1. Introduction
Coverage creams in the pastry and confectionery industry play a very important role in giving softness, texture and presentation to cakes, pies, and desserts. In Ecuador, the pastry sector combined with the bakery sector, for which there are around 5679 businesses, generates sales of USD 306 million per year [
1].
There are cases of creams that cease to have value due to their physicochemical instability—in most cases yielding a phase separation—without being affected by considerable microbiological contamination. The result is a flaccid body of less volume and mass, with alterations in its texture and viscosity (η) characteristics, which makes the cake unattractive to the consumer [
2].
Several studies have been carried out in relation to the evaluation of stabilizers in colloidal systems made up of karaya gum, carob gum and guar gum for ice cream, showing that ice creams formulated with 50% carob gum and 50% guar gum have a lower percentage of melting and less time to the fall of the first drop compared to the control sample. Additionally, stability studies that compared concentrations of 0.3, 0.35 and 0.4% (
w/
w) of a 50% carob gum and 50% guar gum mixture against a control sample that had a commercial stabilizer applied at 0.3% (
w/
w) have shown that the ice cream with 0.4% (
w/
w) showed greater rubberiness, while the control sample showed a greater roughness [
3]. On the other hand, comparative studies between gellan gum and pectin to form fluid gels in fermented milks were carried out to test the viscosimetric and stabilizing properties when compared with that of a sample that did not contain hydrocolloids, in which no fluid gel was formed. The experimental findings proved that, unlike pectin, gellan gum was capable of creating significant yield strength values which improved the stability of colloidal particles and extrindry solid particles added in the milk drink. However, pectin improved the stability when it was combined with gellan gum. The formation of the fluid gel can be attributed to the permanent interactions that occur between gellan gum and milk proteins, forming gels of “hairy particles” as transient interactions between gelled particles [
4].
In the case of creams, it is usual to extend their shelf life by adding modified starch from corn, rice, potato or wheat [
5,
6]. However, the limitations of use are the properties of application in terms of pH, temperature and amount of water, since they frequently do not provide the desired results in the final product due to variations in the process conditions and the raw materials used for each formulation [
5].
To prevent the coalescence and flocculation of the colloidal particles found in the food matrix, certain compounds (known as stabilizants) can be added to certain foods. In this context, gums (which are polysaccharides of a very high molecular weight) have the ability to increase the viscosity of the colloidal system, and can also act as cryoprotectants [
6]. Additionally, they have the ability to retain, swell and gel in the presence of heat. Due to the tendency of gels to partially separate in the liquid phase accompanied by a decrease in the volume of the system, it is important to evaluate the volume and syneresis [
7].
It is also pertinent to assess parameters such as the density and bubble size of the cake cream, since it is a food product obtained by whipping processes, for which its matrix has some air incorporated and therefore it is necessary to measure the foam stability; this depends on factors such as Ostwald maturation, which is the diffusion of gas from the smallest bubbles to the largest ones, the coalescence of the bubbles due to the instability of the film that separates them, and the drainage of liquid of the foam layer due to gravity, which in turn is summarized in the excessive thinning of the lamellae inducing the rupture of the bubbles [
8,
9].
Another parameter for measuring the stability of colloidal systems is the rheological behavior, where thixotropy is a relevant property in terms of the firmness and softness of the creams. It occurs when the changes in the microstructure are reversible, which are time-dependent and expressed by the effect of shear stress (τ). This effect is an ideal situation that occurs under certain experimental situations, in comparison with the numerous situations in which food, in its state of flux, presents irreversibility due to its partial or total decomposition of the structure of the food matrix [
10]. Likewise, the thixotropic index (TI), a parameter that allows for evaluating the degree of rupture of the structure, has allowed in previous investigations, such as the one studied by Lavaselly and Rasia (2004) [
11] in emulsions, to identify that there is a correlation between thixotropic properties and viscosity, where it is stated that TI maintains a relationship with the physical stability of colloidal systems [
11].
Uysal et al. [
12] carried out a study related to the rheological behavior of colloidal food systems, in which pastry creams containing pasteurized liquid whole egg (LWE) were evaluated versus control group creams containing unpasteurized LWE. Specific density, foamability and rheological measurements (shear rate vs. shear stress and shear rate vs. viscosity) of the cake creams were performed. As a result, a pseudoplastic behavior was observed in all cream formulations indicating a decrease in apparent viscosity as a function of increasing shear rate [
12]. An increase from 0.29 to 0.49 in cream specific densities was observed as LWE pasteurization parameters [
12]. Furthermore, a decrease in the foamability of creams with LWE was observed as the pasteurization parameters of LWE increased [
12]. The evaluation of the rheological characteristics of the cream for cakes is important because it provides a previous knowledge about the structure of the baked cakes that, together with the density and foamability, demonstrate the importance of its measurement in the stability of colloidal food systems.
The objective of this work was to evaluate the physicochemical properties of coverage creams for cake, considered as a lyophobic colloidal system (little attraction between the dispersed phase and the dispersant phase), in which polysaccharide-type hydrocolloids such as natural gums were used to reduce the separation of phases of the cream. Various gums from different plant sources, such as Pectin Gum (PG), Tara Gum (TG), Arabic Gum (AG), and Carrageenan Gum (CG) were studied to establish which of these works best as a stabilizer for cake cream, considering the temperature conditions in which the product is going to be stored, i.e., at room temperature and in refrigeration.
2. Materials and Methods
The materials and equipment used were: Bohlin Instruments CVO rheometer, analytical balance of appreciation 0.0001 g, optical microscope (Nikon Eclipse E100, Nikon Instruments Inc., Melville, NY, USA), refrigerator, drying oven (Memmert brand, Memmert GmbH + Co. KG, Germany), centrifuge (Danon/IEC HN-SII Centrifuge, American Laboratory Trading, INC., San Diego, CA, USA), mixer (Umco brand, 7 speeds, UMCO S.A., Ecuador), thermometer, stopwatch, hand refractometer (BRIX/ATC brand, Northern Brewer, LLC., United Kingdom), neubauer chamber, Vernier caliper of appreciation 0.01 cm, falcon tubes of 50 mL, graduated cylinders of 100 mL, and syringes of 10 mL.
2.1. Additive Substances
The used additives were natural gums: pectin (unipectine RS 150 ci-trus, Cargill Texturizing Solutions, Saint-Germain-en-Laye, France) acquired from the Casa de los Químicos in Quito; tara gum (tara gum HV, Silvateam, Lima, Peru), obtained from CO-DAN in Quito; gum arabic (gum arabicum medium light, Alpha Trading GMBH, Hamburg, Germany), acquired at the Casa de los Químicos, Quito; carrageenan (carra-geenan MCH 5316, Gelymar, Santiago, Chile) obtained at ADITMAQ in Quito.
2.2. Elaboration of Coverage Creams
The coverage creams for cake were made as reported by De la Traba Luis and García Víctor [
13]: first, preparation of a syrup with sugar and water, between 67° and 69° Brix; second, beating the yolks for 2 min, after which the syrup was added and the mixture was stirred for 2 additional minutes.
The gums were incorporated by two methods. For method 1 (dissolved gum), gum solutions were made in water at 70 °C (1:9) by stirring for 30 s, followed by adding the gum solution to the yolk–syrup mixture and then mixing for 2 min, finally adding the butter and whipping for another 5 min. A concentration of 0.8% by weight was used for all creams, thus preventing the amount of additive from exceeding 1% of the permissible limit of most gums [
14]. For method 2 (powdered gum), first the syrup was prepared with sugar and water, between 67 and 69° Brix; second, the yolks were beaten for 2 min, after which the syrup was added and the mixture was stirred for a further 2 min. Then the gum powder was added to the mixture of yolks and syrup and stirred for 2 min while adding the butter, finally beating the mixture for a further 5 min.
2.3. Evaluation of Visual Acceptability of Cake Creams
The samples were coded for sensory evaluation by 20 untrained judges, which consisted of a preference test between four pairs of creams made with the four gums by the two methods proposed [
15]. Each judge was presented with a pair of creams made with the same gum by the two methods, and they were asked to choose one of the two creams according to consistency. The process was repeated for the other three pairs of creams made with the remaining gums. For each pair, an answer was obtained for a total of 80 results.
2.4. Characterization of Accepted Cake Cream by Physicochemical Parameters
The characterization of the cake cream that was accepted by the judges was carried out in triplicate at room temperature (at 25 °C) and refrigeration (at 4 °C), in different storage periods of up to 28 days: four with the gums added to a concentration of 0.8% and one without presence of gums (blank). See
Table 1. A total of 270 tests were carried out for the parameters: viscosity, density, bubble size, volume and syneresis.
2.4.1. Viscosity
Viscosity was measured using the “Bohlin Instruments CVO” rheometer, with measuring system PP20 (20 mm parallel rotating plate), GAP of 1000 (distance between the turntable and the fixed plate of 1000 µm, that is, the height of the sample during the measurement) at a constant velocity gradient (D) 0.86 s
−1, time of 7.2 s and temperature of 25 °C [
16]. Three repetitions were carried out for each formulation of the creams, at the two temperatures and for each storage time (0, 3, 7, 10, 14, 17, 21, 24, 28 days); the average is reported in all cases.
2.4.2. Density
The density (ρ) was computed from the mass and the volume of the sample in containers of approximately 16 cm3. Three repetitions were carried out for each formulation of the creams, at the two temperatures and for each storage time (0, 3, 7, 10, 14, 17, 21, 24, 28 days); the average is reported in all cases.
2.4.3. Bubble Size
The bubble size (BS) was measured in a Nikon Eclipse E100 optical microscope using the neubauer camera with a 10× and a 40× observation lens, depending on the size of the bubble. A corresponding photograph was taken to later measure the length of the bubble using an appropriate scale. The photographs were taken and processed using the Leica LAS X software (Leica Microsystems, Germany), which was connected to the Nikon Eclipse E100 optical microscope (Nikon Instruments Inc., Melville, NY, USA). The bubble diameter was measured in triplicate of three expanded samples on the neubauer chamber plate for each formulation, temperature, and storage time (0, 3, 7, 10, 14, 17, 21, 24, 28 days). A total of 270 images were obtained.
2.4.4. Volume Variation
The volume change (ΔV) of the creams was measured with a vernier caliper with an appreciation of 0.01 cm. The samples were placed in 100 mL graduated cylinders up to a height of 17 cm with the subsequent measurement of the distance between the initial height and the height of each of the samples at various storage times. The internal diameter of the graduated cylinder was also measured using the same caliper. The ΔV was obtained by Equation (1). Three repetitions were carried out for each formulation of the creams, at the two temperatures and for each storage time (0, 3, 7, 10, 14, 17, 21, 24, 28 days); the average is reported in all cases.
where ΔH = height variation, R = internal radius of the graduated cylinder.
2.4.5. Syneresis
Syneresis (S) was measured by the modified technique of Ginee et al. (Guinee et al., 1995) [
17], which consists of weighing the sample in a falcon tube and centrifuging it at 3000 rpm for 30 min (for this a Danon/IEC HN-SII centrifuge was used); once the phases were separated, the liquid phase was weighted and the percentage of syneresis (% S) was calculated by means of Equation (2) [
18]. Three repetitions were carried out for each formulation of the creams, at the two temperatures and for each storage time (0, 3, 7, 10, 14, 17, 21, 24, 28 days); the average is reported in all cases.
2.4.6. Rheology
The rheology was evaluated by measuring the shear stress and the viscosity of the five creams, using curves of τ vs. D and η vs. D, where τ is expressed in Pascal units (Pa) and D is expressed in (cm/s)/cm or s
−1, in a “going” and “return” process with the “Bohlin Instrument CVO” rheometer, using a PP 20 measuring system, GAP of 1000, at 25 °C temperature and a variable speed gradient in the range of 0 to 15 s
−1, for a total time of 240 s [
16]. In addition, the area between the “going” and “return” curves was measured, for which the Bohlin software was used; this area corresponds to the hysteresis cycle and is called the thixotropic index which is expressed in quadratic units (q.u.). The rheological study was run in large storage time intervals: 0, 7, 14, 21 and 28 days. Three repetitions were carried out for each formulation of the creams, at the two temperatures and for each storage time. A total of 150 tests were carried out for the rheological parameters, the average is reported in all cases.
4. Discussion
As can be seen in
Table 3, in which the average values of the evaluation of physicochemical properties up to 28 days, both at room temperature and refrigeration, can be seen, tara gum provides a higher viscosity (
of TG = 87.09 Pa·s) than the rest of gums and is obviously higher than that of the white cream (
of blank = 15.49 Pa·s), which is interpreted as a gum capable of thickening and stabilizing the ingredients of the covering cream. The increase in viscosity can be explained by the fact that gums need time to interact with the phases of the cream and to be able to form a three-dimensional network of macromolecules [
20]. Furthermore, the magnification is different for each gum due to the differences between their chemical structure [
9,
20,
21]. Moreover, the marked increase in the viscosity of the cream containing TG at 25 °C indicates the formation of a gel as the storage time elapses.
The addition of the gums influenced the density of the creams, causing a spongier texture, due to the inhibition of the loss of incorporated air. The increase in density can be explained by the fact that butter creams lose volume due to separation of the phases of the system. Furthermore, as the temperature increases, this decrease in volume is more pronounced. The cream made with AG had the lowest density, probably due to that during its elaboration it is capable of incorporating and retaining a large amount of air, which causes the same volume to have less mass and therefore density [
22]. In
Table 3, the average value of densities is verified during the 28 days of storage for the two temperatures (
of AG = 0.9555 g/cm
3) and secondly for the pectin cream (
of PG = 0.99 g/cm
3). On the contrary, white cream was the product that obtained the highest density (
of Blank = 1.04 g/cm
3), due to the loss of air due to physicochemical instability (the lower the air volume, the higher the density). As for cream with tara gum, it has a density very similar to that of cream with PG, (
of TG = 0.99 g/cm
3), which indicates that it can also be an alternative as a substitute for AG and PG from the approach to this food quality.
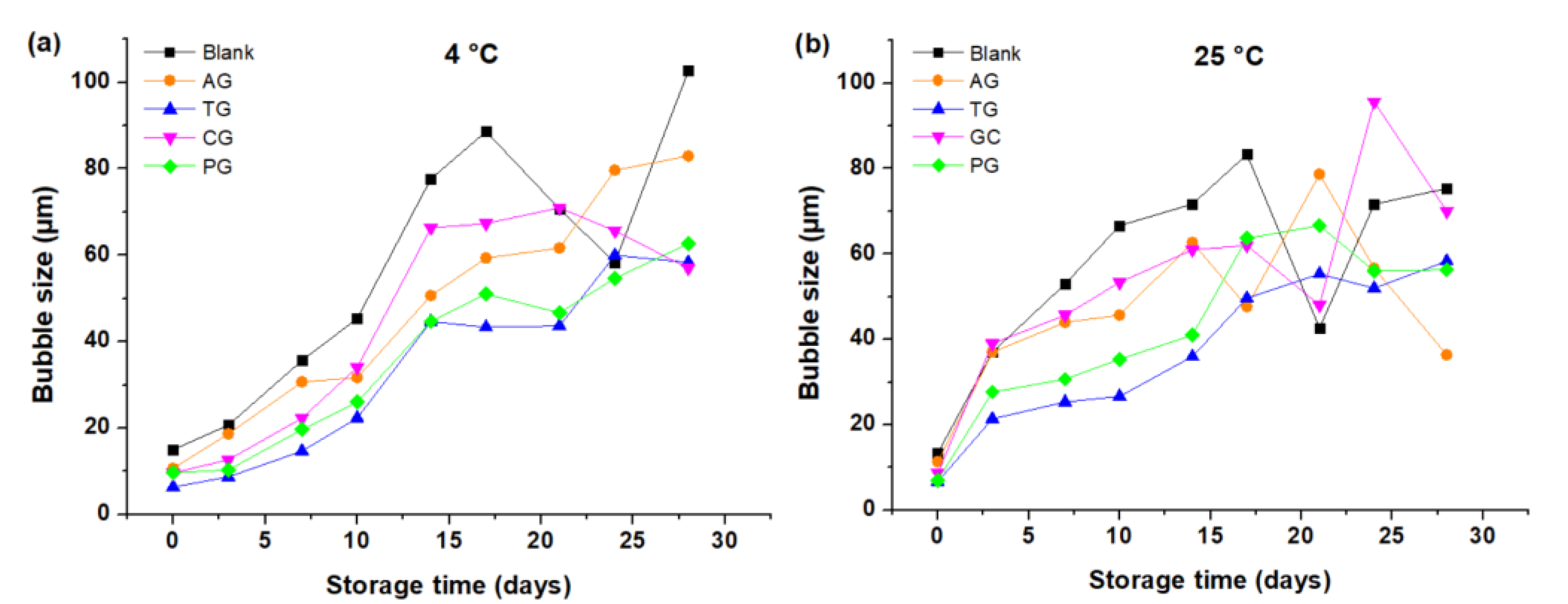
Creams formulated with natural gums caused a reduction in gas diffusion from smaller bubbles to larger ones and also reduced lamellar thinning and bubble ruptures. In
Table 3, the average value of the diameter of the bubbles is lower, firstly for the cream with tara gum ((
) of TG = 35 µm), and secondly for the cream with pectin ((
) of PG = 39 µm). This indicates that tara gum, by improving the viscosity of the colloidal system, also made it possible to stabilize the composition between the liquid phases of the lamellae with the gaseous phase of the bubbles, delaying drainage in the liquid film that separates the bubbles [
6]; the smaller the bubble size, the more stable the cream, which is interpreted in the preservation of its smooth and homogeneous texture. On the contrary, it is verified that the cream without gums has the largest size of bubbles ((
) of 57 µm), on some occasions non-spherical bubbles, and the presence of water masses were seen instead of bubbles, as shown in some images, especially at a temperature of 25 °C. The increase in BS favored by the diffusion phenomenon that allows the bubbles to unite as time passes, and there comes a time when the bubbles may experience thinning due to the drainage of the liquid film that separates them, causing their rupture [
22]. When this happens, the field observed in the microscope shows areas with water and fewer bubbles; the effect on the cream caused a heterogeneous texture, with the presence of lumpy masses and also watery masses.
The change in volume and syneresis of the creams containing gums were lower in relation to the creams without gums. The gums, being made up of hydrocolloid-type molecules, improved viscosity, reducing not only the loss of air, but also they reduced the loss of the liquid phase trapped in the food matrix and its consequent induced water evaporation.
Table 3 shows that the creams containing TG experienced less volume change, and secondly, the cream with PG ((
) of TG = 0.80 cm
3 and (
) of PG = 0.94 cm
3, respectively). It was also found that these gums caused a lower percentage of syneresis (
of PG = 8.07% and
of TG = 9.31%, respectively). Tara gum, like pectin, reduces the separation of liquid, solid and gaseous phases of the topping creams, stabilizing the system and preserving the homogeneity of the ingredients. On the contrary, the creams that did not contain gums experienced greater loss of volume and also greater syneresis ((
) of blank = 1.57 cm
3 and
of blank = 23.44%). This is due to the diversity of the ingredients that are susceptible to separating during storage. In addition, it was possible to verify that the carrageenan added to the creams, although they reduced these physicochemical phenomena (loss of volume and syneresis), are relatively greater than those obtained with the other gums ((
) of CG = 1.18 cm
3 and
of CG = 21.75%), so a good stability of butter-based topping creams could not be ensured.
The decrease in volume can be explained due to the phase separation that the cream undergoes during the storage time, since there is liquid that is released from the matrix of the cream by syneresis of contraction [
7]. There is also a rupture of bubbles in the sample due to drainage of the liquid film in addition to the formation of smaller crystals from the butter fat while it melts [
6], which produces a smaller final volume. By raising the temperature, the cream loses more structure and therefore loses more volume. In the case of cream made with tara gum, there is less volume loss due to the formation of a network of very stable macromolecules that reduces the phase separation.
The increase in syneresis can be explained because creams are thermodynamically unstable systems, which causes the separation of their phases. The amount of released liquid increased during storage and also when the temperature of the system increased [
23]. The observed decrease in syneresis in the previous graphs (
Figure 6a,b) could be caused by dehydration of the sample, which causes some sugar to crystallize and a certain amount of liquid to bind to the cream, increasing the difficulty of the syneresis of the system.
It has been shown that the density is closely related to bubble size with a direct relationship as the storage time of the cream passes. However, it must be emphasized that at 25 °C—in a certain time that depends on each cream—the disappearance of bubbles occurs, interrupting the aforementioned relationship. On the other hand, the densities of the creams presented an inverse relationship to their volume, in contrast with the direct relationship between the density and the volume lost by the sample, as indicated by the results presented in this research. The volume of the creams decreased as small bubbles merge to form larger ones because the space occupied by two bubbles is larger than that that of a single bubble [
7,
9,
22].
The creams made TG and PG had the smallest bubble size ((
) of TG = 35.19 µm and PG = 39.43 µm) as well as the lowest volume loss ((
) of TG = 0.80 cm
3 and PG = 0.94 cm
3), thus demonstrating the direct relationship between these two parameters. As for the cream with AG, the bubble size and the loss of volume are relatively small. However, in the determination of the density, an outstanding performance of this gum was observed because it has a lower density than the rest of creams. This could be explained taking into consideration that since density is closely related to mass and volume, the large amount of air incorporated during beating produces a low-density system which is not sufficiently stable. While time passes, the bubbles fade producing a greater decrease in volume compared with that of creams made with PG and TG. Although GA does not adequately stabilize buttercream, like TG and PG, its low density is an indication of better performance since a greater quantity of the product could be obtained by achieving an adequate whipping process [
24].
A well-known property of fats is their capability to crystallize in different ways while maintaining their chemical composition (polymorphism). The crystallization of a fat influences the size and stability of a food product, since an alpha crystal is smaller, less dense, and also less stable than a beta crystal. Additionally, while sudden temperature changes easily liquefy an unstable glass (which melts), slow temperature changes form stable crystals with higher melting points, which is a desired feature in the confectionery industry [
6]. The syneresis, such as the reduction in the volume of the cream, is also due to this crystallographic change of the butter fat.
In the rheological analysis, as of day 21, the creams stored at 25 °C—with the exception of the one containing TG—displayed a permanent elastic alteration after being subjected to a rheological analysis. This may be due to the fact that certain gums (arabic, pectin and carrageenan) only thicken the system, while others (for example tara gum) gelify the system at concentrations that are within the range used in this work (0.8%) [
24]. This makes tara gum the most suitable for the preparation of butter creams, because during the beating for its preparation the shear will not cause permanent elastic deformation in the cream.
The increase in hysteresis in the case of creams with tara gum and pectin at 4 °C can be explained due to a change in the texture of the food [
25] Meanwhile, the increase in hysteresis at 25 °C in creams with arabic gum, carrageenan or pectin, and in creams without gums, can be originated by a loss of texture in the cream due to the formation of lumps, producing a permanent elastic deformation in the cream [
7,
26]. In the case of cream with tara gum, the very large value of hysteresis could be due to the formation of a gelled system mentioned above. It was also possible to observe a relationship of the area of the curves with the stability of the creams, which is small for the less stable samples, and large for the more stable creams, i.e., the one made with TG (TI = 74.67 and 1559.90 q.u., at 4 °C and 25 °C, respectively).
The various experimental data obtained in the present work and the corresponding statistical analyses indicate that the phase separation in the creams that were made with the four natural gums was reduced compared to that of the cream that did not have gums. This fact indicates that it was possible to increase the stability of butter creams by adding natural gums, with the additional benefit of improving their texture and nutritional value by providing dietary fiber. The butter cream made with TG showed the best viscosity—it is 5.6 times the one of the cream without gums ( without gums = 15.49 Pa·s, with TG = 87.09 Pa·s), has a bubble size 1.6 times smaller compared to that of the cream without gums (() without gums = 57 μm, () with TG = 35 μm), a volume loss 2 times lower than that of the cream without gums (() without gums = 1.57 cm3, (() with TG = 0.80 cm3), a rheology with thixotropic behavior without permanent elastic deformation and higher thixotropic index when compared to those of the cream without gums (cream without gums with plastic and thixotropic behavior, with permanent elastic deformation) (TI max. without gums = 17.40 y 71.78 q.u., TI max. with TG = 74.67 and 1559.90 q.u., at 4 °C and 25 °C, respectively).