The Effects of Using Pineapple Stem Starch as an Alternative Starch Source and Ageing Period on Meat Quality, Texture Profile, Ribonucleotide Content, and Fatty Acid Composition of Longissimus Thoracis of Fattening Dairy Steers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Experimental Cattle and Muscle Collection
2.3. Meat Characteristics
2.3.1. Colour Measurement
2.3.2. Thawing Loss, Cooking Loss and Shear Force Analysis
2.3.3. Texture Profile Analysis
2.4. Ribonucleotide Analysis
2.5. Fatty Acid Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Meat Quality and Texture Profile
3.2. Ribonucleotides
3.3. Fatty Acid Composition
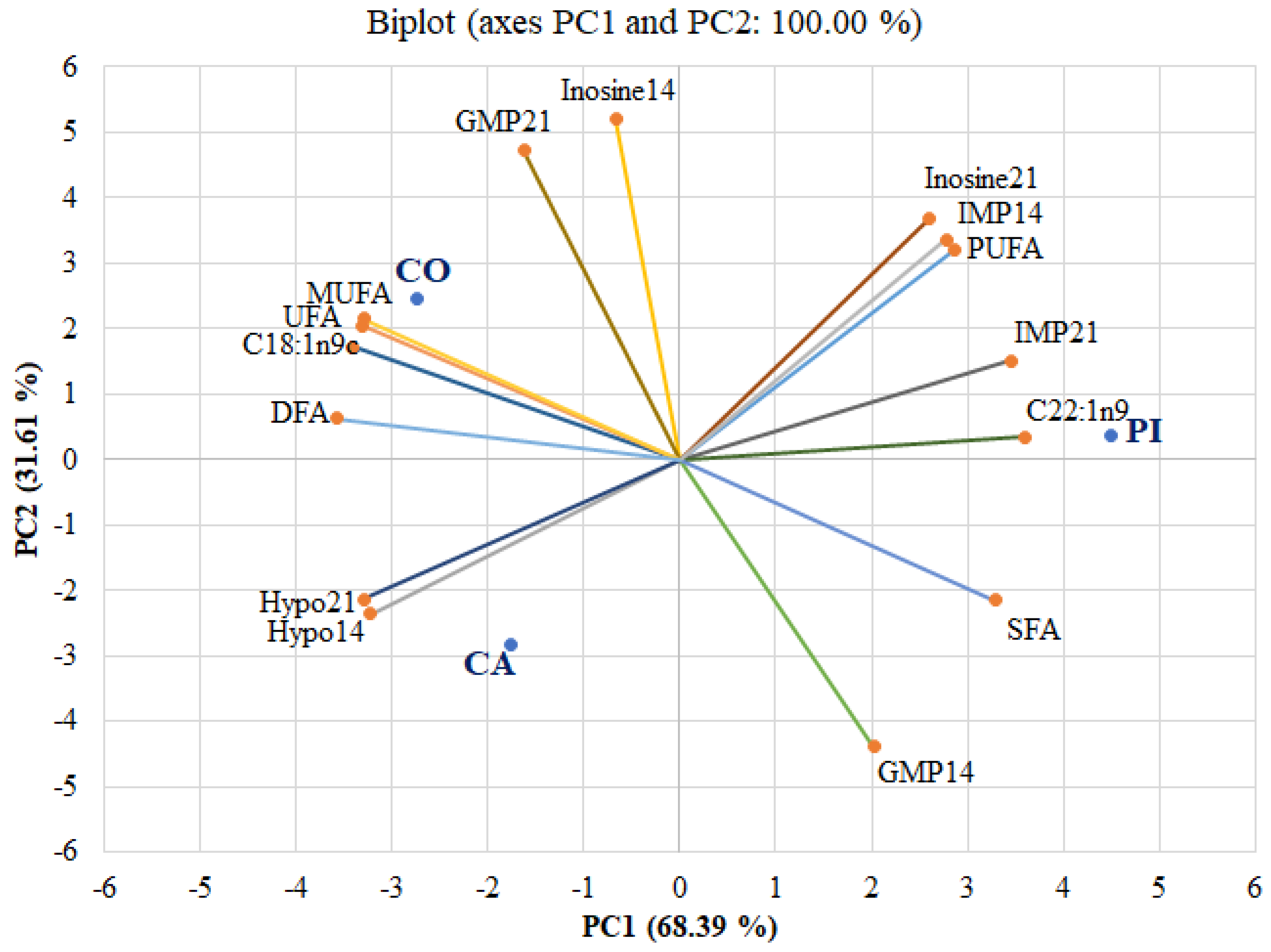
3.4. Ribonucleotide Content and Fatty Acid Composition in Relation to Different Starch Sources in Concentrate and Ageing Period
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Department of Trade Negotiations, Ministry of Commerce, Thailand. Product Profile Pineapple. Available online: https://api.dtn.go.th/files/v3/60fa587cef41404be4216b7f/download (accessed on 1 September 2021).
- Suksathit, S.; Wachirapakorn, C.; Opatpatanakit, Y. Effects of levels of ensiled pineapple waste and pangola hay fed as roughage sources on feed intake, nutrient digestibility and ruminal fermentation of Southern Thai native cattle. Songklanakarin J. Sci. Technol. 2011, 33, 281–289. [Google Scholar]
- Greenwood, P.L. Review: An overview of beef production from pasture and feedlot globally, as demand for beef and the need for sustainable practices increase. Animal 2021, 100295. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-H.; Joo, S.-T. Fatty Acid Profiles, Meat Quality, and Sensory Palatability of Grain-fed and Grass-fed Beef from Hanwoo, American, and Australian Crossbred Cattle. Food Sci. Anim. Resour. 2017, 37, 153–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Beak, S.-H.; Jung, D.J.S.; Kim, S.Y.; Jeong, I.H.; Piao, M.Y.; Kang, H.J.; Fassah, D.M.; Na, S.W.; Yoo, S.P.; et al. Genetic, management, and nutritional factors affecting intramuscular fat deposition in beef cattle—A review. Asian Australas. J. Anim. Sci. 2018, 31, 1043–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duckett, S.K.; Neel, J.P.S.; Lewis, R.M.; Fontenot, J.P.; Clapham, W.M. Effects of forage species or concentrate finishing on animal performance, carcass and meat quality1,2. J. Anim. Sci. 2013, 91, 1454–1467. [Google Scholar] [CrossRef] [Green Version]
- Khongpradit, A.; Boonsaen, P.; Homwong, N.; Matsuba, K.; Kobayashi, Y.; Sawanon, S. Effect of Starch Source in Concentrate Diets and of Days on Feed on Growth Performance, Carcass Characteristics, Meat Quality, and Economic Return of Feedlot Steers. Tropic. Anim. Health Product. 2021, in press. [Google Scholar]
- Sawanon, S.; Boonsaen, P.; Khongpradit, A. A Guide for High. Quality Beef Production from Fattening Male Dairy Cattle; Department of Animal Science, Faculty of Agriculture, Kamphaeng Saen Campus; Kasetsart University: Nakhon Pathom, Thailand, 2017. [Google Scholar]
- Duff, G.C.; McMurphy, C.P. Feeding Holstein steers from start to finish. Vet. Clin. North Am. Food Anim. Pract. 2007, 23, 281–297. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Na, Y. Effects of a pineapple (Ananas comosus L.) cannery by-product on growth performance and carcass characteristics in finishing Hanwoo steers. Anim. Biosci. 2021, 34, 233–242. [Google Scholar] [CrossRef]
- Pintadis, S.; Boonsaen, P.; Hattakum, C.; Homwong, N.; Sawanon, S. Effects of concentrate levels and pineapple stem on growth performance, carcass and meat quality of dairy steers. Trop. Anim. Health Prod. 2020, 52, 1911–1917. [Google Scholar] [CrossRef]
- Hattakum, C.; Kanjanapruthipong, J.; Nakthong, S.; Wongchawalit, J.; Piamya, P.; Sawanon, S. Pineapple stem by-product as a feed source for growth performance, ruminal fermentation, carcass and meat quality of Holstein steers. S. Afr. J. Anim. Sci. 2019, 49, 147. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crop. Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Khongpradit, A.; Boonsaen, P.; Homwong, N.; Suzuki, Y.; Koike, S.; Sawanon, S.; Kobayashi, Y. Effect of pineapple stem starch feeding on rumen microbial fermentation, blood lipid profile, and growth performance of fattening cattle. Anim. Sci. J. 2020, 91, e13459. [Google Scholar] [CrossRef] [PubMed]
- Felderhoff, C.; Lyford, C.; Malaga, J.; Polkinghorne, R.; Brooks, C.; Garmyn, A.; Miller, M. Beef Quality Preferences: Factors Driving Consumer Satisfaction. Foods 2020, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flowers, S.; McFadden, B.R.; Carr, C.C.; Mateescu, R.G. Consumer preferences for beef with improved nutrient profile1. J. Anim. Sci. 2019, 97, 4699–4709. [Google Scholar] [CrossRef]
- Gilmore, L.A.; Walzem, R.L.; Crouse, S.F.; Smith, D.R.; Adams, T.H.; Vaidyanathan, V.; Cao, X.; Smith, S.B. Consumption of High-Oleic Acid Ground Beef Increases HDL-Cholesterol Concentration but Both High- and Low-Oleic Acid Ground Beef Decrease HDL Particle Diameter in Normocholesterolemic Men. J. Nutr. 2011, 141, 1188–1194. [Google Scholar] [CrossRef] [Green Version]
- Muroya, S.; Oe, M.; Ojima, K.; Watanabe, A. Metabolomic approach to key metabolites characterizing postmortem aged loin muscle of Japanese Black (Wagyu) cattle. Asian Australas. J. Anim. Sci. 2019, 32, 1172–1185. [Google Scholar] [CrossRef] [Green Version]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of Inosine Monophosphate and Its Degradation Products during Aging of Pork of Different Qualities in Relation to Basic Taste and Retronasal Flavor Perception of the Meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef]
- Koohmaraie, M.; Geesink, G. Contribution of postmortem muscle biochemistry to the delivery of consistent meat quality with particular focus on the calpain system. Meat Sci. 2006, 74, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.; Stromer, M.; Robson, R. Identification of the 30 kDa polypeptide in post mortem skeletal muscle as a degradation product of troponin-T. Biochimie 1994, 76, 369–375. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Hughes, J.M.; Clarke, F.M.; Purslow, P.P.; Warner, R.D. Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Compr. Rev. Food Sci. Food Saf. 2019, 19, 44–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeller, S.; Miller, R.; Edwards, K.; Zerby, H.; Logan, K.; Aldredge, T.; Stahl, C.; Boggess, M.; Box-Steffensmeier, J. Consumer perceptions of pork eating quality as affected by pork quality attributes and end-point cooked temperature. Meat Sci. 2010, 84, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Joo, S.-T.; Warner, R. Consumer Acceptability of Intramuscular Fat. Food Sci. Anim. Resour. 2016, 36, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Ledward, D.A.; Johnston, D.E.; Knight, M.K. The Chemistry of Muscle-Based Foods; Royal Society of Chemistry: Cambridge, UK, 1992. [Google Scholar]
- Mancini, R.; Ramanathan, R. Effects of postmortem storage time on color and mitochondria in beef. Meat Sci. 2014, 98, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Colle, M.; Richard, R.; Killinger, K.; Bohlscheid, J.; Gray, A.; Loucks, W.; Day, R.; Cochran, A.; Nasados, J.; Doumit, M. Influence of extended aging on beef quality characteristics and sensory perception of steaks from the gluteus medius and longissimus lumborum. Meat Sci. 2015, 110, 32–39. [Google Scholar] [CrossRef]
- Chaosap, C.; Sivapirunthep, P.; Sitthigripong, R.; Tavitchasri, P.; Maduae, S.; Kusee, T.; Setakul, J.; Adeyemi, K. Meat quality, post-mortem proteolytic enzymes, and myosin heavy chain isoforms of different Thai native cattle muscles. Anim. Biosci. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chaosap, C.; Sitthigripong, R.; Sivapirunthep, P.; Pungsuk, A.; Adeyemi, K.; Sazili, A.Q. Myosin heavy chain isoforms expression, calpain system and quality characteristics of different muscles in goats. Food Chem. 2020, 321, 126677. [Google Scholar] [CrossRef]
- Palka, K. The influence of post-mortem ageing and roasting on the microstructure, texture and collagen solubility of bovine semitendinosus muscle. Meat Sci. 2002, 64, 191–198. [Google Scholar] [CrossRef]
- Nishimura, T. Mechanism Involved in the Improvement of Meat Taste during Postmortem Aging. Food Sci. Technol. Int. 1998, 4, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Jones, N.R. Meat and fish flavors; significance of ribomononucleotides and their metabolites. J. Agric. Food Chem. 1969, 17, 712–716. [Google Scholar] [CrossRef]
- Melton, S.L.; Black, J.M.; Davis, G.W.; Backus, W.R. Flavor and Selected Chemical Components of Ground Beef from Steers Backgrounded on Pasture and Fed Corn up to 140 Days. J. Food Sci. 1982, 47, 699–704. [Google Scholar] [CrossRef]
- Theurer, C.B. Grain Processing Effects on Starch Utilization by Ruminants. J. Anim. Sci. 1986, 63, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, W.; Tan, Z. Effects of dietary starch types on rumen fermentation and blood profile in goats. Czech. J. Anim. Sci. 2016, 61, 32–41. [Google Scholar] [CrossRef] [Green Version]
- Smith, S.B.; Crouse, J.D. Relative Contributions of Acetate, Lactate and Glucose to Lipogenesis in Bovine Intramuscular and Subcutaneous Adipose Tissue. J. Nutr. 1984, 114, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Griinari, J.M.; Corl, B.A.; Lacy, S.H.; Chouinard, P.Y.; Nurmela, K.V.V.; Bauman, D.E. Conjugated Linoleic Acid Is Synthesized Endogenously in Lactating Dairy Cows by Δ9-Desaturase. J. Nutr. 2000, 130, 2285–2291. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of δ6, δ5, and δ9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef]
| Trait | Treatment (T) 1 | Ageing (A) | RMSE 2 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| CO | CA | PI | 14 Days | 21 Days | T | A | T × A | ||
| Meat Quality | |||||||||
| Colour | |||||||||
| L* | 35.32 | 36.51 | 36.41 | 35.67 | 36.49 | 2.32 | 0.347 | 0.268 | 0.933 |
| a* | 14.46 | 15.29 | 14.78 | 14.96 | 14.72 | 1.79 | 0.509 | 0.676 | 0.339 |
| b* | 41.36 | 42.13 | 42.24 | 41.44 | 42.36 | 3.21 | 0.739 | 0.358 | 0.641 |
| Thawing loss (%) | 3.31 | 3.48 | 3.93 | 3.08 b | 4.06 a | 1.18 | 0.430 | 0.019 | 0.777 |
| Cooking loss (%) | 19.23 | 21.01 | 21.73 | 19.60 | 21.72 | 4.22 | 0.287 | 0.121 | 0.712 |
| Shear force (kg) | 5.72 | 4.97 | 5.03 | 5.41 | 5.07 | 1.08 | 0.151 | 0.328 | 0.949 |
| Texture Profile | |||||||||
| Hardness (N/cm2) | 40.57 | 42.74 | 48.85 | 40.92 | 47.19 | 22.80 | 0.615 | 0.392 | 0.890 |
| Springiness (cm) | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.00 | 0.473 | 0.694 | 0.636 |
| Gumminess (N/cm2) | 24.29 | 23.95 | 26.38 | 22.98 | 26.76 | 12.60 | 0.863 | 0.351 | 0.931 |
| Chewiness (N/cm) | 23.48 | 23.94 | 25.95 | 22.27 | 26.64 | 12.46 | 0.858 | 0.275 | 0.945 |
| Cohesiveness(ratio) | 0.57 | 0.59 | 0.55 | 0.57 | 0.56 | 0.06 | 0.206 | 0.880 | 0.980 |
| Trait 1 | Treatment (T) 2 | Ageing (A) | RMSE 3 | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| CO | CA | PI | 14 Days | 21 Days | T | A | T x A | ||
| Hypo 4 | 31.76 | 33.52 | 27.61 | 27.13 d | 34.80 c | 9.80 | 0.294 | 0.019 | 0.507 |
| Inosine | 39.18 | 36.43 | 38.06 | 40.31 | 35.47 | 12.12 | 0.848 | 0.216 | 0.900 |
| IMP 5 | 71.82 b | 55.42 b | 107.2 a | 107.44 a | 48.86 b | 35.20 | 0.002 | <0.0001 | 0.629 |
| GMP 6 | 2.49 | 3.09 | 3.10 | 3.47 c | 2.33 d | 1.67 | 0.552 | 0.039 | 0.079 |
| Trait | Starch Source 1 | RMSE | p-Value | |||
|---|---|---|---|---|---|---|
| CO | CA | PI | ||||
| Fatty Acid Composition (% of Total Fatty Acids) | ||||||
| Lauric acid | C12:0 | 0.18 | 0.19 | 0.17 | 0.05 | 0.776 |
| Myristic acid | C14:0 | 4.37 | 4.84 | 4.83 | 0.46 | 0.133 |
| Myristoleic acid | C14:1 | 1.55 | 1.81 | 1.54 | 0.45 | 0.501 |
| Pentadecylic acid | C15:0 | 0.25 | 0.25 | 0.24 | 0.08 | 0.961 |
| Palmitic acid | C16:0 | 27.81 | 27.90 | 29.59 | 1.57 | 0.092 |
| Palmitoleic acid | C16:1 | 6.28 | 6.23 | 6.10 | 0.81 | 0.917 |
| Margaric acid | C17:0 | 0.57 | 0.55 | 0.60 | 0.08 | 0.477 |
| Heptadecenoic acid | C17:1 | 0.55 | 0.51 | 0.56 | 0.11 | 0.657 |
| Stearic acid | C18:0 | 10.93 | 11.39 | 10.68 | 1.38 | 0.650 |
| Oleic acid | C18:1n9c | 43.70 a | 42.36 ab | 40.65 b | 1.91 | 0.027 |
| Linoleic acid | C18:2n6c | 1.15 | 1.10 | 1.18 | 0.24 | 0.821 |
| α-Linolenic acid | C18:3n3 | 0.23 | 0.23 | 0.18 | 0.07 | 0.383 |
| Heneicosylic acid | C21:0 | 0.24 | 0.23 | 0.19 | 0.10 | 0.580 |
| Erucic acid | C22:1n9 | 0.45 b | 0.47 b | 0.74 a | 0.10 | 0.021 |
| Arachidonic acid | C20:4n6 | 0.15 | 0.18 | 0.19 | 0.07 | 0.659 |
| Lignoceric acid | C24:0 | 0.11 | 0.13 | 0.17 | 0.08 | 0.413 |
| Nervonic acid | C24:1 | 1.49 | 1.64 | 2.40 | 0.82 | 0.116 |
| Total fatty acid 2 | 8.60 | 9.37 | 9.07 | 1.82 | 0.748 | |
| SFA | 44.45 | 45.49 | 46.45 | 1.99 | 0.200 | |
| MUFA | 54.02 | 53.01 | 51.99 | 2.02 | 0.202 | |
| PUFA | 1.53 | 1.51 | 1.55 | 0.27 | 0.956 | |
| UFA | 55.55 | 54.51 | 53.55 | 1.99 | 0.200 | |
| DFA | 66.48 | 65.91 | 64.22 | 1.76 | 0.072 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaosap, C.; Sahatsanon, K.; Sitthigripong, R.; Sawanon, S.; Setakul, J. The Effects of Using Pineapple Stem Starch as an Alternative Starch Source and Ageing Period on Meat Quality, Texture Profile, Ribonucleotide Content, and Fatty Acid Composition of Longissimus Thoracis of Fattening Dairy Steers. Foods 2021, 10, 2319. https://doi.org/10.3390/foods10102319
Chaosap C, Sahatsanon K, Sitthigripong R, Sawanon S, Setakul J. The Effects of Using Pineapple Stem Starch as an Alternative Starch Source and Ageing Period on Meat Quality, Texture Profile, Ribonucleotide Content, and Fatty Acid Composition of Longissimus Thoracis of Fattening Dairy Steers. Foods. 2021; 10(10):2319. https://doi.org/10.3390/foods10102319
Chicago/Turabian StyleChaosap, Chanporn, Katatikarn Sahatsanon, Ronachai Sitthigripong, Suriya Sawanon, and Jutarat Setakul. 2021. "The Effects of Using Pineapple Stem Starch as an Alternative Starch Source and Ageing Period on Meat Quality, Texture Profile, Ribonucleotide Content, and Fatty Acid Composition of Longissimus Thoracis of Fattening Dairy Steers" Foods 10, no. 10: 2319. https://doi.org/10.3390/foods10102319






