Maintaining the Balance of Intestinal Flora through the Diet: Effective Prevention of Illness
Abstract
:1. Introduction
2. Gut Flora and Brain–Gut Axis
2.1. Gut Flora
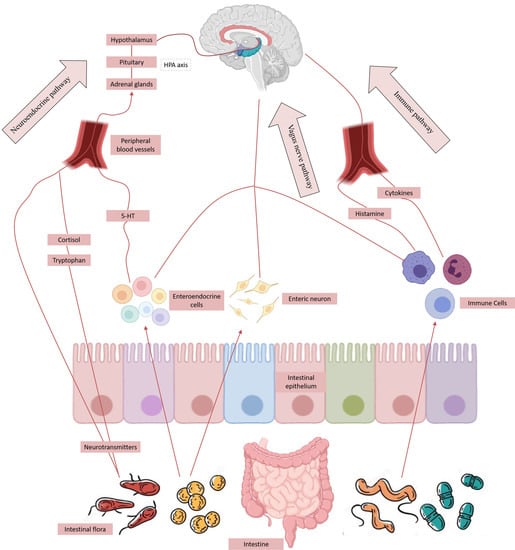
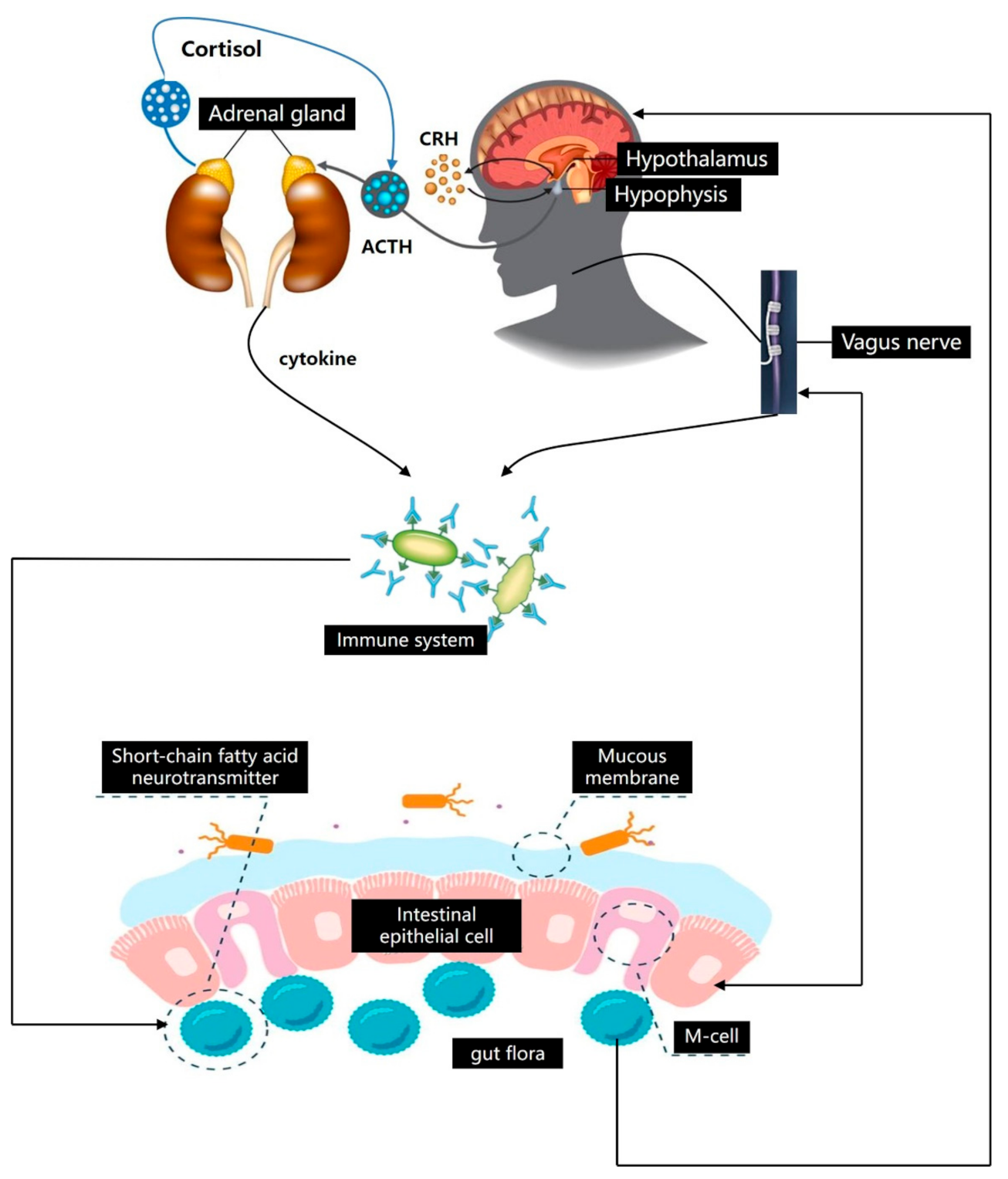
2.2. Bi-Directional Regulation Mechanism of Brain–Gut Axis
2.3. Gut–Brain Axis Influences the Distribution of Intestinal Microflora
3. Gut Flora and Mental Illness
3.1. Dual Relationship between Intestinal Microflora and Depression
3.2. Relationship between Intestinal Microflora and AD
3.3. Gut Flora and Parkinson
4. Dietary Intervention: Improving Intestinal Microbiota Imbalance to Prevent Mental Illness
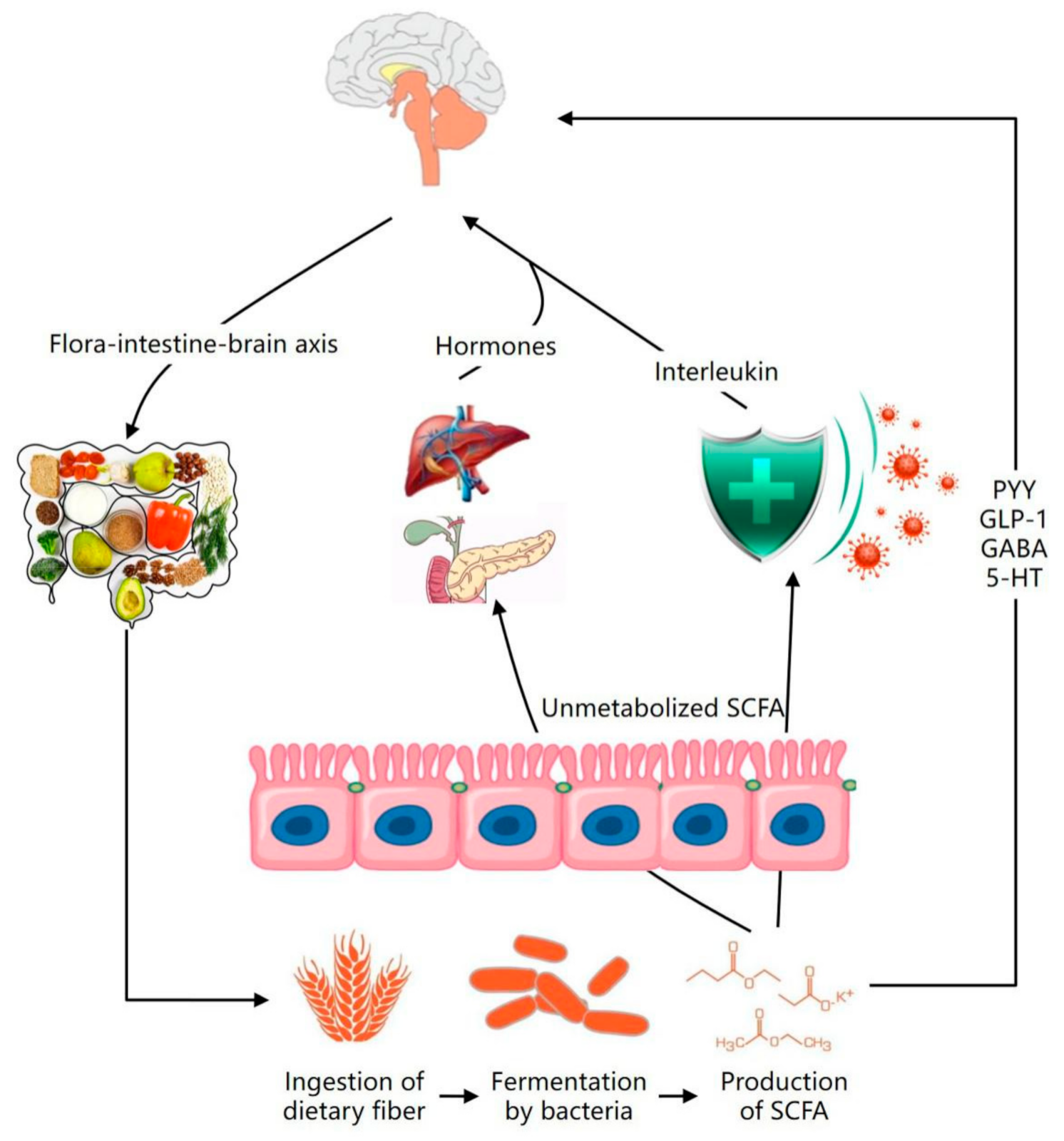
4.1. Regulation of Dietary Fiber on Intestinal Flora: An Effective Method to Prevent Mental Disease
4.2. Regulation of Tea Polyphenols on Intestinal Flora—Effective Prevention of Mental Diseases
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, B.; Kim, M.; Yun, C. Regulation of gastrointestinal immunity by metabolites. Nutrients 2021, 13, 167. [Google Scholar] [CrossRef]
- Gianfranco, N.; Larisa, R.; Gabriele, M.; Gloria, L.; Alessandro, F.; Francesco, F. The baseline structure of the enteric nervous system and its role in Parkinson’s disease. Life 2021, 11, 732. [Google Scholar]
- Wu, S.; Liu, X.; Jiang, R.; Yan, X.; Ling, Z. Roles and mechanisms of gut microbiota in patients with Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 650047–650064. [Google Scholar] [CrossRef]
- Alina, W.; Lukasz, P.; Wieslaw, J. Gut microbiota in depression: A focus on Ketamine. Front. Behav. Neurosci. 2021, 15, 693362. [Google Scholar]
- Tracey, B.; Julie, D.; Jane, C.; Nicole, R.; Christine, B.; Pramod, G. The microbiome-gut-brain axis and resilience to developing anxiety or depression under stress. Microorganisms 2021, 9, 723. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Rohitha, M.; Andrew, S.; Eric, S. Systemic disease associations with disorders of gut–brain interaction and gastrointestinal transit: A review. Clin. Exp. Gastroenterol. 2021, 14, 249–257. [Google Scholar]
- Pan, W.; Jiang, P.; Zhao, J.; Shi, H.; Zhang, P.; Yang, X.; Biazik, J.; Hu, M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54–71. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Stavropoulou, E.; Bezirtzoglou, E.E.; Tsakris, A. Potential elimination of human gut resistome by exploiting the benefits of functional foods. Front. Microbiol. 2020, 11, 50. [Google Scholar] [CrossRef] [Green Version]
- Rajesh, J.; Pravin, M. Dietary fiber in poultry nutrition and their effects on nutrient utilization, performance, gut health, and on the environment: A review. J. Anim. Sci. Biotechnol. 2021, 12, 51–67. [Google Scholar]
- Kaur, A.P.; Bhardwaj, S.; Dhanjal, D.S.; Nepovimova, E.; CruzMartins, N.; Kuca, K.; Chopra, C.; Singh, R.; Kumar, H.; Sen, F.; et al. Plant prebiotics and their role in the amelioration of diseases. Biomolecules 2021, 11, 440. [Google Scholar] [CrossRef]
- Shen, G.; Wu, J.; Ye, B.; Qi, N. Gut microbiota-derived metabolites in the development of diseases. Can. J. Infect. Dis. Med Microbiol. 2021, 2021, 6658674. [Google Scholar] [CrossRef] [PubMed]
- Serrano, W.; Olaechea, R.M.; Tarazona, U.I. Comparative analysis of gut microbiota in two populations of argopecten purpuratus (Lamarck, 1819) based on 16S rRNA gene amplicon data. Microbiol. Resour. Announc. 2020, 9, 1481–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, Y. Research progress of relationship between intestinal flora and allergic diseases. E3s Web Conf. 2019, 5, 78–95. [Google Scholar] [CrossRef]
- Nayla, M.; Khansa, A.; Khalid, M.; Aftab, A.; Munir, A.; Iltaf, S.; Ahlam, K.; Fadwa, M. Hidden role of gut microbiome dysbiosis in schizophrenia: Antipsychotics or psychobiotics as therapeutics? Int. J. Mol. Sci. 2021, 22, 7671. [Google Scholar] [CrossRef]
- Montiel, A.J.; Cervantes, R.M.; Bravo, R.G.; Pacheco, L.G. The microbiota-gut-brain axis: Neurobehavioral correlates, health and sociality. Front. Integr. Neurosci. 2013, 7, 70–79. [Google Scholar]
- Yan, W.; Lloyd, H.K. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar]
- Julie, D.; Faye, C.; Raylene, R.; Willem, H.; Mohamad, B.; Katherine, P.; Andrew, M.; Linda, C. The chemo-gut study: Investigating the long-term effects of chemotherapy on gut microbiota, metabolic, immune, psychological and cognitive parameters in young adult Cancer survivors; study protocol. BMC Cancer 2019, 19, 1243. [Google Scholar] [CrossRef] [Green Version]
- Stilling, R.M.; Dinan, T.G.; Cryan, J. Microbial genes, brain & behaviour-epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014, 13, 69–86. [Google Scholar]
- Wang, S.; Yu, Y.; Khosrow, A. Role of gut microbiota in neuroendocrine regulation of carbohydrate and lipid metabolism via the microbiota-gut-brain-liver axis. Microorganisms 2020, 8, 527. [Google Scholar] [CrossRef] [Green Version]
- Mark, O.; Shams, T.; Bushra, S.; Benjamin, M.; George, P. The microbiota-gut-brain axis-heart shunt part II: Prosaic foods and the brain-heart connection in Alzheimer disease. Microorganisms 2020, 8, 493. [Google Scholar] [CrossRef] [Green Version]
- Emeran, A.M. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011, 2, 453–466. [Google Scholar]
- Karakan, T.; Ozkul, C.; Akkol, E.K.; Bilici, S.; SobarzoSánchez, E.; Capasso, R. Gut-Brain-Microbiota axis: Antibiotics and functional gastrointestinal disorders. Nutrients 2021, 13, 389. [Google Scholar] [CrossRef]
- Philip, S. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar]
- Carmela, C.; Marzia, S.; Giovanna, T. Gut-brain axis: Focus on neurodegeneration and mast cells. Appl. Sci. 2020, 10, 1828. [Google Scholar] [CrossRef] [Green Version]
- Yasmine, B.; Shruti, N. Compartmentalized and systemic control of tissue immunity by commensals. Nat. Immunol. 2013, 14, 646–653. [Google Scholar]
- Chase, M.; Matt, V. The fragility of probiotic bifidobacterium longum NCC3001 use for depression in patients with irritable bowel syndrome. Gastroenterology 2018, 154, 764–769. [Google Scholar]
- Afifa, A.; Payard, I.; Rolland, C.; Harkat, C.; Braniste, V.; Théodorou, V.; Tompkins, T.A. Bifidobacterium longum and lactobacillus helveticus synergistically suppress stress-related visceral hypersensitivity through hypothalamic-pituitary-adrenal axis modulation. J. Neurogastroenterol. Motil. 2018, 24, 138–146. [Google Scholar]
- Mark, L. Microbial endocrinology in the microbiome-gut-brain axis: How bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013, 9, e1003726. [Google Scholar] [CrossRef]
- Donatella, M.; Beatrice, B.; Stefania, P.; Elisabetta, P.; Alessandro, A.; Maria, F.B.; Lucia, M.; Barbara, C.; Filippo, B.; Federico, M. The Microbiota/Microbiome and the Gut–Brain Axis: How Much Do They Matter in Psychiatry? Life 2021, 11, 760–769. [Google Scholar]
- Rudzki, L.; Maes, M. The microbiota-gut-immune-glia axis in major depression. Mol. Neurobiol. 2020, 57, 4269–4295. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, J.; Yuan, G.; Long, L.; Zhou, Y. Bioinformatics analysis and identification of potential genes related to pathogenesis of cervical intraepithelial neoplasia. J. Cancer 2020, 11, 2150–2157. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Zhou, N.; Ma, W.; Gu, X.; Chen, B.; Zeng, Y.; Yang, L.; Zhou, M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019, 10, 2947–2957. [Google Scholar] [CrossRef]
- Luo, Y.; Zeng, B.; Zeng, L.; Du, X.; Li, B.; Huo, R.; Liu, L.; Wang, H.; Dong, M.; Pan, J.; et al. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the Hippocampus. Transl. Psychiatry 2018, 8, 187–195. [Google Scholar] [CrossRef]
- Hannah, S. Depression linked to the microbiome. Nat. Med. 2019, 25, 358. [Google Scholar]
- Jiang, H.; Pan, L.; Zhang, X.; Zhang, Z.; Zhou, Y.; Ruan, B. Altered gut bacterial-fungal interkingdom networks in patients with current depressive episode. Brain Behav. 2020, 10, e01677. [Google Scholar] [CrossRef]
- Lin, P.; Ding, B.; Feng, C.; Yin, S.; Zhang, T.; Qi, X.; Lv, H.; Guo, X.; Dong, K.; Zhu, Y.; et al. Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 2017, 207, 300–304. [Google Scholar] [CrossRef]
- Averina, V.; Yana, Z.; Roman, Y.; Alexey, K.; Valeriya, U.; Anna, M.; George, K.; Valery, D.; Vladimir, C. Bacterial metabolites of human gut microbiota correlating with depression. Int. J. Mol. Sci. 2020, 21, 9234. [Google Scholar] [CrossRef] [PubMed]
- Sarah, D.; Gerard, C.; Michael, B.; Felice, N.J. The gut microbiome and diet in psychiatry: Focus on depression. Curr. Opin. Psychiatry 2015, 28, 1–6. [Google Scholar]
- Filipe, D.V.; Petia, K.D.; Daisy, G.; Jennifer, V.; Carine, Z.; Adeline, D.; Fredrik, B.; Gilles, M. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar]
- Elaine, Y.H.; Sara, W.M.; Sophia, H.; Gil, S.; Embriette, R.H.; Tyler, M.; Julian, A.C.; Janet, C.; Sarah, E.R.; Joseph, F.P.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar]
- Schatzberg, A.F.; Keller, J.; Tennakoon, L.; Lembke, A.; Williams, G.; Kraemer, F.B.; Sarginson, J.E.; Lazzeroni, L.C.; Murphy, G. HPA axis genetic variation, cortisol and psychosis in major depression. Mol. Psychiatry 2014, 19, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Luna, R.A.; Foster, J.A. Gut brain axis: Diet microbiota interactions and implications for modulation of anxiety and depression. Curr. Opin. Biotechnol. 2015, 32, 35–41. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in pafients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef] [Green Version]
- Williams, B.B.; Van, A.H.; Cimermancic, P.; Donia, M.S.; Zimmermann, M.; Taketani, M.; Ishihara, A.; Kashyap, P.C.; Fraser, J.S.; Fischbach, M.A. Discovery and characterization of gut microbiota decarboxylases that can produce the neurotransmitter tryptamine. Cell Host Microbe 2014, 16, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Herrp, K. Reimagining Alzheimer’s disease—An age-based hypothesis. J. Neurosci. 2010, 30, 16755–16762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marta, S.; Breno, S.D.; Jerzy, L. Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 2017, 54, 8071–8089. [Google Scholar]
- Alessandro, V.; Sara, D.T.; Adriana, M. Sexual differentiation of microglia. Front. Neuroendocr. 2019, 52, 156–164. [Google Scholar]
- Aurelie, L.P.; Gilles, D.; Eric, H.F.; Anis, L.; Graham, P.; Jacek, M.W.; Tamas, F. Role of the peripheral innate immune system in the development of Alzheimer’s disease. Exp. Gerontol. 2017, 107, 59–66. [Google Scholar]
- Tohidpour, A.; Morgun, A.V.; Boitsova, E.B.; Malinovskaya, N.A.; Martynova, G.P.; Khilazheva, E.D.; Kopylevich, N.V.; Gertsog, G.E.; Salmina, A.B. Neuroinflammation and infection: Molecular mechanisms associated with dysfunction of neurovascular Unit. Cell Infect. Microbiol. 2017, 7, 276–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Wang, X.; Geng, M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018, 7, 2–19. [Google Scholar] [CrossRef] [Green Version]
- Megan, W.B.; Ishraq, A.; Scott, J.B.; Rajiv, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar]
- Mark, K.; Courtney, A.M.; Daniel, M.F.; Krista, M.H.; Stephen, J.H.; David, S.; Gavin, R. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology 2010, 35, 870–880. [Google Scholar]
- Harishankar, P.Y.; Yun, L. The development of treatment for Parkinson’s disease. Adv. Parkinson’s Dis. 2015, 4, 59–78. Adv. Parkinson’s Dis. 2015, 4, 59–78. [Google Scholar]
- Sakakibara, R. Gastrointestinal dysfunction in movement disorders. Neurol. Sci. 2021, 42, 1355–1365. [Google Scholar] [CrossRef]
- Graz, A.; Urbas, S.; Antoniuk, T.; Adamczewska, K.; Bien, T.; Siuda, J. Comparative analysis of non-motor symptoms in patients with Parkinson’s disease and atypical Parkinsonisms. Clin. Neurol. Neurosurg. 2020, 197, 106088. [Google Scholar]
- Yu, Q.; Yu, S.; Zuo, L.; Lian, T.; Hu, Y.; Wang, R.; Piao, Y.; Guo, P.; Liu, L.; Jin, Z.; et al. Parkinson disease with constipation: Clinical features and relevant factors. Sci. Rep. 2018, 8, 197–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Jiang, Q.; Zhao, S.; Yan, B.; Zhou, X. Impact of buckwheat fermented milk combined with high-fat diet on rats’ gut microbiota and short-chain fatty acids. J. Food Sci. 2019, 84, 3833–3842. [Google Scholar] [CrossRef]
- Sun, Q.; Cheng, L.; Zeng, X.; Zhang, X.; Wu, Z.; Weng, P. The modulatory effect of plant polysaccharides on gut flora and the implication for neurodegenerative diseases from the perspective of the microbiota-gut-brain axis. Int. J. Biol. Macromol. 2020, 164, 1484–1492. [Google Scholar] [CrossRef]
- Yuuki, O.; Vassilis, P. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology 2016, 151, 836–844. [Google Scholar]
- Reyes, J.F.; EkmarkLewen, S.; Perdiki, M.; Klingstedt, T.; Hoffmann, A.; Wiechec, E.; Nilsson, P.; Nilsson, P.R.; Alafuzoff, I.; Hallbeck, M. Accumulation of alpha-synuclein within the liver, potential role in the clearance of brain pathology associated with Parkinson’s disease. Acta Neuropathol. Commun. 2021, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, L.; Zeng, X.; Zhang, X.; Liu, Y.; Wu, Z.; Weng, P. The intervention of unique plant polysaccharides-Dietary fiber on depression from the gut-brain axis. Int. J. Biol. Macromol. 2020, 170, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Kidane, D. Gut microbiota imbalance and base excision repair dynamics in colon cancer. J. Cancer 2016, 7, 1421–1430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzeciak, P.; Herbet, M. Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Konrad, K.; Renata, W.; Emilia, F.; Agnieszka, N. Effects of physical and chemical factors on the structure of gluten, gliadins and glutenins as studied with spectroscopic methods. Molecules 2021, 26, 508. [Google Scholar] [CrossRef]
- Ghada, A.S. Dietary fiber, Atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [Green Version]
- Sabina, L.; Vasiliki, K.; Fanny, B.; Phillip, P.; Nicholas, A.P.; Eric, C.M.; Robert, R.; Glenn, G.; Bjorge, W. Wood-derived dietary fibers promote beneficial human gut microbiota. mSphere 2019, 4, 4–18. [Google Scholar]
- Celestine, W.; Philip, H.; Lynnette, F. Potential benefits of dietary fiber intervention in inflammatory bowel disease. Int. J. Mol. Sci. 2016, 17, 919. [Google Scholar] [CrossRef] [Green Version]
- Cronin, P.; Joyce, S.A.; Paul, W.O.; Eibhlis, M. Dietary fibre modulates the gut microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Chung, J.; Jeong, J.H.; Song, J. Resveratrol modulates the gut-brain axis: Focus on Glucagon-Like Peptide-1, 5-HT and gut microbiota. Front. Aging Neurosci. 2020, 12, 588044. [Google Scholar] [CrossRef]
- Mishima, Y.; Ishihara, S. Molecular mechanisms of microbiota-mediated pathology in irritable bowel syndrome. Int. J. Mol. Sci. 2020, 21, 8864. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, S.; Li, S.; Zhang, L.; Wu, L.; Zhu, H.; Zhang, Y.; Liu, J. Role of 5-Hydroxytryptamine and intestinal flora on depressive-like behavior induced by lead exposure in Rats. BioMed Res. Int. 2021, 12, 1–14. [Google Scholar]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Maggi, F.; Giron, M.; Giaroni, C.; Baj, A. Impact of microbial metabolites on microbiota-gut-brain axis in inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jia, Q.; Song, L.; Duan, L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: A systematic review and meta-analysis. Medicine 2019, 98, 14513–14526. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Cates, A.N.; Gillespie, A.L.; Godlewska, B.R.; Scaife, J.C.; Wright, L.; Cowen, P.; Harmer, C. Translating the promise of 5HT4 receptor agonists for the treatment of depression. Psychol. Med. 2020, 13, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Browning, K.N.; Carson, K.E. Central neurocircuits regulating food intake inresponse to gut inputs-preclinical evidence. Nutrients 2021, 13, 908. [Google Scholar] [CrossRef]
- Mark, L.; Ashley, C.; Joshua, L.; Ai, Y.; Alexandra, P.; Jay, J.; Gregory, P.; Mihai, C. Resistant starch alters the microbiota-gut brain axis: Implications for dietary modulation of behavior. PLoS ONE 2016, 11, e0146406. [Google Scholar] [CrossRef]
- Shohei, A.; Yuko, A.; Yoko, N.; Mitsuru, Y.; Sohsaku, Y.; Takahisa, K.; Kazushige, C.; Taiga, T.; Masaki, H.; Atsushi, A.; et al. Fiber-rich barley increases butyric acid-producing bacteria in the human gut microbiota. Metabolites 2021, 11, 559–607. [Google Scholar]
- Hellas, C.; Philip, C.C. Defining a healthy diet: Evidence for the role of contemporary dietary patterns in health and disease. Nutrients 2020, 12, 334. [Google Scholar] [CrossRef] [Green Version]
- Noemi, R.; Esther, N.; Natalia, G.Z.; Ligia, E.D.; Sonia, G.M.; Ascension, M. Microbiota and lifestyle: A special focus on diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- Agusti, A.; Garcia, P.; Lopez, I.; Campillo, I.; Maes, M.; Romaní, M.; Sanz, Y. Interplay between the gut-brain axis, obesity and cognitive function. Front. Neurosci. 2018, 12, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chen, Y.; Cheng, M.; Zhang, X.; Zheng, X.; Zhang, Z. The modulatory effect of polyphenols from green tea, oolong tea and black tea on human intestinal microbiota in vitro. J. Food Sci. Technol. 2018, 55, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, K.; Kałdunska, J.; Kochman, J.; Janda, K. Chemical profile and antioxidant activity of the kombucha beverage derived from white, green, black and red tea. Antioxidants 2020, 9, 447. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yang, C.; Zhang, X.; Wang, Y. The relationship between host circadian rhythms and intestinal microbiota: A new cue to improve health by tea polyphenols. Crit. Rev. Food Sci. Nutr. 2020, 61, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Gradisar, H.; Pristovsek, P.; Plaper, A.; Jerala, R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J. Med. Chem. 2007, 50, 264–271. [Google Scholar] [CrossRef]
- Thida, K.; Sakunnee, B.; Yingmanee, T. Efficacy of kombucha obtained from green, oolong, and black teas on inhibition of pathogenic Bacteria, antioxidation, and toxicity on colorectal cancer cell line. Microorganisms 2019, 7, 700. [Google Scholar] [CrossRef] [Green Version]
- Ana, M.; Clara, S. A review on the biological activity of camellia species. Molecules 2021, 26, 2178. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar]
- Selma, M.V.; Espin, J.; Tomas, F. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombo, F. Bioavailability of dietary polyphenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 10, 18–36. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Sang, S.; Zhu, N.; Lu, H.; Sheng, S.; Lee, M.; Ho, C.; Yang, C. Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats. Chem. Res. Toxicol. 2002, 15, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Bond, T.; Derbyshire, E. Tea compounds and the gut microbiome: Findings from trials and mechanistic studies. Nutrients 2019, 11, 2364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarewicz, M.; Drod, L.; Tarko, T. The Interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2018, 10, 188. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, Z.; Xu, L.; Zhang, X. Maintaining the Balance of Intestinal Flora through the Diet: Effective Prevention of Illness. Foods 2021, 10, 2312. https://doi.org/10.3390/foods10102312
Zhang L, Zhang Z, Xu L, Zhang X. Maintaining the Balance of Intestinal Flora through the Diet: Effective Prevention of Illness. Foods. 2021; 10(10):2312. https://doi.org/10.3390/foods10102312
Chicago/Turabian StyleZhang, Li, Zhenying Zhang, Lei Xu, and Xin Zhang. 2021. "Maintaining the Balance of Intestinal Flora through the Diet: Effective Prevention of Illness" Foods 10, no. 10: 2312. https://doi.org/10.3390/foods10102312