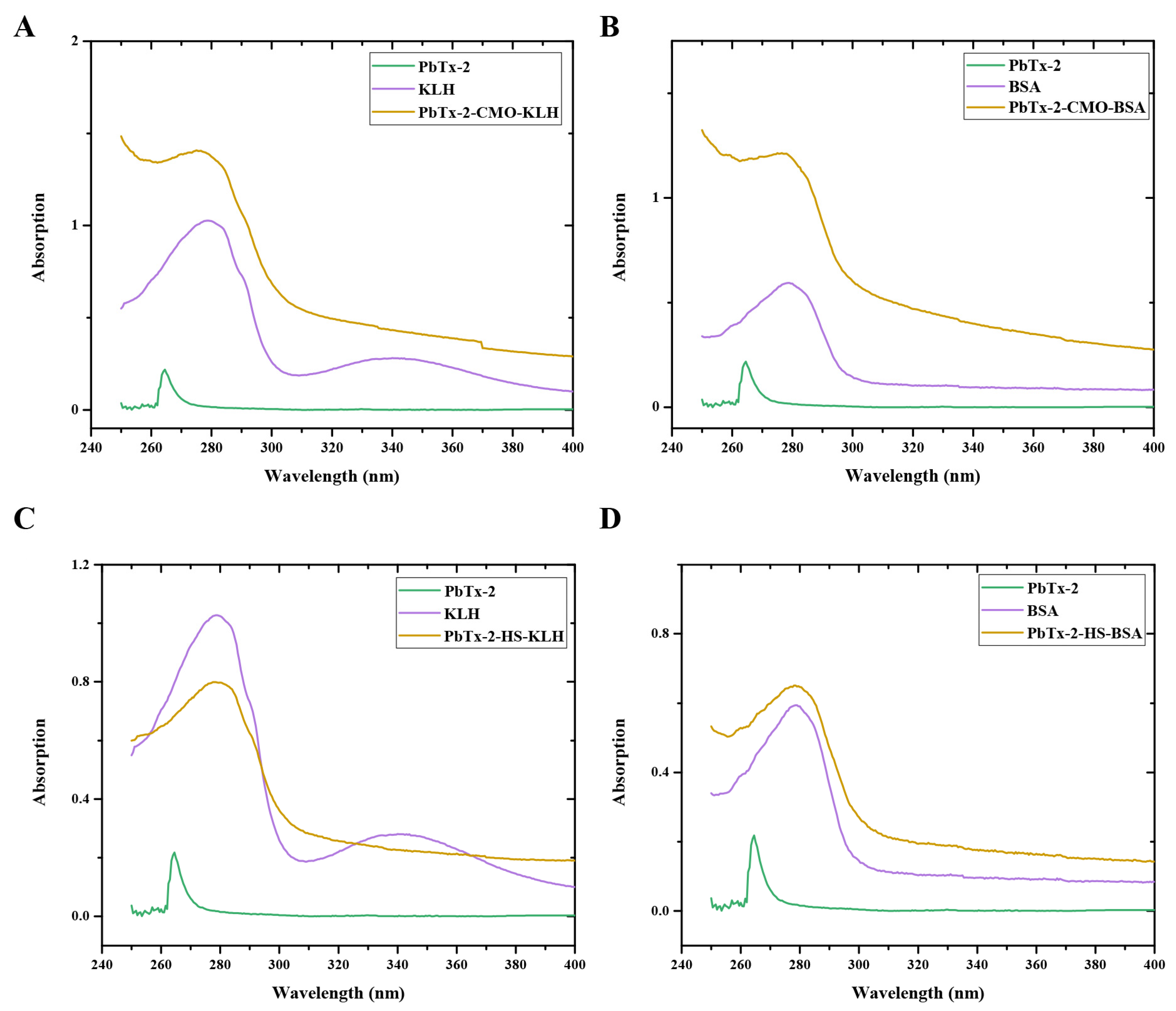
2.2. Preparation of PbTx-2-Protein Conjugates
2.3. Preparation of mAbs
2.4. ELISA and icELISA Protocols
2.5. Sample Preparation
2.6. Molecule Alignment and Electrostatic Potential Analysis
3. Results and Discussion
3.1. Hapten Design, Synthesis, and Conjugate Preparation
3.2. Antibody Production and Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Özogul, F.; Hamed, I. Marine-based toxins and their health risk. In Food Quality: Balancing Health and Disease; Academic Press: Cambridge, MA, USA, 2018; pp. 109–144. [Google Scholar]
- Radwan, F.F.Y.; Wang, Z.H.; Ramsdell, J.S. Identification of a rapid detoxification mechanism for brevetoxin in rats. Toxicol. Sci. 2005, 85, 839–846. [Google Scholar] [CrossRef]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar]
- Hoagland, P.; Jin, D.; Beet, A.; Kirkpatrick, B.; Reich, A.; Ullmann, S.; Fleming, L.E.; Kirkpatrick, G. The human health effects of Florida red tide (FRT) blooms: An expanded analysis. Environ. Int. 2014, 68, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Baden, D.G.; Bourdelais, A.J.; Jacocks, H.; Michelliza, S.; Naar, J. Natural and derivative brevetoxins: Historical background, multiplicity, and effects. Environ. Health Perspect. 2005, 113, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Shin, C.; Hwang, J.Y.; Yoon, J.H.; Kim, S.H.; Kang, G.J. Simultaneous determination of neurotoxic shellfish toxins (brevetoxins) in commercial shellfish by liquid chromatography tandem mass spectrometry. Food Control. 2018, 91, 365–371. [Google Scholar] [CrossRef]
- Wunschel, D.S.; Valenzuela, B.R.; Kaiser, B.L.D.; Victry, K.; Woodruff, D. Method development for comprehensive extraction and analysis of marine toxins: Liquid-liquid extraction and tandem liquid chromatography separations coupled to electrospray tandem mass spectrometry. Talanta 2018, 187, 302–307. [Google Scholar] [CrossRef]
- Dom, I.; Bire, R.; Hort, V.; Lavison-Bompard, G.; Nicolas, M.; Guerin, T. Extended targeted and non-targeted strategies for the analysis of marine toxins in mussels and oysters by (LC-HRMS). Toxins 2018, 10, 375. [Google Scholar] [CrossRef] [Green Version]
- McCall, J.R.; Jacocks, H.M.; Baden, D.G.; Bourdelais, A.J. Development of a competitive fluorescence-based synaptosome binding assay for brevetoxins. Harmful Algae 2012, 19, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Yasumoto, T. Chemiluminescent receptor binding assay for ciguatoxins and brevetoxins using acridinium brevetoxin-b2. Toxins 2019, 11, 580. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Li, Y.S.; Pan, F.G.; Zhang, Y.Y.; Lu, S.Y.; Ren, H.L.; Li, Z.H.; Liu, Z.S.; Zhang, J.H. Development of a new monoclonal antibody based direct competitive enzyme-linked immunosorbent assay for detection of brevetoxins in food samples. Food Chem. 2010, 118, 467–471. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, F.G.; Li, Y.S.; Zhang, Y.Y.; Zhang, J.H.; Lu, S.Y.; Ren, H.L.; Liu, Z.S. Colloidal gold probe-based immunochromatographic assay for the rapid detection of brevetoxins in fishery product samples. Biosens. Bioelectron. 2009, 24, 2744–2747. [Google Scholar] [CrossRef]
- Lai, W.; Wei, Q.; Zhuang, J.; Lu, M.; Tang, D. Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens. Bioelectron. 2016, 80, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Naar, J.; Branaa, P.; Bottein-Dechraoui, M.Y.; Chinain, M.; Pauillac, S. Polyclonal and monoclonal antibodies to PbTx-2-type brevetoxins using minute amount of hapten-protein conjugates obtained in a reversed micellar medium. Toxicon 2001, 39, 869–878. [Google Scholar] [CrossRef]
- Li, H.; Ma, S.; Zhang, X.; Li, C.; Dong, B.; Mujtaba, M.G.; Wei, Y.; Liang, X.; Yu, X.; Wen, K.; et al. Generic hapten synthesis, broad-specificity monoclonal antibodies preparation, and ultrasensitive elisa for five antibacterial synergists in chicken and milk. J. Agric. Food Chem. 2018, 66, 11170–11179. [Google Scholar] [CrossRef] [PubMed]
- Mari, G.M.; Li, H.; Dong, B.; Yang, H.; Talpur, A.; Mi, J.; Guo, L.; Yu, X.; Ke, Y.; Han, D.; et al. Hapten synthesis, monoclonal antibody production and immunoassay development for direct detection of 4-hydroxybenzehydrazide in chicken, the metabolite of nifuroxazide. Food Chem. 2021, 355, 129598. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Li, D.; Chen, X.; Li, Z.; Gao, H.; Cao, L.; Li, S.; Hou, Y. Development of an indirect competitive enzyme-linked immunosorbent assay for screening ethopabate residue in chicken muscle and liver. RSC Adv. 2017, 7, 36072–36080. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Sheng, F.; Kong, D.; Wang, Y.; Pan, Y.; Chen, M.; Tao, Y.; Liu, Z.; Ahmed, S.; Yuan, Z.; et al. Broad-spectrum monoclonal antibody and a sensitive multi-residue indirect competitive enzyme-linked immunosorbent assay for the antibacterial synergists in samples of animal origin. Food Chem. 2019, 280, 20–26. [Google Scholar] [CrossRef]
- Huang, J.X.; Yao, C.Y.; Yang, J.Y.; Li, Z.F.; He, F.; Tian, Y.X.; Wang, H.; Xu, Z.L.; Shen, Y.D. Design of novel haptens and development of monoclonal antibody-based immunoassays for the simultaneous detection of tylosin and tilmicosin in milk and water samples. Biomolecules 2019, 9, 770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keeffe, M.; Crabbe, P.; Salden, M.; Wichers, J.; Van Peteghem, C.; Kohen, F.; Pieraccini, G.; Moneti, G. Preliminary evaluation of a lateral flow immunoassay device for screening urine samples for the presence of sulphamethazine. J. Immunol. Methods 2003, 278, 117–126. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, K.; Wang, Z.; Jiang, H.; Beier, R.C.; Shen, J. An ultra-sensitive monoclonal antibody-based fluorescent microsphere immunochromatographic test strip assay for detecting aflatoxin M1 in milk. Food Control 2016, 60, 588–595. [Google Scholar] [CrossRef]
- Zhang, X.; Eremin, S.A.; Wen, K.; Yu, X.; Li, C.; Ke, Y.; Jiang, H.; Shen, J.; Wang, Z. Fluorescence polarization immunoassay based on a new monoclonal antibody for the detection of the zearalenone class of mycotoxins in maize. J. Agric. Food Chem. 2017, 65, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Song, M.; Yu, X.; Wang, Z.; Ke, Y.; Jiang, H.; Li, J.; Shen, J.; Wen, K. Development of a new broad-specific monoclonal antibody with uniform affinity for aflatoxins and magnetic beads-based enzymatic immunoassay. Food Control. 2017, 79, 309–316. [Google Scholar] [CrossRef]
- Peng, D.; Chang, F.; Wang, Y.; Chen, D.; Liu, Z.; Zhou, X.; Feng, L.; Yuan, Z. Development of a sensitive monoclonal-based enzyme-linked immunosorbent assay for monitoring T-2 toxin in food and feed. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 683–692. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, M.; Shi, W.; Li, C.; Zhang, S.; Shen, J. New haptens and antibodies for ractopamine. Food Chem. 2015, 183, 111–114. [Google Scholar] [CrossRef] [PubMed]



| Immunogen: PbTx-2-CMO-KLH/Coating Antigen PbTx-2-CMO-BSA a | ||||||
|---|---|---|---|---|---|---|
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Absorbance of PbTx at 0 mg/kg | 1.64 ± 0.06 | 0.52 ± 0.07 | 0.74 ± 0.07 | 0.76 ± 0.08 | 0.98 ± 0.07 | 1.542 ± 0.06 |
| Absorbance of PbTx at 0.10 mg/kg c | 0.51 ± 0.02 | 0.15 ± 0.01 | 0.17 ± 0.01 | 0.20 ± 0.03 | 0.29 ± 0.05 | 0.63 ± 0.06 |
| Absorbance of PbTx at 1.00 mg/kg c | 0.11 ± 0.02 | 0.07 ± 0.01 | 0.08 ± 0.02 | 0.09 ± 0.01 | 0.11 ± 0.01 | 0.14 ± 0.01 |
| Inhibition ratio by 0.10 mg/kg | 68.90% | 71.15% | 77.03% | 84.80% | 70.41% | 59.14% |
| Inhibition ratio by 1.00 mg/kg | 93.29% | 86.54% | 89.19% | 88.16% | 88.78% | 90.92% |
| Immunogen: PbTx-2-HS-KLH/Coating Antigen PbTx-2-HS-BSA b | ||||||
| No. | 1 | 2 | 3 | 4 | 5 | 6 |
| Absorbance of PbTx at 0 mg/L | 2.39 ± 0.05 | 0.71 ± 0.04 | 0.28 ± 0.01 | 1.07 ± 0.08 | 1.68 ± 0.14 | 0.79 ± 0.01 |
| Absorbance of PbTx at 0.50 mg/L c | 2.13 ± 0.07 | 0.63 ± 0.05 | 0.17 ± 0.01 | 0.59 ± 0.01 | 1.14 ± 0.07 | 0.69 ± 0.04 |
| Absorbance of PbTx at 5.00 mg/L c | 1.77 ± 0.03 | 0.58 ± 0.07 | 0.16 ± 0.05 | 0.34 ± 0.06 | 0.96 ± 0.06 | 0.49 ± 0.01 |
| Inhibition ratio by 0.50 mg/kg | 10.88% | 14.86% | 39.29% | 44.86% | 32.14% | 12.66% |
| Inhibition ratio by 5.00 mg/kg | 25.94% | 18.31% | 42.86% | 68.22% | 42.86% | 37.97% |
| Compound | PbTx-2 | CR (%) | PbTx-1 | CR (%) | PbTx-3 | CR (%) |
|---|---|---|---|---|---|---|
| mAb 6D8 a | 786.72 | 100 | 806.52 | 97.92 | 726.51 | 108.29 |
| mAb 9G8 a | 434.54 | 100 | 424.61 | 102.33 | 398.62 | 109.01 |
| mAb 1D3 b | 78.53 | 100 | 80.62 | 97.41 | 69.84 | 112.44 |
| mAb 1D3 c | 60.71 | 100 | 52.61 | 115.40 | 51.83 | 117.13 |
| Toxin | Concentration (μg/kg) | Recovery of Toxins from Oyster |
|---|---|---|
| PbTx-2 | 200 | 108.33 ± 3.82% |
| 400 | 91.25 ± 3.31% | |
| 800 | 91.21 ± 1.25% | |
| PbTx-1 | 200 | 115.00 ± 5.00% |
| 400 | 92.92 ± 3.82% | |
| 800 | 91.04 ± 3.21% | |
| PbTx-3 | 200 | 113.17 ± 4.25% |
| 400 | 94.42 ± 3.12% | |
| 800 | 89.08 ± 1.12% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ding, M.; Zhang, C.; Mao, Y.; Wang, Y.; Li, P.; Jiang, H.; Wang, Z.; Yu, X. Development of a New Monoclonal Antibody against Brevetoxins in Oyster Samples Based on the Indirect Competitive Enzyme-Linked Immunosorbent Assay. Foods 2021, 10, 2398. https://doi.org/10.3390/foods10102398
Zhang X, Ding M, Zhang C, Mao Y, Wang Y, Li P, Jiang H, Wang Z, Yu X. Development of a New Monoclonal Antibody against Brevetoxins in Oyster Samples Based on the Indirect Competitive Enzyme-Linked Immunosorbent Assay. Foods. 2021; 10(10):2398. https://doi.org/10.3390/foods10102398
Chicago/Turabian StyleZhang, Xiya, Mingyue Ding, Chensi Zhang, Yexuan Mao, Youyi Wang, Peipei Li, Haiyang Jiang, Zhanhui Wang, and Xuezhi Yu. 2021. "Development of a New Monoclonal Antibody against Brevetoxins in Oyster Samples Based on the Indirect Competitive Enzyme-Linked Immunosorbent Assay" Foods 10, no. 10: 2398. https://doi.org/10.3390/foods10102398
APA StyleZhang, X., Ding, M., Zhang, C., Mao, Y., Wang, Y., Li, P., Jiang, H., Wang, Z., & Yu, X. (2021). Development of a New Monoclonal Antibody against Brevetoxins in Oyster Samples Based on the Indirect Competitive Enzyme-Linked Immunosorbent Assay. Foods, 10(10), 2398. https://doi.org/10.3390/foods10102398








