Sprouting Time Affects Sorghum (Sorghum bicolor [L.] Moench) Functionality and Bread-Baking Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Composition and Enzymatic Activity of Sorghum Flour
2.3. Sorghum Flour Functionality
2.3.1. Water (WAC) and Oil (OAC) Absorption Capacity
2.3.2. Swelling Power (Sp) and Pasting Properties
2.4. Protein Features
2.4.1. Kafirin Extraction and Electrophoresis Analysis (SDS-PAGE)
2.4.2. Protein Solubility and Thiol Accessibility
2.5. Sorghum–Wheat Blend Functionality
2.6. Bread Making
2.7. Bread Properties
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition and Enzymatic Activities
3.2. Functional Properties
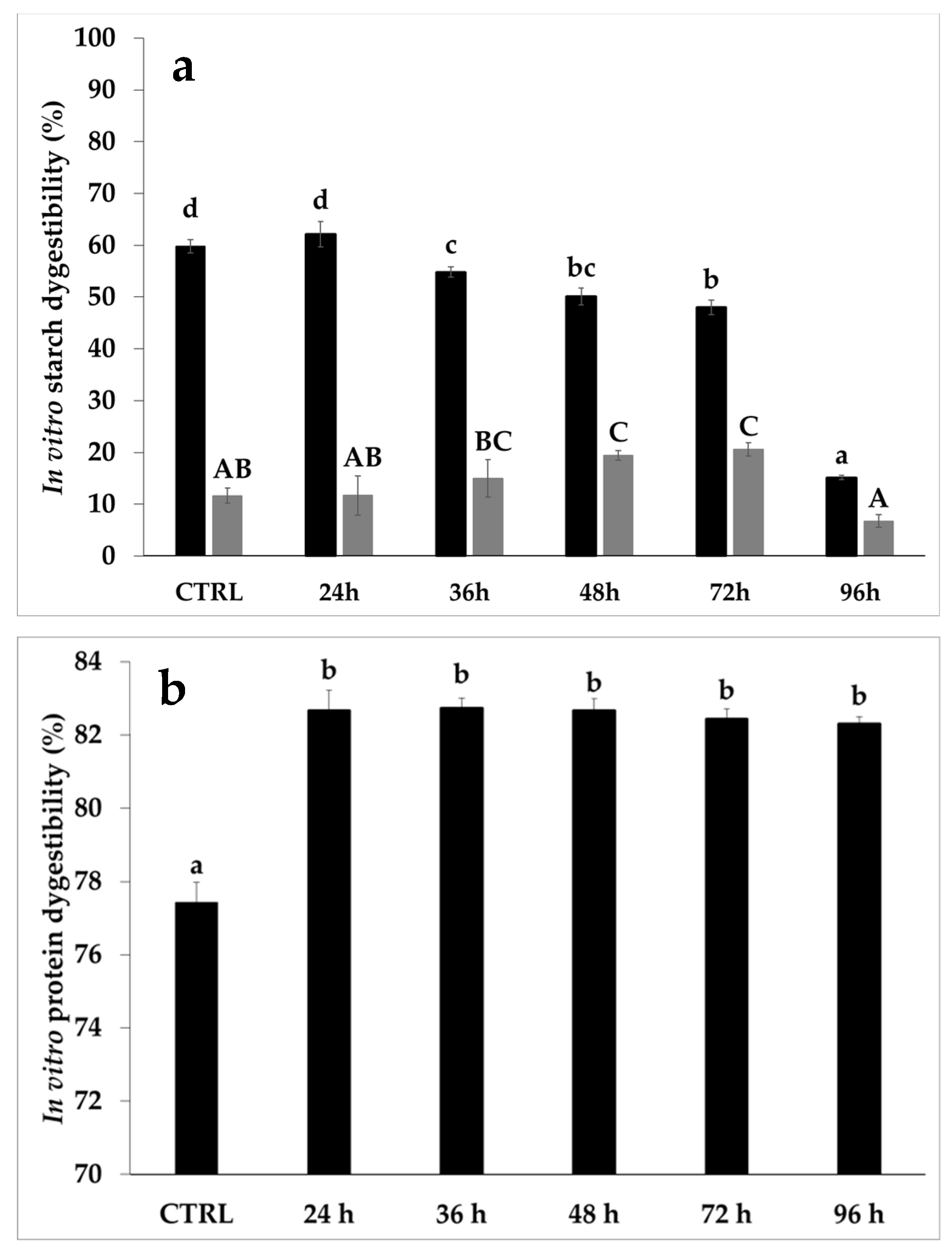
3.3. Protein Features
3.4. Effects of Sprouting on Sorghum–Wheat Blend Functionality
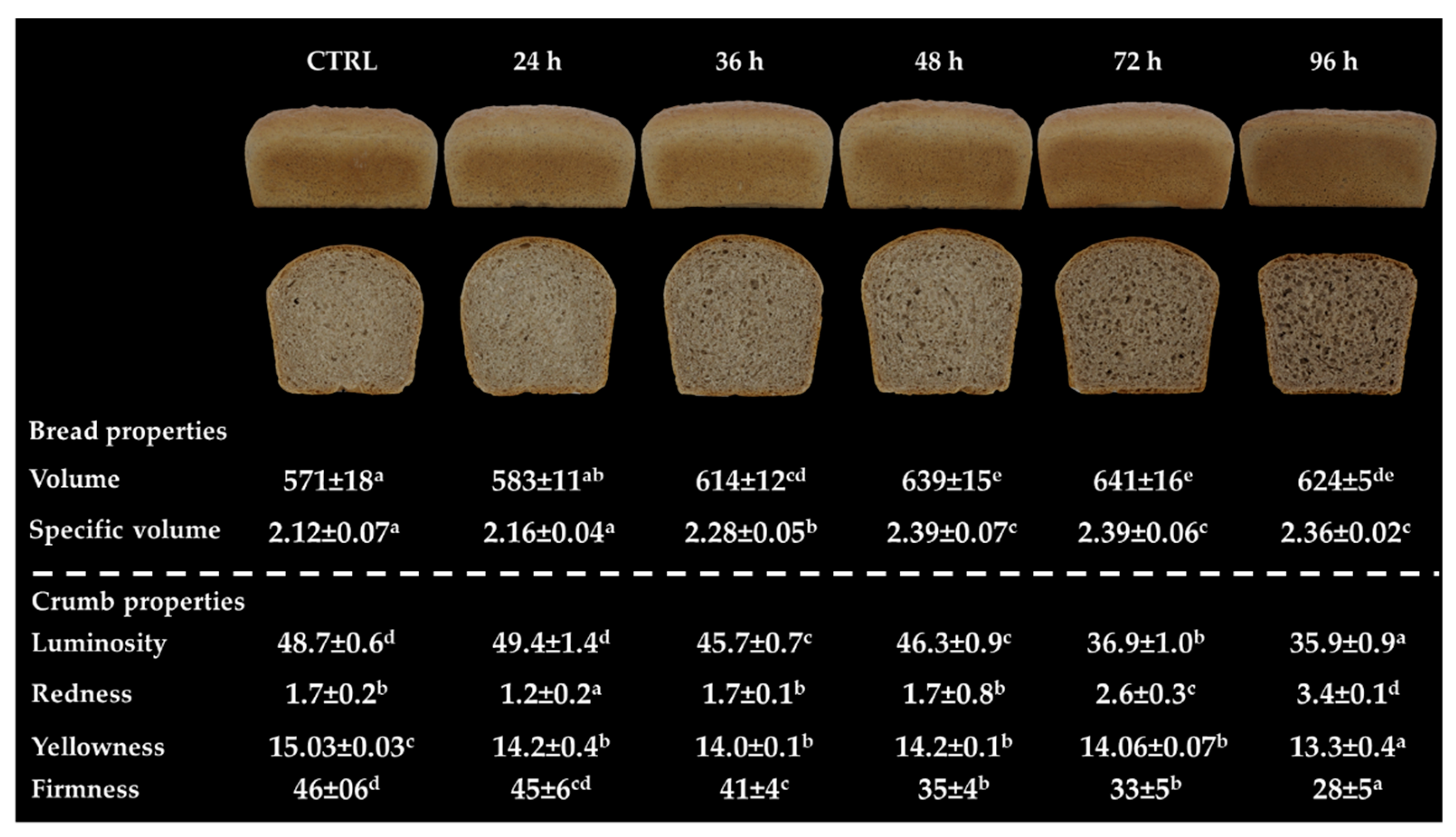
3.5. Bread Properties
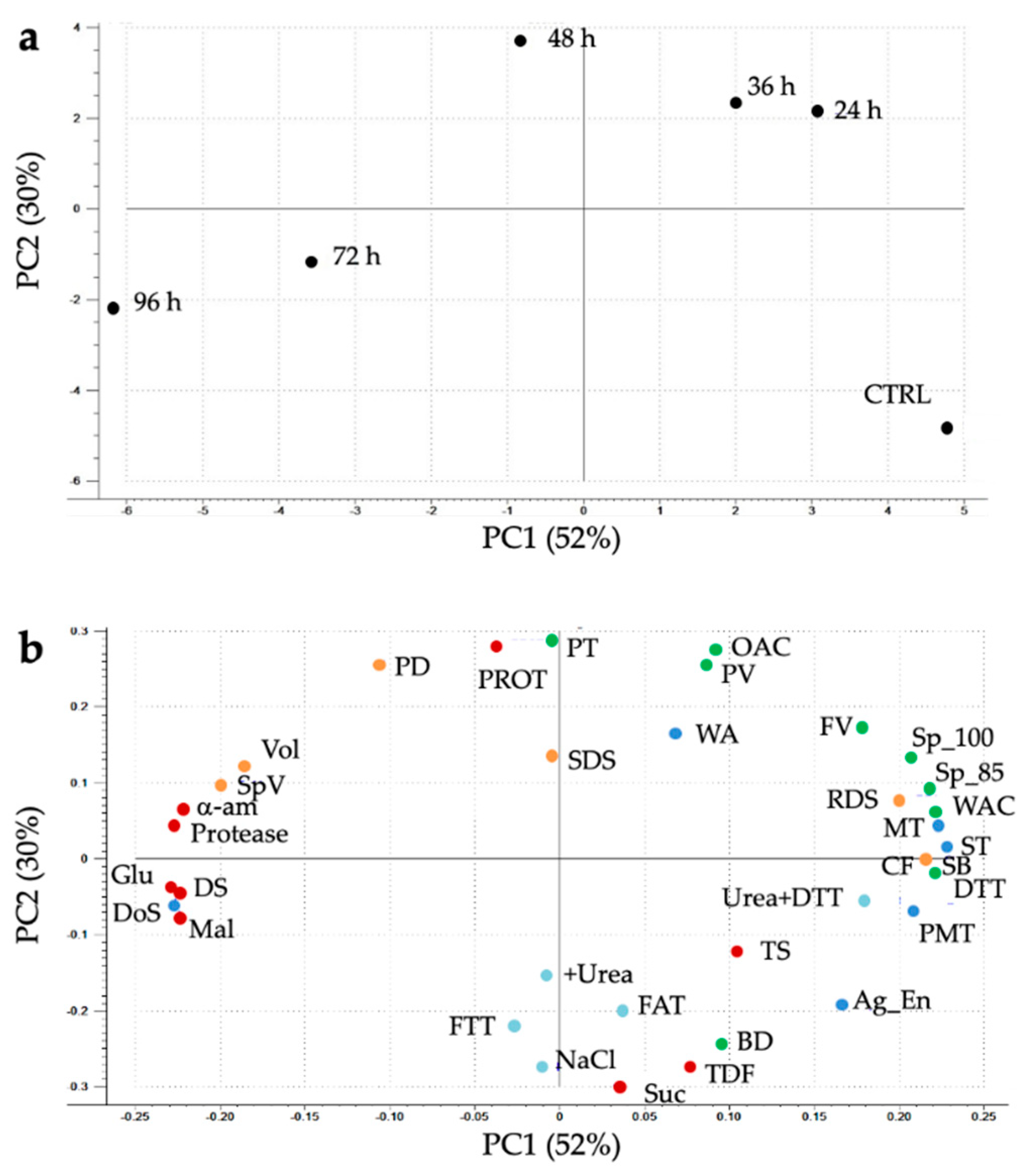
3.6. Principal Component Analysis (PCA)
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α-am | α-amylase activity |
| Ag_En | Aggregation Energy |
| ANOVA | Analysis of Variance |
| BD | Breakdown |
| CF | Crumb Firmness |
| DDT | Dough Development Time |
| DoS | Degree of Softening |
| DS | Damaged Starch |
| DTT | Dithiothreitol |
| FAT | Free Accessible Thiols |
| FFT | Free Total Thiols |
| FU | Farinograph Units |
| FV | Final Viscosity |
| Glu | D-glucose |
| GPU | GlutoPeak Units |
| Mal | Maltose |
| MT | Maximum Torque |
| OAC | Oil Absorption Capacity |
| PD | Protein Digestibility |
| PMT | Peak Maximum Time |
| Prot | Protein |
| PT | Peak Temperature |
| PV | Peak Viscosity |
| RDS | Rapidly Digestible Starch |
| RVA | Rapid Visco Analyzer |
| SB | Setback |
| SDS | Slowly Digestible Starch |
| SDS-PAG | Sodium Dodecyl Sulfate |
| Sp | Swelling power |
| SpV | Specific Volume |
| ST | Stability |
| Suc | Sucrose |
| TDF | Total Dietary Fiber |
| TS | Total Starch |
| Vol | Volume |
| WA | Water Absorption |
| WAC | Water Absorption Capacity |
References
- Phattanakulkaewmorie, N.; Paseephol, T.; Moongngarm, A. Chemical compositions and physico-chemical properties of malted sorghum flour and characteristics of gluten free bread. World Acad. Sci. Eng. Technol. 2011, 81, 454–460. [Google Scholar]
- Hugo, L.F.; Rooney, L.W.; Taylor, J.R.N. Fermented sorghum as a functional ingredient in composite breads. Cereal Chem. J. 2003, 80, 495–499. [Google Scholar] [CrossRef]
- Schober, T.J.; Bean, S.R.; Boyle, D.L. Gluten-free dorghum bread improved by sourdough fermentation: Biochemical, rheological, and microstructural background. J. Agric. Food Chem. 2007, 55, 5137–5146. [Google Scholar] [CrossRef]
- Marengo, M.; Bonomi, F.; Marti, A.; Pagani, M.A.; Elkhalifa, A.E.O.; Iametti, S. Molecular features of fermented and sprouted sorghum flours relate to their suitability as components of enriched gluten-free pasta. LWT-Food Sci. Technol. 2015, 63, 511–518. [Google Scholar] [CrossRef]
- Renzetti, S.; Dal Bello, F.; Arendt, E.K. Microstructure, fundamental rheology and baking characteristics of batters and breads from different gluten-free flours treated with a microbial transglutaminase. J. Cereal Sci. 2008, 48, 33–45. [Google Scholar] [CrossRef]
- Renzetti, S.; Arendt, E.K. Effects of oxidase and protease treatments on the breadmaking functionality of a range of gluten-free flours. Eur. Food Res. Technol. 2009, 229, 307–317. [Google Scholar] [CrossRef]
- Teferra, T.F.; Awika, J.M. Sorghum as a healthy global food security crop: Opportunities and challenges. Cereal Foods World 2019, 64, 1–8. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.H.; Marx, D.B.; Wilson, J.D.; Buchanan, B.B.; Lemaux, P.G.; Pedersen, J.F. Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain. Plant Sci. 2010, 179, 598–611. [Google Scholar] [CrossRef] [Green Version]
- Oria, M.P.; Hamaker, B.R.; Axtell, J.D.; Huang, C.-P. A highly digestible sorghum mutant cultivar exhibits a unique folded structure of endosperm protein bodies. Proc. Natl. Acad. Sci. 2000, 97, 5065–5070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Mesa-Stonestreet, N.J.; Alavi, S.; Bean, S.R. Sorghum proteins: The concentration, isolation, modification, and food applications of kafirins. J. Food Sci. 2010, 75, 90–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, F. Structure, physicochemical properties, modifications, and uses of sorghum starch. Compr. Rev. Food Sci. Food Saf. 2014, 13, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Anglani, C. Sorghum for human food—A review. Plant Foods Hum. Nutr. 1998, 52, 85–95. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Influence of grain germination on functional properties of sorghum flour. Food Chem. 2010, 121, 387–392. [Google Scholar] [CrossRef]
- Marchini, M.; Marti, A.; Folli, C.; Prandi, B.; Ganino, T.; Conte, P.; Fadda, C.; Mattarozzi, M.; Carini, E. Sprouting of sorghum (Sorghum bicolor [L.] Moench): Effect of drying treatment on protein and starch features. Foods 2021, 10, 407. [Google Scholar] [CrossRef]
- Yi, C.; Li, Y.; Ping, J. Germination of sorghum grain results in significant changes in paste and texture properties. J. Texture Stud. 2017, 48, 386–391. [Google Scholar] [CrossRef]
- Ocheme, O.B.; Adedeji, O.E.; Lawal, G.; U. M., Z. Effect of germination on functional properties and degree of starch gelatinization of sorghum flour. J. Food Res. 2015, 4, 159. [Google Scholar] [CrossRef] [Green Version]
- Elmaki, H.B.; Babiker, E.E.; El Tinay, A.H. Changes in chemical composition, grain malting, starch and tannin contents and protein digestibility during germination of sorghum cultivars. Food Chem. 1999, 64, 331–336. [Google Scholar] [CrossRef]
- Correia, I.; Nunes, A.; Barros, A.S.; Delgadillo, I. Protein profile and malt activity during sorghum germination. J. Sci. Food Agric. 2008, 88, 2598–2605. [Google Scholar] [CrossRef]
- Marchini, M.; Carini, E.; Cataldi, N.; Boukid, F.; Blandino, M.; Ganino, T.; Vittadini, E.; Pellegrini, N. The use of red lentil flour in bakery products: How do particle size and substitution level affect rheological properties of wheat bread dough? Lebensm. Wiss. Technol. 2021, 136, 110299. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.R.; El-Beltagi, H.S.; Abd El-Salam, S.M.; Omran, A.A. Protein solubility, digestibility and fractionation after germination of sorghum varieties. PLoS ONE 2012, 7, e31154. [Google Scholar] [CrossRef] [Green Version]
- Lemmens, E.; Moroni, A.V.; Pagand, J.; Heirbaut, P.; Ritala, A.; Karlen, Y.; Lê, K.; den Broeck, H.C.; Brouns, F.J.P.H.; Brier, N.; et al. Impact of cereal seed sprouting on its nutritional and technological properties: A critical review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 305–328. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Rehal, J.; Kaur, A.; Jyot, G. Enhancement of attributes of cereals by germination and fermentation: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1575–1589. [Google Scholar] [CrossRef] [PubMed]
- AACC International. Method 44-15.02. Moisture—Air-Oven Methods. Approved November 3; In Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC International. Method 76-13.01. Total Starch Assay Procedure (Megazyme Amyloglucosidase/α-Amylase Method). Approved November 3; In Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC International. Method 76-31.01. Determination of Damaged Starch--Spectrophotometric Method. Approved November 3; In Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC International. Method 46-12.01. Crude Protein—Kjeldahl Method, Boric Acid Modification. Approved November 3; In Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- AOAC. Method 991.43. Total, Soluble, and Insoluble Dietary Fibre in Foods. In Official Methods of Analysis, 17th ed.; Association of Analytical: Washington, DC, USA, 2003. [Google Scholar]
- AACC International. Method 22-05.01. Measurement of α-Amylase in Cereal Grains and Flours—Amylazyme Method. Approved November 3; In Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- AACC International. Method 22-62.01. Measurement of Proteolytic Activity—Spectrophotometric Method. Approved November 3; In Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Zhang, G.; Hamaker, B.R. Low α-amylase starch digestibility of cooked sorghum flours and the effect of protein. Cereal Chem. J. 1998, 75, 710–713. [Google Scholar] [CrossRef]
- ICC International Association for Cereal Science and Technology. Standard No. 162. In Rapid Pasting Method using the Newport Rapid Visco Analyser; ICC: Vienna, Austria, 1996. [Google Scholar]
- Carter, R.; Reck, D.R. Low temperature solvent extraction process for producing high purity zein. United States Pat. Trademark Off. 1970, 28, 131–134. [Google Scholar] [CrossRef]
- Espinosa-Ramírez, J.; Serna-Saldívar, S.O. Functionality and characterization of kafirin-rich protein extracts from different whole and decorticated sorghum genotypes. J. Cereal Sci. 2016, 70, 57–65. [Google Scholar] [CrossRef]
- Suárez-Estrella, D.; Cardone, G.; Buratti, S.; Pagani, M.A.; Marti, A. Sprouting as a pre-processing for producing quinoa-enriched bread. J. Cereal Sci. 2020, 96, 103111. [Google Scholar] [CrossRef]
- ICC International. Method for Using the Brabender Farinograph. In Association for Cereal Science and Technology. Standard No. 115/1; ICC: Vienna, Austria, 1992. [Google Scholar]
- ICC International. Method for Test Baking of Wheat Flours. In Association for Cereal Science and Technology. Standard No. 131; ICC: Vienna, Austria, 1992. [Google Scholar]
- AACC International. Method 74-09.01. Measurement of Bread Firmness by Universal Testing Machine. Approved November 3; In Approved Methods of Analysis, 11th ed.; Cereals & Grains Association: St. Paul, MN, USA, 1999. [Google Scholar]
- Englyst, K.N.; Hudson, G.J.; Englyst, H.N. Starch analysis in food. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd: Chichester, UK, 2006; pp. 1–17. ISBN 9780470027318. [Google Scholar]
- Hsu, H.W.; Vavak, D.L.; Satterlee, L.D.; Miller, G.A. A multienzyme technique for estimating protein digestibility. J. Food Sci. 1977, 42, 1269–1273. [Google Scholar] [CrossRef]
- Vilakati, N.; MacIntyre, U.; Oelofse, A.; Taylor, J.R.N. Influence of micronization (infrared treatment) on the protein and functional quality of a ready-to-eat sorghum-cowpea African porridge for young child-feeding. LWT-Food Sci. Technol. 2015, 63, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Grassi, S.; Cardone, G.; Bigagnoli, D.; Marti, A. Monitoring the sprouting process of wheat by non-conventional approaches. J. Cereal Sci. 2018, 83, 180–187. [Google Scholar] [CrossRef]
- Cardone, G.; Grassi, S.; Scipioni, A.; Marti, A. Bread-making performance of durum wheat as affected by sprouting. Lebensm. Wiss. Technol. 2020, 134, 110021. [Google Scholar] [CrossRef]
- Ku, Y.; Jansen, O.; Oles, C.J.; Lazar, E.Z.; Rader, J.I. Precipitation of inulins and oligoglucoses by ethanol and other solvents. Food Chem. 2003, 81, 125–132. [Google Scholar] [CrossRef]
- Hager, A.-S.; Taylor, J.P.; Waters, D.M.; Arendt, E.K. Gluten free beer—A review. Trends Food Sci. Technol. 2014, 36, 44–54. [Google Scholar] [CrossRef]
- Rosa, M.; Mirna, H.; Hilal, M.; González, J.; Prado, F. Changes in soluble carbohydrates and related enzymes induced by low temperature during early developmental stages of quinoa (Chenopodium quinoa) seedlings. J. Plant Physiol. 2004, 161, 683–689. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D.; Gawlik-Dziki, U. Starch and protein analysis of wheat bread enriched with phenolics-rich sprouted wheat flour. Food Chem. 2017, 228, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Cardone, G.; D’Incecco, P.; Pagani, M.A.; Marti, A. Sprouting improves the bread-making performance of whole wheat flour ( Triticum aestivum L.). J. Sci. Food Agric. 2020, 100, 2453–2459. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.; Cardone, G.; Pagani, M.; Casiraghi, M. Flour from sprouted wheat as a new ingredient in bread-making. LWT-Food Sci. Technol. 2018, 89, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, M.; Maeda, T.; Tsukahara, K.; Kayahara, H.; Morita, N. Application of pregerminated brown rice for breadmaking. Cereal Chem. J. 2004, 81, 450–455. [Google Scholar] [CrossRef]
- Charoenthaikij, P.; Jangchud, K.; Jangchud, A.; Prinyawiwatkul, W.; No, H.K.; King, J.M. Physicochemical properties and consumer acceptance of wheat-germinated brown rice bread during storage time. J. Food Sci. 2010, 75, S333–S339. [Google Scholar] [CrossRef]
- Charoenthaikij, P.; Jangchud, K.; Jangchud, A.; Prinyawiwatkul, W.; Tungtrakul, P. Germination conditions affect selected quality of composite wheat-germinated brown rice flour and bread formulations. J. Food Sci. 2010, 75, S312–S318. [Google Scholar] [CrossRef]
- Park, S.H.; Morita, N. Dough and breadmaking properties of wheat flour substituted by 10% with germinated quinoa flour. Food Sci. Technol. Int. 2005, 11, 471–476. [Google Scholar] [CrossRef]
- Horstmann, S.W.; Atzler, J.J.; Heitmann, M.; Zannini, E.; Lynch, K.M.; Arendt, E.K. A comparative study of gluten-free sprouts in the gluten-free bread-making process. Eur. Food Res. Technol. 2019, 245, 617–629. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Arendt, E.K. Oat malt as a baking ingredient—A comparative study of the impact of oat, barley and wheat malts on bread and dough properties. J. Cereal Sci. 2012, 56, 747–753. [Google Scholar] [CrossRef]
- Mäkinen, O.E.; Zannini, E.; Arendt, E.K. Germination of oat and quinoa and evaluation of the malts as gluten free baking ingredients. Plant Foods Hum. Nutr. 2013, 68, 90–95. [Google Scholar] [CrossRef]
- Bhol, S.; John Don Bosco, S. Influence of malted finger millet and red kidney bean flour on quality characteristics of developed bread. LWT-Food Sci. Technol. 2014, 55, 294–300. [Google Scholar] [CrossRef]
- Hallén, E.; İbanoğlu, Ş.; Ainsworth, P. Effect of fermented/germinated cowpea flour addition on the rheological and baking properties of wheat flour. J. Food Eng. 2004, 63, 177–184. [Google Scholar] [CrossRef]
- Marengo, M.; Carpen, A.; Bonomi, F.; Casiraghi, M.C.; Meroni, E.; Quaglia, L.; Iametti, S.; Pagani, M.A.; Marti, A. Macromolecular and micronutrient profiles of sprouted chickpeas to be used for integrating cereal-based food. Cereal Chem. J. 2017, 94, 82–88. [Google Scholar] [CrossRef]
- Correia, I.; Nunes, A.; Barros, A.S.; Delgadillo, I. Comparison of the effects induced by different processing methods on sorghum proteins. J. Cereal Sci. 2010, 51, 146–151. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Some physicochemical properties of flour from germinated sorghum grain. J. Food Sci. Technol. 2013, 50, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Sharma, S. Bioactive components and functional properties of biologically activated cereal grains: A bibliographic review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3051–3071. [Google Scholar] [CrossRef] [PubMed]
- Suárez-Estrella, D.; Bresciani, A.; Iametti, S.; Marengo, M.; Pagani, M.A.; Marti, A. Effect of sprouting on proteins and starch in quinoa (Chenopodium quinoa Willd.). Plant Foods Hum. Nutr. 2020, 75, 635–641. [Google Scholar] [CrossRef]
- Žilić, S.; Janković, M.; Barać, M.; Pešić, M.; Konić-Ristić, A.; Hadži-Tašković Šukalović, V. Effects of enzyme activities during steeping and sprouting on the solubility and composition of proteins, their bioactivity and relationship with the bread making quality of wheat flour. Food Funct. 2016, 7, 4323–4331. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, H.; Guo, X.; Qian, H. The impact of germination on the characteristics of brown rice flour and starch. J. Sci. Food Agric. 2012, 92, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Ahmed, R.; Ali, R.; Khan, M.S.; Sayeed, S.A.; Saeed, J. Effect of proteases & carbohydrases on dough rheology and end quality of cookie. Am. J. Food Sci. Nutr. Res. 2015, 2, 62–66. [Google Scholar]
- Cornejo, F.; Caceres, P.J.; Martínez-Villaluenga, C.; Rosell, C.M.; Frias, J. Effects of germination on the nutritive value and bioactive compounds of brown rice breads. Food Chem. 2015. [Google Scholar] [CrossRef] [Green Version]



| CTRL | 24 h | 36 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|---|---|
| Total starch | 77.5 ± 1.9 b | 73.2 ± 1.2 a | 75.0 ± 2.1 ab | 75.2 ± 2.5 ab | 73.7 ± 0.4 a | 74.3 ± 1.4 ab |
| Damaged starch | 9.4 ± 0.5 a | 9.6 ± 0.2 a | 9.6 ± 0.3 a | 11.5 ± 0.3 b | 12.7 ± 0.5 c | 13.6 ± 0.3 d |
| Maltose | 0.2 ± 0.1 a | 0.12 ± 0.03 a | 0.54 ± 0.09 b | 1.31 ± 0.03 c | 2.23 ± 0.03 d | 3.5 ± 0.3 e |
| Sucrose | 0.64 ± 0.05 d | 0.25 ± 0.05 a | 0.24 ± 0.01 a | 0.22 ± 0.08 a | 0.36 ± 0.05 b | 0.45 ± 0.03 c |
| D-glucose | 0.24 ± 0.01 a | 0.25 ± 0.01 a | 0.29 ± 0.08 a | 0.47 ± 0.02 b | 0.58 ± 0.01 c | 0.70 ± 0.01 d |
| Protein | 8.8 ± 0.1 a | 9.0 ± 0.1 ab | 9.1 ± 0.1 b | 9.1 ± 0.1 b | 9.0 ± 0.1 ab | 8.9 ± 0.1 ab |
| Total fiber | 7.2 ± 0.2 a | 6.9 ± 0.4 a | 6.8 ± 0.5 a | 6.7 ± 0.2 a | 7.0 ± 0.5 a | 6.9 ± 0.1 a |
| Insoluble | 82 | 78 | 85 | 82 | 89 | 90 |
| Soluble | 18 | 22 | 15 | 18 | 11 | 10 |
| α-amylase | 0.07 ± 0.02 a | 2.7 ± 0.6 b | 3.0 ± 0.4 bc | 3.7 ± 0.4 c | 4.5 ± 0.4 d | 6.2 ± 0.6 e |
| Protease | 1.3 ± 0.1 a | 2.5 ± 0.1 b | 2.7 ± 0.1 bc | 3.0 ± 0.1 c | 3.9 ± 0.2 d | 4.3 ± 0.2 e |
| CTRL | 24 h | 36 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|---|---|
| Hydration Properties | ||||||
| WAC | 1.50 ± 0.01 d | 1.48 ± 0.03 cd | 1.50 ± 0.01 d | 1.42 ± 0.03 bc | 1.38 ± 0.04 ab | 1.33 ± 0.02 a |
| OAC | 1.07 ± 0.02 a | 1.28 ± 0.02 b | 1.29 ± 0.04 b | 1.31 ± 0.04 cd | 1.08 ± 0.05 a | 1.03 ± 0.03 a |
| Swelling Power (Sp) | ||||||
| 85 °C | 7.2 ± 0.4 c | 6.7 ± 0.1 bc | 6.3 ± 0.2 bc | 6.3 ± 0.2 bc | 6.4 ± 0.1 bc | 5.4 ± 0.3 a |
| 100 °C | 10.2 ± 0.1 bc | 10.8 ± 0.6 bc | 10.2 ± 0.5 bc | 9.7 ± 0.2 c | 5.7 ± 0.4 b | 3.5 ± 0.4 a |
| Pasting properties in water | ||||||
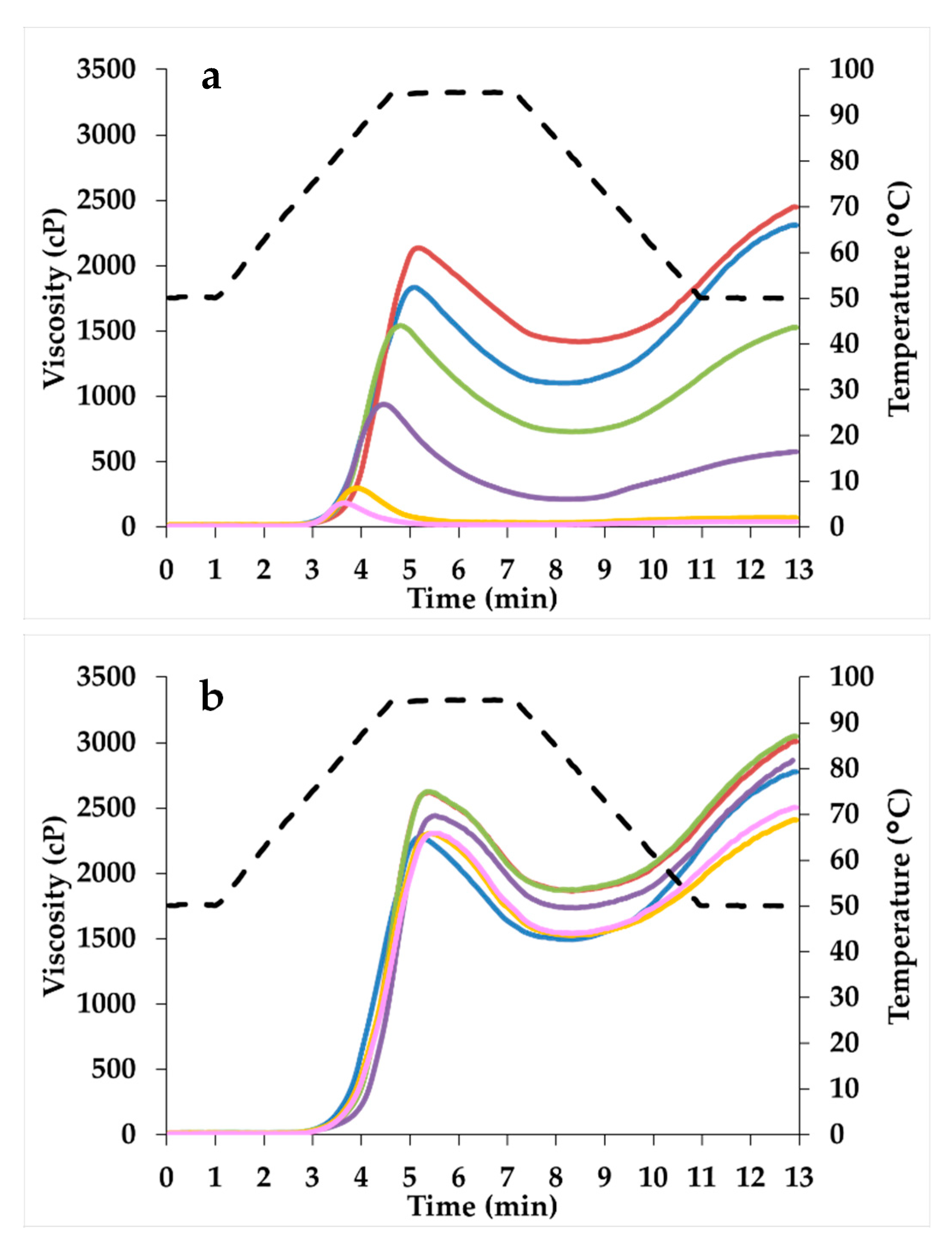
| Viscosity peak | 1858 ± 21 e | 2131 ± 7 f | 1593 ± 44 d | 945 ± 4 c | 306 ± 11 b | 187 ± 1 a |
| Peak temperature | 77.8 ± 0.5 a | 81.2 ± 0.6 c | 79.3 ± 0.4 b | 78.0 ± 0.5 ab | 78.0 ± 0.5 ab | 77.5 ± 0.1 a |
| Breakdown | 739 ± 8 d | 706 ± 10 c | 826 ± 11 e | 720 ± 6 cd | 276 ± 13 b | 166 ± 2 a |
| Final viscosity | 2392 ± 75 d | 2509 ± 65 d | 1606 ± 67 c | 602 ± 22 b | 70 ± 2 a | 43 ± 1 a |
| Setback | 1273 ± 58 e | 1084 ± 55 d | 838 ± 35 c | 378 ± 14 b | 40 ± 1 a | 23 ± 1 a |
| CTRL | 24 h | 36 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|---|---|
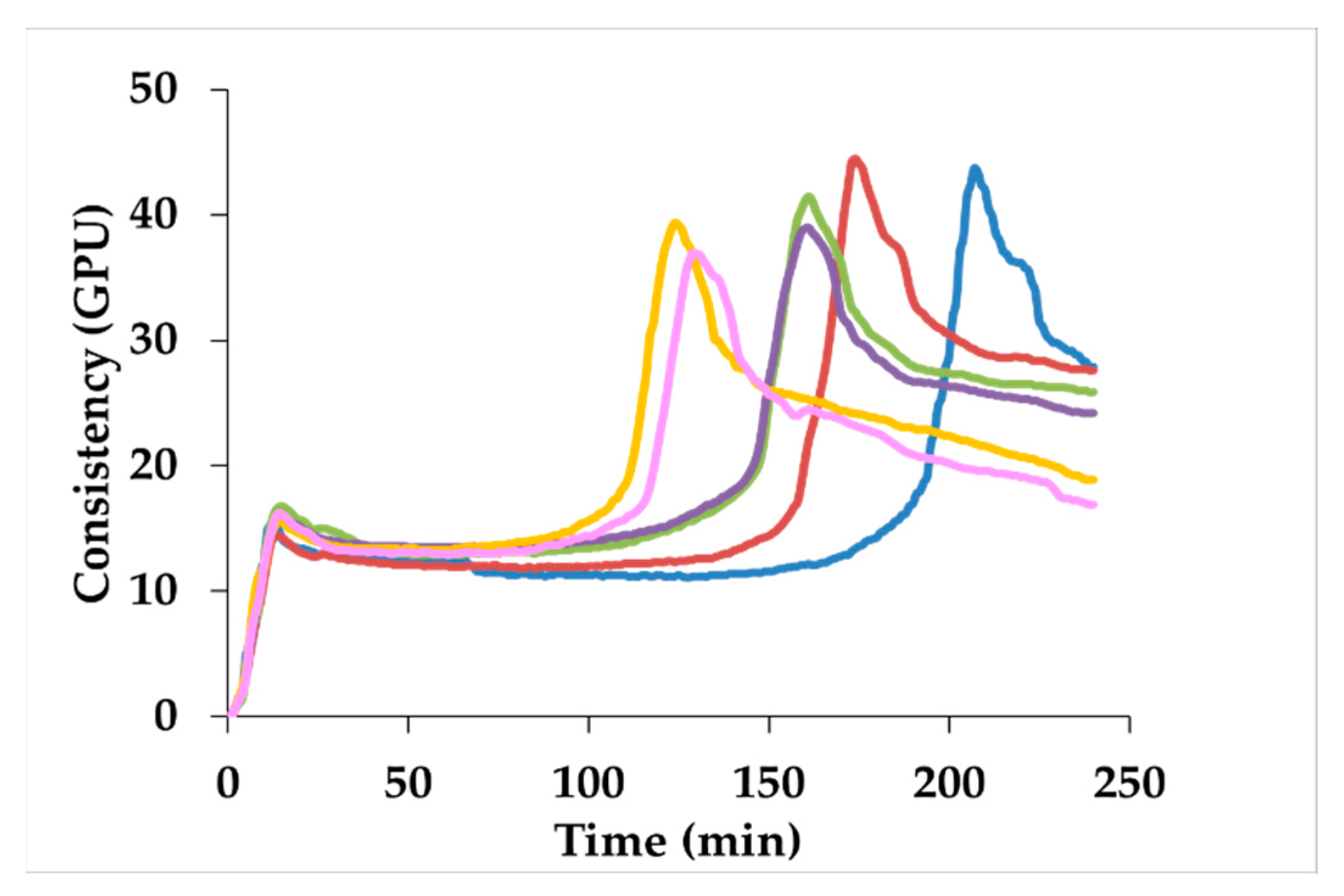
| Gluten aggregation properties | ||||||
| Maximum torque | 42.9 ± 1.0 b | 43.5 ± 1.5 b | 43.0 ± 1.3 b | 40.3 ± 1.2 ab | 39.5 ± 0.1 a | 37.6 ± 1.5 a |
| Peak maximum time | 217 ± 14 c | 180 ± 8 b | 166 ± 5 b | 167 ± 6 b | 125 ± 3 a | 135 ± 6 a |
| Aggregation energy | 1063 ± 8 c | 1075 ± 26 c | 1073 ± 22 c | 1029 ± 15 bc | 978 ± 5 ab | 935 ± 21 a |
| Mixing properties | ||||||
| Water absorption | 61.6 ± 0.3 b | 61.7 ± 0.3 b | 61.5 ± 0.2 b | 61.9 ± 0.1 b | 61.6 ± 0.3 b | 61.4 ± 0.2 b |
| Dough development time | 3.0 ± 1.0 b | 3.0 ± 0.1 b | 2.7 ± 0.2 ab | 2.1 ± 0.2 ab | 2.0 ± 0.1 ab | 1.9 ± 0.2 a |
| Stability | 9.5 ± 0.1 cd | 9.0 ± 0.6 cd | 7.3 ± 0.4 c | 5.3 ± 0.4 b | 2.9 ± 0.6 a | 2.2 ± 0.3 a |
| Degree of softening | 57 ± 4 a | 62 ± 6 a | 84 ± 1 b | 108 ± 1 c | 167 ± 8 d | 191 ± 5 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardone, G.; Rumler, R.; Speranza, S.; Marti, A.; Schönlechner, R. Sprouting Time Affects Sorghum (Sorghum bicolor [L.] Moench) Functionality and Bread-Baking Performance. Foods 2021, 10, 2285. https://doi.org/10.3390/foods10102285
Cardone G, Rumler R, Speranza S, Marti A, Schönlechner R. Sprouting Time Affects Sorghum (Sorghum bicolor [L.] Moench) Functionality and Bread-Baking Performance. Foods. 2021; 10(10):2285. https://doi.org/10.3390/foods10102285
Chicago/Turabian StyleCardone, Gaetano, Rubina Rumler, Sofia Speranza, Alessandra Marti, and Regine Schönlechner. 2021. "Sprouting Time Affects Sorghum (Sorghum bicolor [L.] Moench) Functionality and Bread-Baking Performance" Foods 10, no. 10: 2285. https://doi.org/10.3390/foods10102285
APA StyleCardone, G., Rumler, R., Speranza, S., Marti, A., & Schönlechner, R. (2021). Sprouting Time Affects Sorghum (Sorghum bicolor [L.] Moench) Functionality and Bread-Baking Performance. Foods, 10(10), 2285. https://doi.org/10.3390/foods10102285







