Geographical and Varietal Traceability of Chinese Jujubes Based on Physical and Nutritional Characteristics
Abstract
:1. Introduction
2. Materials and Methods
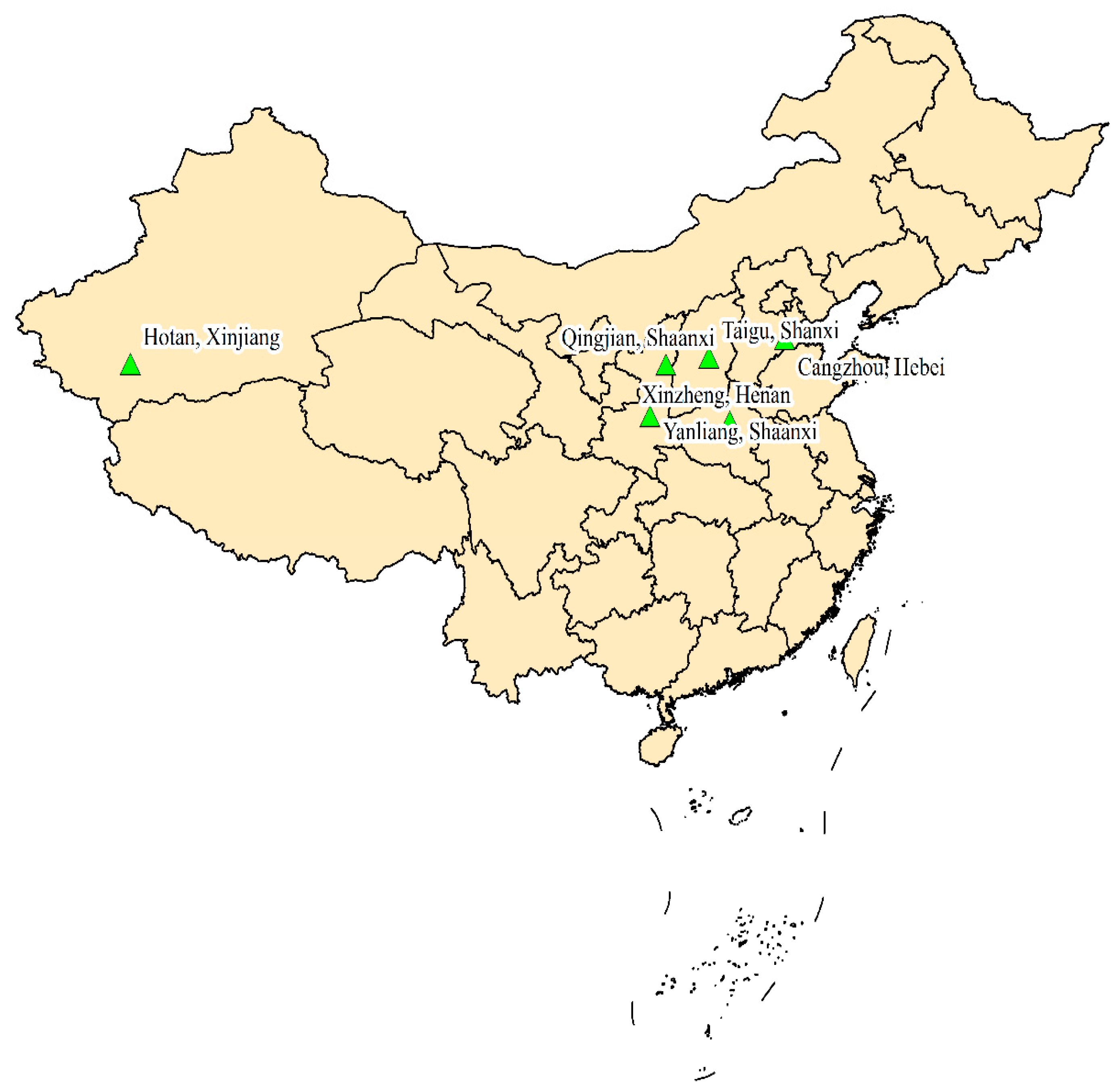
2.1. Sampling Information
2.2. Quality Parameter Determination
2.2.1. Fresh Fruit Mass Determination
2.2.2. Determination of Shape Ratio of Fruits
2.2.3. Edible Fruit Rate Determination
2.2.4. Moisture Content Determination
2.2.5. Soluble Sugar Determination
2.2.6. Mineral Element Determination
2.2.7. Ascorbic Acid Determination
2.2.8. Dietary Fiber Determination
2.2.9. cAMP Determination
2.3. Statistical Analyses
3. Results
3.1. Differences in Chinese Jujube Samples from Different Categories
3.2. CA of Quality Parameters
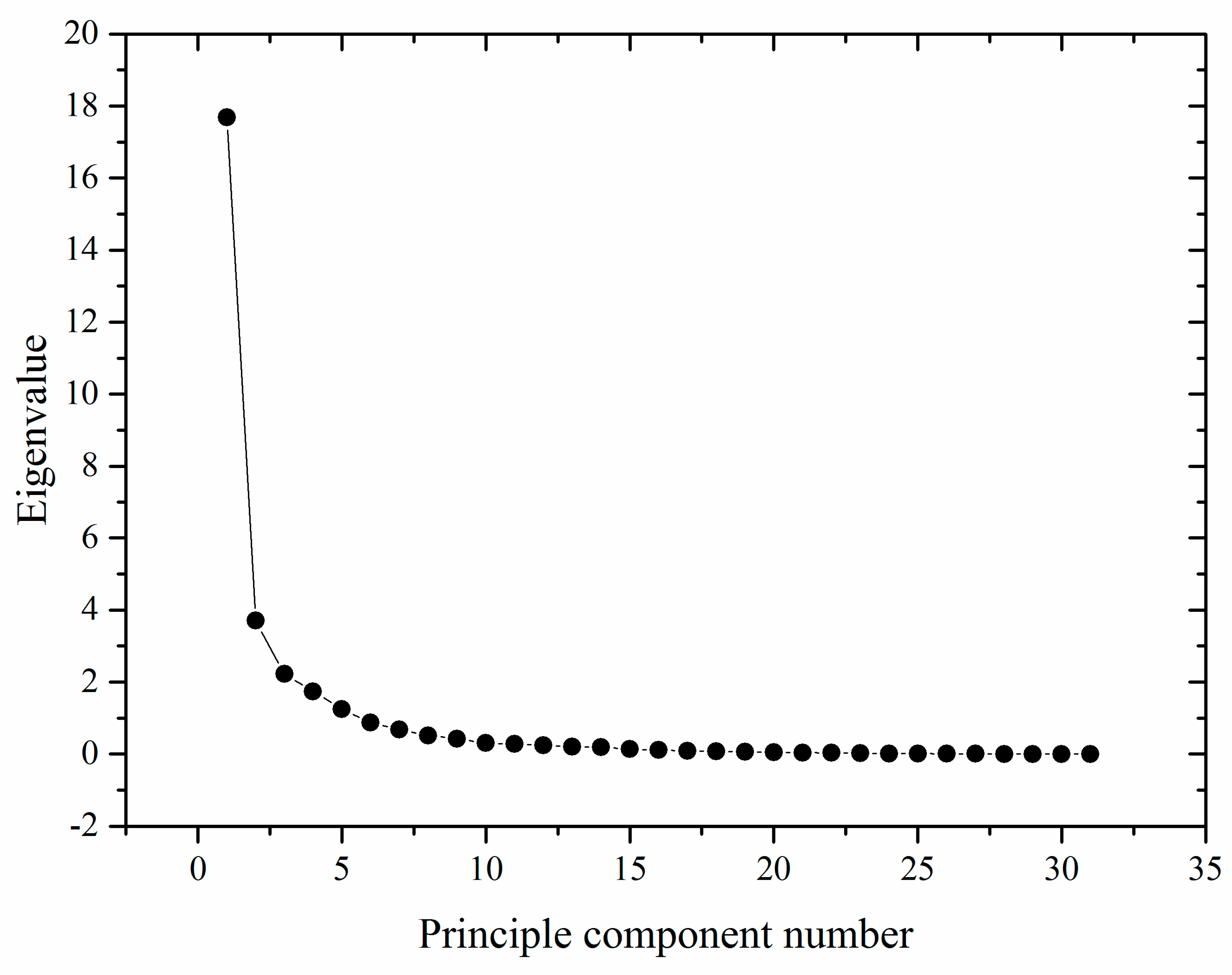
3.3. PCA Analysis for Main Parameter Selection and Quality Evaluation
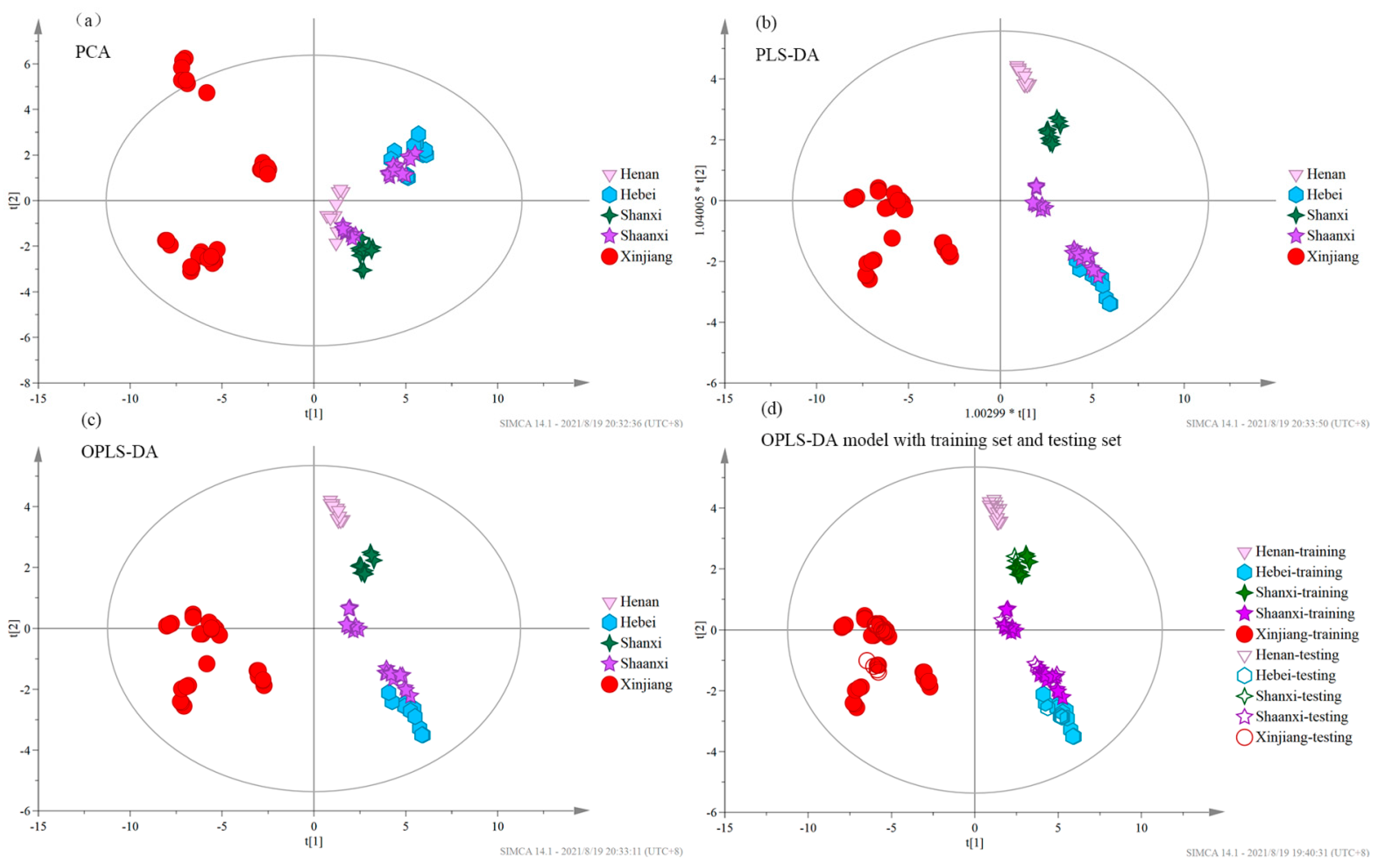
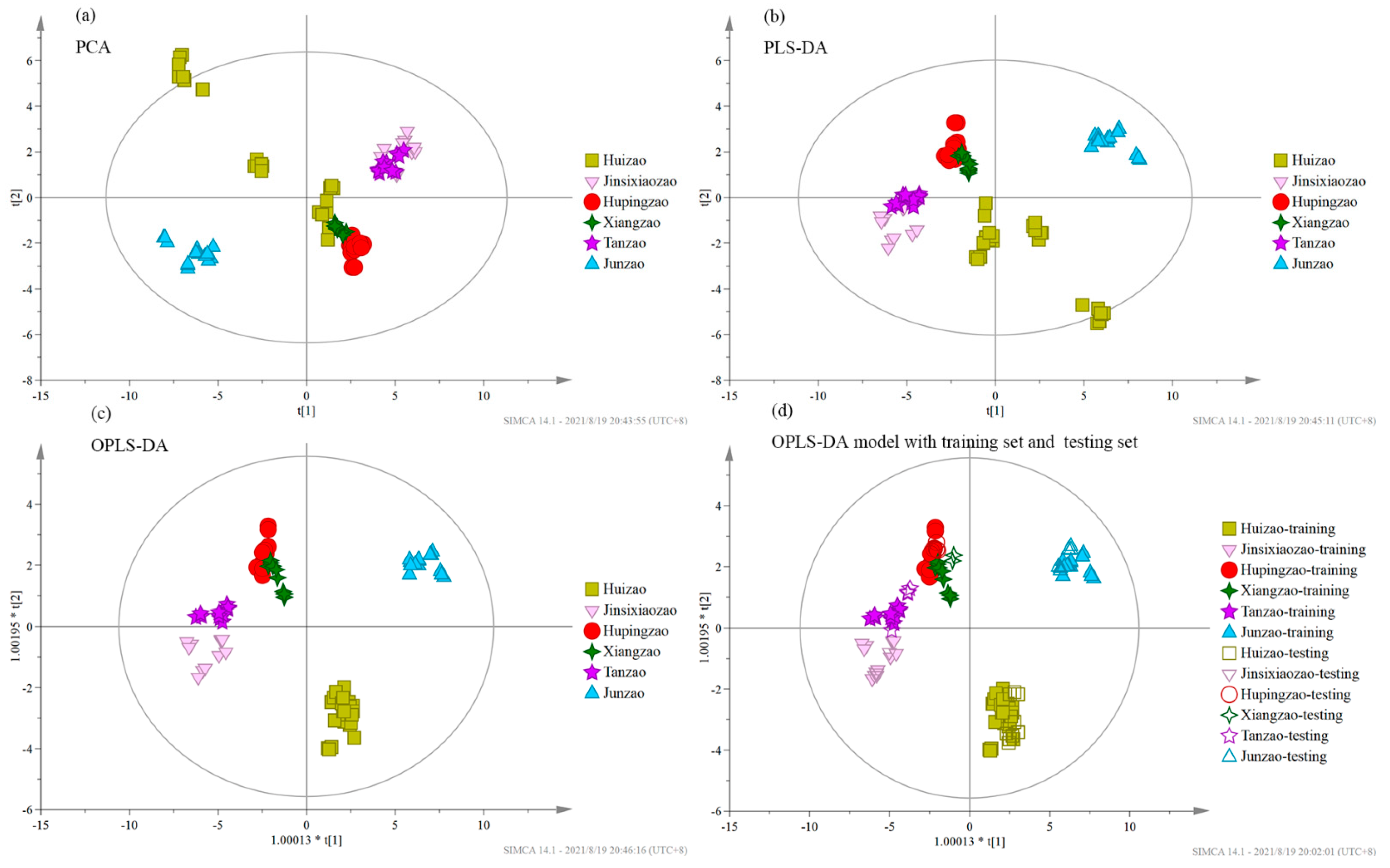
3.4. Chemometric Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, X.X.; Liu, H.M.; Yan, Y.Y.; Fan, L.Y.; Yang, J.N.; Wang, X.D.; Qin, G.Y. Structural characterization and antioxidant activity of polysaccharides extracted from jujube using subcritical water. LWT 2020, 117, 108645. [Google Scholar] [CrossRef]
- Sheng, J.P.; Shen, L. Chinese jujube (Ziziphus jujuba Mill.) and Indian jujube (Ziziphus mauritiana Lam.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E.M., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 299–326e. [Google Scholar]
- Tang, Q.; Li, C.; Ge, Y.; Li, X.; Cheng, Y.; Hou, J.; Li, J. Exogenous application of melatonin maintains storage quality of jujubes by enhancing anti-oxidative ability and suppressing the activity of cell wall-degrading enzymes. LWT 2020, 127, 109431. [Google Scholar] [CrossRef]
- Lin, X.; Zhou, L.; Li, T.; Brennan, C.; Fu, X.; Liu, R.H. Phenolic content, antioxidant and antiproliferative activities of six varieties of white sesame seeds (Sesamum indicum L.). RSC Adv. 2017, 7, 5751–5758. [Google Scholar] [CrossRef] [Green Version]
- Pon, Y.L.; Auersperg, N.; Wong, A.S. Gonadotropins regulate N-cadherin-mediated human ovarian surface epithelial cell survival at both post-translational and transcriptional levels through a cyclic AMP/protein kinase A pathway. J. Biol. Chem. 2005, 280, 15438–15448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.; Fan, D.; Fu, B.; Zhou, J.; Li, H. Comparison of physical and chemical composition of three chinese jujube (Ziziphusjujuba Mill.) cultivars cultivated in four districts of Xinjiang region in China. Food Sci. Technol. 2019, 39, 912–921. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Chen, Q.; Bi, J.; Meng, X.; Wu, X.; Qiao, Y.; Lyu, Y. GC/MS coupled with MOS e-nose and flash GC e-nose for volatile characterization of Chinese jujubes as affected by different drying methods. Food Chem. 2020, 331, 127201. [Google Scholar] [CrossRef]
- Gao, Q.H.; Wu, P.T.; Liu, J.R.; Wu, C.S.; Parry, J.W.; Wang, M. Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Sci. Hortic. 2011, 130, 67–72. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, Y.; Zhang, W.; Yang, S.; Ye, Z.; Zhang, G. Variation of the light stable isotopes in the superior and inferior grains of rice (Oryzasativa L.) with different geographical origins. Food Chem. 2016, 209, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Dai, B.; Wang, B.; Zha, Y.; Song, Q. Traceability in food processing: Problems, methods, and performance evaluations-a review. Crit. Rev. Food Sci. Nutr. 2020, 1–14. [Google Scholar] [CrossRef]
- Gonzalez-Dominguez, R.; Sayago, A.; Akhatou, I.; Fernandez-Recamales, A. Multi-chemical profiling of strawberry as a traceability tool to investigate the effect of cultivar and cultivation conditions. Foods 2020, 9, 96. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wang, Q.; Li, J.; Zhao, S.; Qie, M.; Wu, X.; Bai, Y.; Zhao, Y. Study on the origin traceability of Tibet highland barley (Hordeum vulgare L.) based on its nutrients and mineral elements. Food Chem. 2021, 346, 128928. [Google Scholar] [CrossRef]
- Sun, L.; Ma, X.; Jin, H.Y.; Fan, C.J.; Li, X.D.; Zuo, T.T.; Ma, S.C.; Wang, S. Geographical origin differentiation of Chinese Angelica by specific metal element fingerprinting and risk assessment. Environ. Sci. Pollut. Res. Int. 2020, 27, 45018–45030. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Mao, X.H.; Zhang, J.Y. Effects of different extraction methods on cAMP from wild jujube. Food Sci. Technol. 2018, 43, 192–195. [Google Scholar]
- Varra, M.O.; Ghidini, S.; Zanardi, E.; Badiani, A.; Ianieri, A. Authentication of European sea bass according to production method and geographical origin by light stable isotope ratio and rare earth elements analyses combined with chemometrics. Ital. J. Food Saf. 2019, 8, 7872. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gou, X.; Niu, P.; Sun, L.; Guo, Y. Multivariate data analysis of the physicochemical and phenolic properties of not from concentrate apple luices to explore the alternative cultivars in juice production. Food Anal. Methods 2018, 11, 1735–1747. [Google Scholar] [CrossRef]
- Chen, L.; Yu, F.; Sun, S.; Liu, X.; Sun, Z.; Cao, W.; Liu, S.; Li, Z.; Xue, C. Evaluation indicators of Ruditapes philippinarum nutritional quality. J. Food Sci.Technol. 2021, 58, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.L.; Chen, J.N.; Han, H.T.; Liu, C.J.; Gao, Q.; Li, J.H.; Li, D.J.; Tanokura, M.; Liu, C.Q. Multivariate analyses of the physicochemical properties of turnip (Brassicarapa L.) chips dried using different methods. Dry. Technol. 2019, 38, 411–419. [Google Scholar] [CrossRef]
- Shin, E.C.; Pegg, R.B.; Phillips, R.D.; Eitenmiller, R.R. Interrelationships among tocopherols of commercial Runner market type peanuts grown in the United States. Int. J. Food Sci. Technol. 2010, 45, 2622–2628. [Google Scholar] [CrossRef]
- Bi, J.F.; Wang, X.; Chen, Q.Q.; Liu, X.; Wu, X.Y.; Wang, Q.; Lv, J.; Yang, A.J. Evaluation indicators of explosion puffing Fuji apple chips quality from different Chinese origins. LWT—Food Sci. Technol. 2015, 60, 1129–1135. [Google Scholar] [CrossRef]
- Liu, C.J.; Wang, H.O.; Xue, Y.L.; Zhang, Z.Y.; Niu, L.Y.; Li, D.J.; Jiang, N.; Cui, L.; Liu, C.Q. Screening quality evaluation factors of freezedried peach (Prunus persica L. Batsch) powders from different ripening time cultivars. J. Food Qual. 2017, 2017, 7213694. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Yangming, H.; Gorska-Horczyczak, E.; Wierzbicka, A.; Jelen, H.H. Rapid analysis of Baijiu volatile compounds fingerprint for their aroma and regional origin authenticity assessment. Food Chem. 2021, 337, 128002. [Google Scholar] [CrossRef] [PubMed]
- Cheaitou, A.; van Delft, C.; Dallery, Y.; Jemai, Z. Two-period production planning and inventory control. Int. J. Prod. Econ. 2009, 118, 118–130. [Google Scholar] [CrossRef]
- Jia, C.; Shan, C.; Zhou, T.; Xiangyang, L.I.; Peng, W.U.; Sun, Y. Comparison of fruit nutritional traits of major cultivars of Chinese cherry (Prunus pseudocerasus Lindl.). Food Sci. 2019, 40, 244–250. [Google Scholar]
- Jiang, T.; He, F.; Han, S.; Chen, C.; Zhang, Y.; Che, H. Characterization of cAMP as an anti-allergic functional factor in Chinese jujube (Ziziphus jujuba Mill.). J. Funct. Foods 2019, 60, 103414. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayad, A.A.; Williams, L.L.; Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O. Date fruit: A review of the chemical and nutritional compounds, functional effects and food application in nutrition bars for athletes. Int. J. Food Sci. Technol. 2020, 56, 1503–1513. [Google Scholar] [CrossRef]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Review of dried fruits: Phytochemicals, antioxidant efficacies, and health benefits. J. Funct. Foods 2016, 21, 113–132. [Google Scholar] [CrossRef]
- Hassan, I.; Cotrozzi, L.; Haiba, N.S.; Basahi, J.; Hammam, E. Trace elements in the fruits of date palm (Phoenix dactylifera L.) in Jeddah City, Saudi Arabia. Agrochimica 2017, 61, 75–93. [Google Scholar]
- Hasnaoui, A.; Elhoumaizi, M.A.; Asehraou, A.; Sindic, M.; Hakkou, A. Chemical composition and microbial quality of dates grown in Figuig oasis of Marocco. Int. J. Agric. Biol. 2010, 12, 311–314. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Qian, D.; Tang, Y.; Wu, D.; Su, S.; Wang, H.; Zhao, Y. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphu sjujuba) fruit during ripening. Food Chem. 2015, 167, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.H.; Wu, C.S.; Yu, J.G.; Wang, M.; Ma, Y.J.; Li, C.L. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphusjujuba Mill.) selections. J. Food Sci. 2012, 77, C1218–C1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, X.; Zhang, K.; Zhang, Y.; Song, Y.; Wang, F. Fatty acid composition of 21 cultivars of Chinese jujube fruits (Ziziphusjujuba Mill.). J. Food Meas. Charact. 2020, 15, 1225–1240. [Google Scholar] [CrossRef]
- Reche, J.; Almansa, M.S.; Hernandez, F.; Carbonell-Barrachina, A.A.; Legua, P.; Amoros, A. Fatty acid profile of peel and pulp of Spanish jujube (Ziziphusjujuba Mill.) fruit. Food Chem. 2019, 295, 247–253. [Google Scholar] [CrossRef]
- Rahimi, A.; Banakar, A.; Zareiforush, H.; Beygvand, M.; Montazeri, M. Classification of Jujube fruits using different data mining methods. Researcher 2014, 6, 52–61. [Google Scholar]
- Guo, Y.; Ni, Y.; Kokot, S. Evaluation of chemical components and properties of the jujube fruit using near infrared spectroscopy and chemometrics. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2016, 153, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, L.; Trygg, J.; Wold, S. CV-ANOVA for significance testing of PLS and OPLS® models. J. Chemom. 2008, 22, 594–600. [Google Scholar] [CrossRef]
| Quality Parameters | Henan Huizao | Hebei Jinsixiaozao | Shanxi Hupingzao | Shaanxi Xiangzao | Shaanxi Tanzao | Xinjiang Junzao | Xinjiang Huizao | |
|---|---|---|---|---|---|---|---|---|
| Sample number | 15 | 15 | 15 | 12 | 15 | 21 | 18 | |
| Physical parameters | Fresh mass (g) | 10.41 ± 0.72 d | 7.50 ± 0.80 d | 18.08 ± 2.41 b | 14.63 ± 0.73 c | 10.26 ± 1.42 d | 25.96 ± 3.66 a | 7.84 ± 3.00 d |
| Shape ratio | 1.45 ± 0.07 ab | 1.35 ± 0.02 bc | 1.28 ± 0.12 bc | 1.18 ± 0.02 c | 1.28 ± 0.32 bc | 1.57 ± 0.10 a | 1.40 ± 0.06 ab | |
| Edible rate (%) | 92.67 ± 0.22 c | 92.58 ± 0.70 c | 95.47 ± 0.30 a | 94.29 ± 0.21 b | 93.70 ± 1.13 b | 95.41 ± 0.99 a | 93.82 ± 0.49 b | |
| Soluble sugar (%) | 24.28 ± 1.16 c | 19.94 ± 1.88 d | 23.26 ± 1.08 c | 23.93 ± 1.00 c | 22.66 ± 2.58 c | 37.93 ± 0.72 b | 39.77 ± 0.61 a | |
| Moisture content (%) | 65.18 ± 2.71 a | 65.12 ± 0.80 a | 68.75 ± 2.45 a | 67.66 ± 0.72 a | 64.41 ± 0.72 a | 41.35 ± 0.45 b | 38.46 ± 6.59 b | |
| Nutritional parameters | Ascorbic acid (mg/100 g) | 331.80 ± 1.88 a | 330.40 ± 18.08 a | 168.20 ± 30.33 b | 113.00 ± 16.73 c | 186.40 ± 10.67 b | 70.50 ± 2.74 d | 34.17 ± 13.64 e |
| Na (mg/kg) | 3.78 ± 0.58 c | 56.04 ± 16.42 c | 27.00 ± 13.06 c | 24.38 ± 3.76 c | 9.12 ± 2.06 c | 149.95 ± 21.11 b | 357.05 ± 127.88 a | |
| Mg (mg/kg) | 243.80 ± 12.52 c | 208.60 ± 25.39 cd | 147.80 ± 10.57 f | 182.25 ± 11.15 de | 168.40 ± 5.77 de | 305.00 ± 35.89 b | 400.33 ± 75.58 a | |
| K (mg/kg) | 3652.20 ± 366.73 c | 2908.40 ± 220.97 c | 3024.40 ± 278.62 c | 3229.00 ± 298.67 c | 2066.40 ± 280.09 d | 6307.17 ± 637.33 b | 7183.67 ± 1156.97 a | |
| Mn (mg/kg) | 2.34 ± 0.19 ab | 1.70 ± 0.30 c | 2.08 ± 0.38 bc | 1.65 ± 0.10 c | 2.24 ± 0.50 ab | 2.67 ± 0.36 a | 2.67 ± 0.36 a | |
| Fe (mg/kg) | 5.10 ± 0.38 c | 4.50 ± 0.49 c | 4.22 ± 0.63 c | 3.60 ± 0.59 c | 7.64 ± 6.37 bc | 10.97 ± 1.68 ab | 14.57 ± 3.65 a | |
| Cu (mg/kg) | 1.39 ± 0.23 b | 1.23 ± 0.23 bc | 1.27 ± 0.12 bc | 1.25 ± 0.13 bc | 1.06 ± 0.21 bc | 0.88 ± 0.22 c | 1.81 ± 0.54 a | |
| Zn (mg/kg) | 2.46 ± 0.59 cd | 3.48 ± 0.47 a | 3.18 ± 0.64 ab | 1.98 ± 0.22 d | 3.34 ± 0.54 ab | 2.67 ± 0.72 bc | 3.30 ± 0.45 ab | |
| dietary fiber (%) | 4.54 ± 0.03 f | 6.23 ± 0.04 c | 6.07 ± 0.03 d | 5.94 ± 0.04 e | 6.10 ± 0.03 d | 6.64 ± 0.04 b | 6.76 ± 0.15 a | |
| cAMP (mg/kg) | 45.15 ± 20.05 c | 41.92 ± 11.29 c | 93.01 ± 13.95 b | 57.06 ± 21.07 c | 41.96 ± 4.59 c | 213.29 ± 34.14 a | 90.86 ± 12.99 b | |
| Total amino acid (mg/100 g) | 1.16 ± 0.10 cd | 1.04 ± 0.06 d | 1.08 ± 0.05 cd | 1.36 ± 0.04 b | 1.22 ± 0.03 bc | 1.81 ± 0.10 a | 1.82 ± 0.24 a | |
| X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | X9 | X10 | X11 | X12 | X13 | X14 | X15 | X31 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X1 | 1 | |||||||||||||||
| X2 | −0.777 ** | 1 | ||||||||||||||
| X3 | 0.319 | −0.381 | 1 | |||||||||||||
| X4 | 0.393 | −0.067 | 0.217 | 1 | ||||||||||||
| X5 | 0.351 | −0.593 | 0.798 | 0.016 | 1 | |||||||||||
| X6 | −0.945 ** | 0.698 | −0.205 | −0.496 | −0.216 | 1 | ||||||||||
| X7 | 0.788 | −0.624 | −0.032 | 0.221 | 0.137 | −0.742 | 1 | |||||||||
| X8 | 0.836 | −0.496 | −0.049 | 0.444 | −0.001 | −0.882 | 0.734 | 1 | ||||||||
| X9 | 0.928 | −0.661 | 0.205 | 0.439 | 0.233 | −0.934 | 0.745 | 0.931 | 1 | |||||||
| X10 | 0.622 | −0.423 | 0.208 | 0.562 | 0.219 | −0.666 | 0.422 | 0.666 | 0.657 ** | 1 | ||||||
| X11 | 0.783 | −0.599 | −0.014 | 0.366 | 0.08 | −0.826 | 0.621 | 0.793 | 0.765 | 0.729 | 1 | |||||
| X12 | 0.19 | −0.111 | −0.587 | 0.038 | −0.395 | −0.258 | 0.267 | 0.430 ** | 0.367 | 0.201 | 0.344 * | 1 | ||||
| X13 | −0.008 | 0.06 | −0.256 | 0.117 | −0.085 | −0.097 | 0.158 | 0.169 | 0.045 | 0.315 | 0.296 | 0.192 | 1 | |||
| X14 | 0.553 | −0.717 | 0.23 | 0.071 | 0.425 ** | −0.603 | 0.600 | 0.390 | 0.487 | 0.203 | 0.517 | −0.039 | 0.313 | 1 | ||
| X15 | 0.678 | −0.586 | 0.761 | 0.458 | 0.598 | −0.654 ** | 0.315 | 0.38 | 0.627 | 0.409 | 0.408 | −0.232 | −0.201 | 0.483 | 1 | |
| X31 | 0.913 | −0.796 | 0.272 | 0.389 | 0.305 | −0.926 | 0.627 | 0.805 | 0.894 | 0.569 | 0.766 | 0.222 | −0.13 | 0.562 | 0.689 | 1 |
| Quality Parameter | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| Soluble sugar (X1, %) | 0.859 | 0.366 | 0.180 | 0.167 | −0.007 |
| Ascorbic acid (X2, mg/100 g) | −0.590 | −0.671 | −0.283 | 0.176 | −0.087 |
| Fresh mass (X3, g g) | 0.039 | 0.177 | 0.922 | 0.161 | −0.128 |
| Shape ratio (X4) | 0.319 | −0.068 | 0.123 | 0.792 | 0.023 |
| Edible rate (X5, %) | 0.114 | 0.345 | 0.800 | −0.166 | 0.110 |
| Moisture content (X6, %) | −0.848 | −0.332 | −0.085 | −0.303 | −0.067 |
| Na (X7, mg/kg) | 0.842 | 0.120 | −0.021 | −0.164 | 0.226 |
| Mg (X8, mg/kg) | 0.915 | 0.069 | −0.152 | 0.266 | 0.049 |
| K (X9, mg/kg) | 0.932 | 0.182 | 0.079 | 0.228 | −0.015 |
| Mn (X10, mg/kg) | 0.470 | 0.230 | 0.034 | 0.597 | 0.304 |
| Fe (X11, mg/kg) | 0.684 | 0.376 | −0.148 | 0.327 | 0.280 |
| Cu (X12, mg/kg) | 0.399 | 0.030 | −0.718 | −0.020 | 0.062 |
| Zn (X13, mg/kg) | 0.057 | −0.105 | −0.168 | 0.139 | 0.919 |
| Dietary fiber (X14, %) | 0.525 | 0.388 | 0.327 | −0.204 | 0.462 |
| cAMP (X15, mg/kg) | 0.453 | 0.317 | 0.868 | 0.343 | −0.136 |
| Aspartate (X16, g/100 g) | 0.568 | 0.255 | 0.474 | 0.360 | −0.007 |
| Threonine (X17, g/100 g) | 0.710 | 0.426 | 0.280 | 0.392 | 0.066 |
| Serine (X18, g/100 g) | 0.659 | 0.433 | 0.231 | 0.259 | −0.021 |
| Glutamate (X19, g/100 g) | 0.694 | 0.638 | −0.100 | 0.162 | 0.075 |
| Proline (X20, g/100 g) | 0.658 | 0.362 | −0.127 | 0.168 | −0.251 |
| Glycine (X21, g/100 g) | 0.608 | 0.592 | 0.280 | 0.213 | −0.020 |
| Alanine (X22, g/100 g) | 0.554 | 0.691 | 0.258 | 0.031 | 0.011 |
| Valine (X23, g/100 g) | 0.641 | 0.646 | 0.286 | 0.093 | 0.077 |
| Isoleucine (X24, g/100 g) | 0.561 | 0.695 | −0.032 | 0.228 | −0.271 |
| Leucine (X25, g/100 g) | 0.563 | 0.737 | 0.116 | 0.190 | −0.048 |
| Tyrosine (X26, g/100 g) | 0.092 | 0.736 | 0.121 | −0.348 | −0.085 |
| Phenylalanine (X27, g/100 g) | 0.406 | 0.779 | 0.182 | 0.156 | −0.066 |
| Lysine (X28, g/100 g) | 0.329 | 0.738 | 0.277 | 0.295 | −0.120 |
| Histidine (X29, g/100 g) | 0.404 | 0.704 | 0.289 | −0.056 | 0.196 |
| Arginine (X30, g/100 g) | −0.326 | 0.749 | 0.081 | 0.119 | 0.455 |
| Total amino acid (X31, mg/100 g) | 0.794 | 0.518 | 0.116 | 0.222 | −0.152 |
| (a) | ||||||||||||
| Data set | Region | Sample Number | Discriminant Accuracy (%) | Henan | Hebei | Shanxi | Shaanxi | Xinjiang | ||||
| Training set | Henan | 11 | 100 | 11 | 0 | 0 | 0 | 0 | ||||
| Hebei | 11 | 100 | 0 | 11 | 0 | 0 | 0 | |||||
| Shanxi | 11 | 100 | 0 | 0 | 11 | 0 | 0 | |||||
| Shaanxi | 20 | 100 | 0 | 0 | 0 | 20 | 0 | |||||
| Xinjiang | 28 | 100 | 0 | 0 | 0 | 0 | 28 | |||||
| Testing set | Henan | 4 | 100 | 4 | 0 | 0 | 2 | 0 | ||||
| Hebei | 4 | 75 | 0 | 3 | 0 | 1 | 0 | |||||
| Shanxi | 4 | 100 | 0 | 0 | 4 | 0 | 0 | |||||
| Shaanxi | 7 | 71.43 | 0 | 2 | 0 | 5 | 0 | |||||
| Xinjiang | 11 | 100 | 0 | 0 | 0 | 0 | 11 | |||||
| (b) | ||||||||||||
| Data set | Region | Sample Number | Discriminant Accuracy (%) | Huizao | Jinsixiaozao | Hupingzao | Xiangzao | Tanzao | Junzao | |||
| Training set | Huizao | 24 | 100 | 11 | 0 | 0 | 0 | 0 | 0 | |||
| Jinsixiaozao | 11 | 100 | 0 | 11 | 0 | 0 | 0 | 0 | ||||
| Hupingzao | 11 | 100 | 0 | 0 | 11 | 0 | 0 | 0 | ||||
| Xiangzao | 9 | 100 | 0 | 0 | 0 | 11 | 0 | 0 | ||||
| Tanzao | 11 | 100 | 0 | 0 | 0 | 0 | 11 | 0 | ||||
| Junzao | 15 | 100 | 0 | 0 | 0 | 0 | 0 | 15 | ||||
| Testing set | Huizao | 9 | 100 | 9 | 0 | 0 | 0 | 0 | 0 | |||
| Jinsixiaozao | 4 | 100 | 0 | 4 | 0 | 0 | 0 | 0 | ||||
| Hupingzao | 4 | 75 | 0 | 0 | 3 | 1 | 0 | 0 | ||||
| Xiangzao | 3 | 100 | 0 | 0 | 0 | 3 | 0 | 0 | ||||
| Tanzao | 4 | 75 | 0 | 1 | 0 | 0 | 3 | 0 | ||||
| Junzao | 6 | 100 | 0 | 0 | 0 | 0 | 0 | 6 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Li, L.; Zhang, G.; Jiang, N.; Ouyang, X.; Wang, M. Geographical and Varietal Traceability of Chinese Jujubes Based on Physical and Nutritional Characteristics. Foods 2021, 10, 2270. https://doi.org/10.3390/foods10102270
Wu L, Li L, Zhang G, Jiang N, Ouyang X, Wang M. Geographical and Varietal Traceability of Chinese Jujubes Based on Physical and Nutritional Characteristics. Foods. 2021; 10(10):2270. https://doi.org/10.3390/foods10102270
Chicago/Turabian StyleWu, Linxia, Ling Li, Guoguang Zhang, Nan Jiang, Xihui Ouyang, and Meng Wang. 2021. "Geographical and Varietal Traceability of Chinese Jujubes Based on Physical and Nutritional Characteristics" Foods 10, no. 10: 2270. https://doi.org/10.3390/foods10102270






