Formulation of Heat-Induced Whey Protein Gels for Extrusion-Based 3D Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Gel Preparation
2.4. 3D Printing
2.5. Gel Stiffness
2.6. Rheological Analysis
2.7. Yield Stress
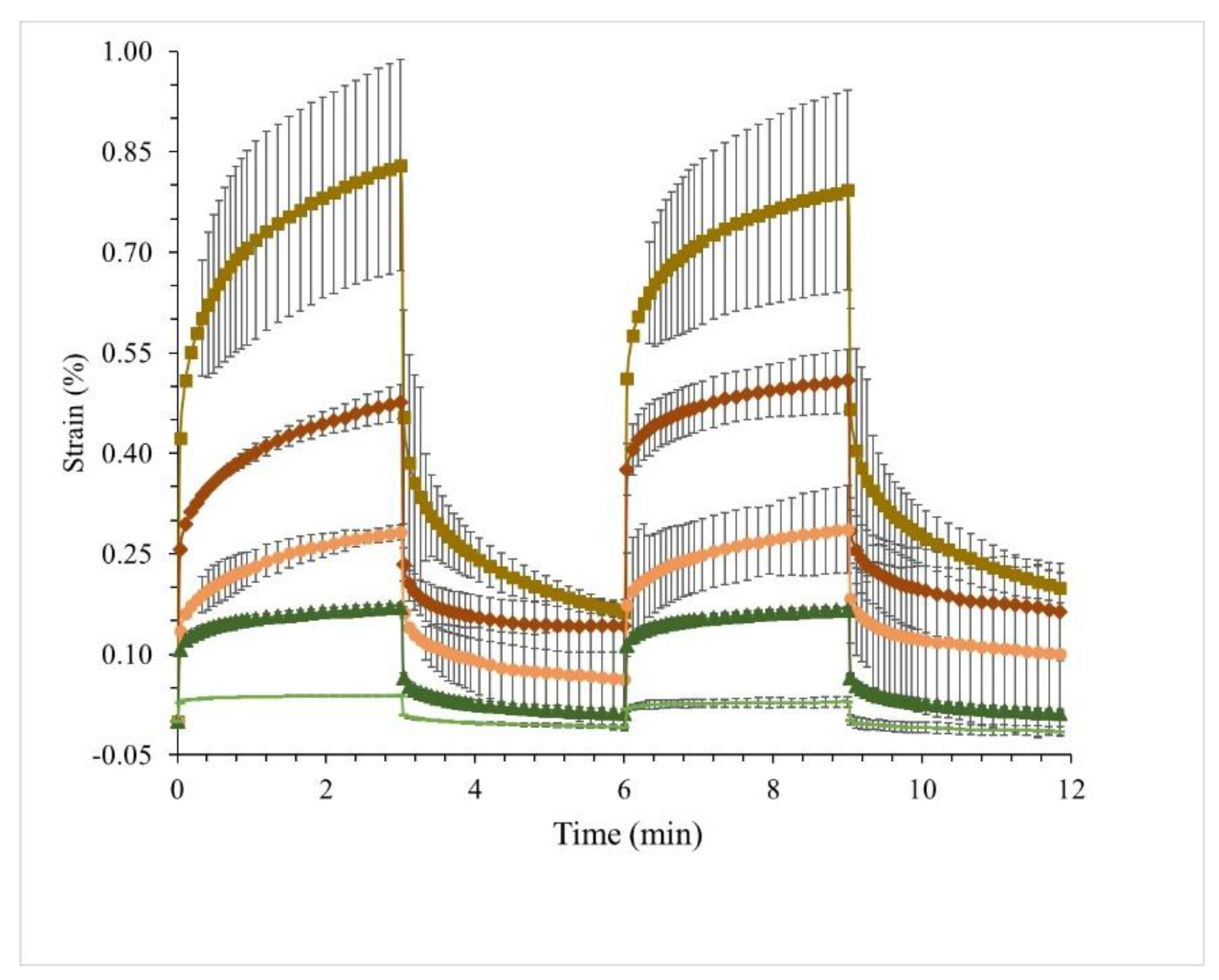
2.8. Creep–Recovery
2.9. Statistical Analysis
3. Results and Discussion
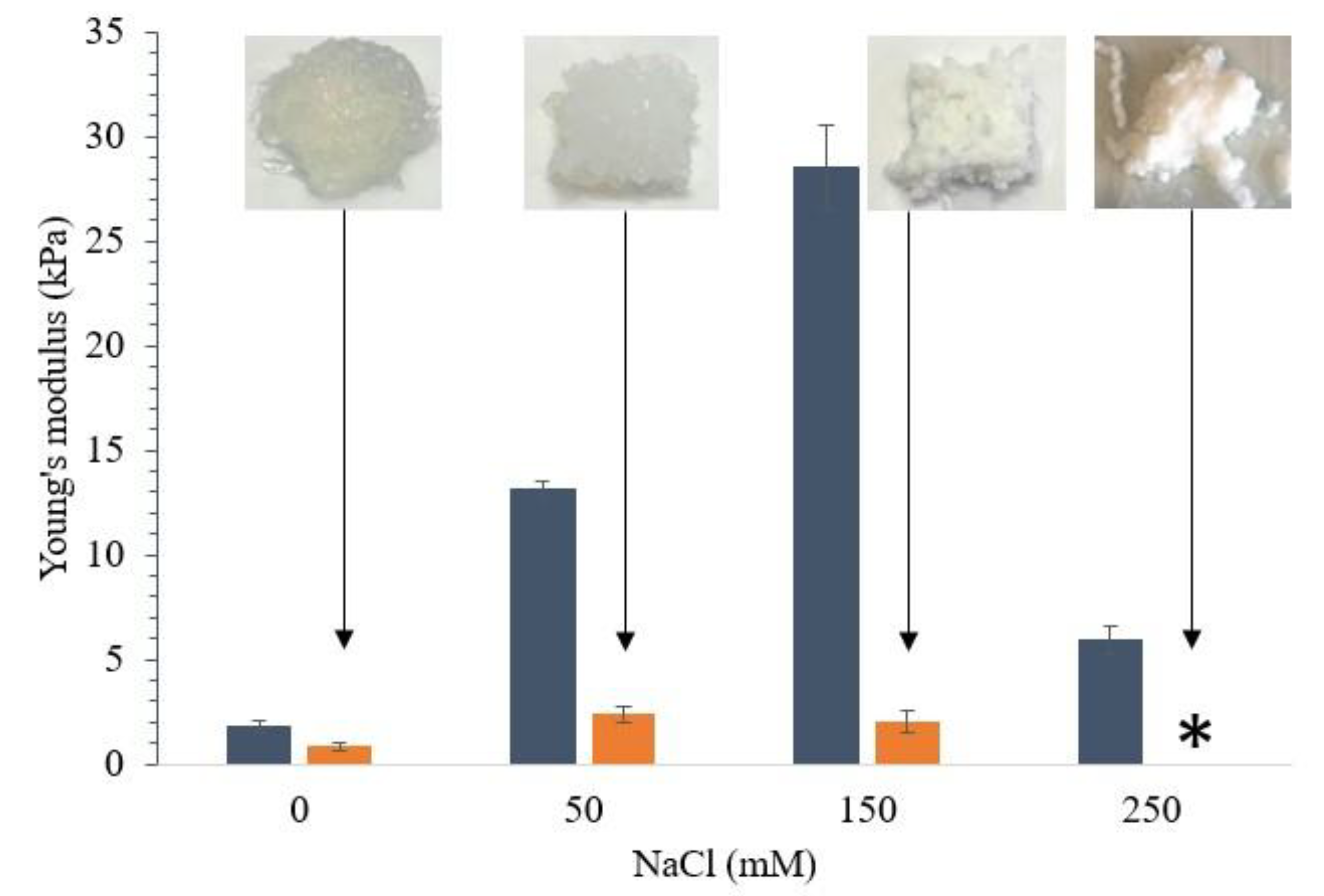
3.1. Effect of Ionic Strength on Printability of WPI Gels
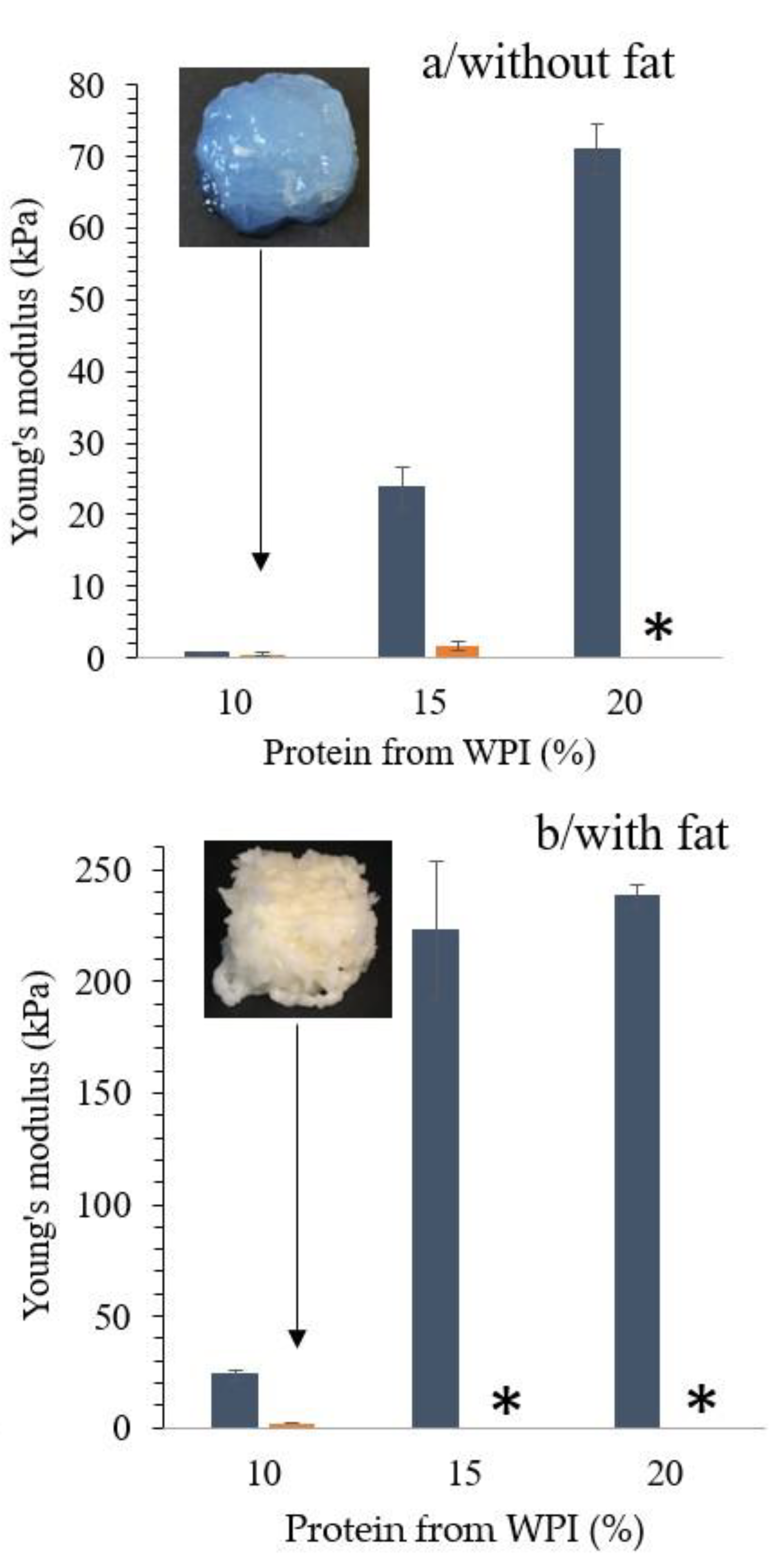
3.2. Effect of WPI Concentration and Addition of Fat on Printability of WPI Emulsion Gels
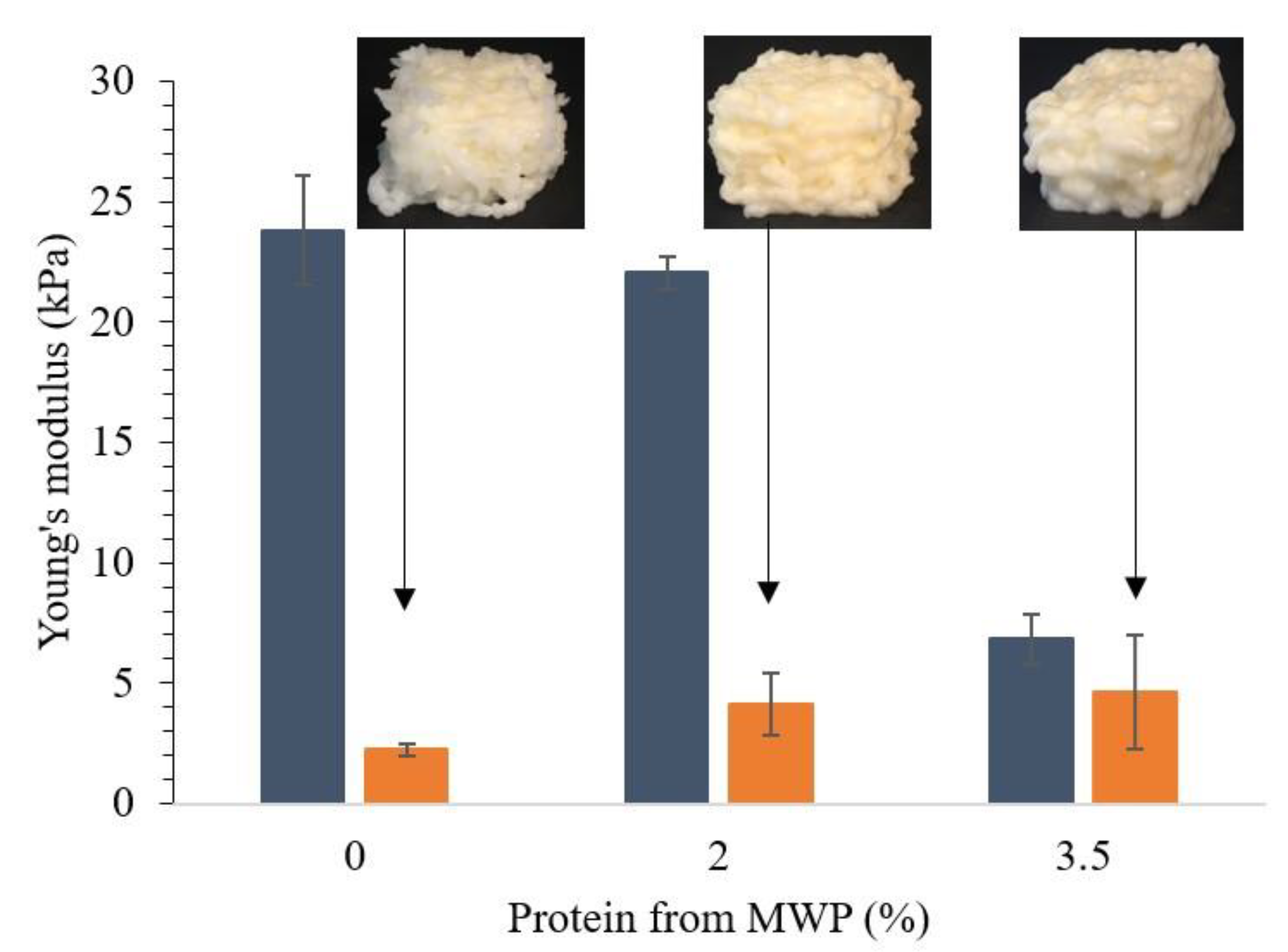
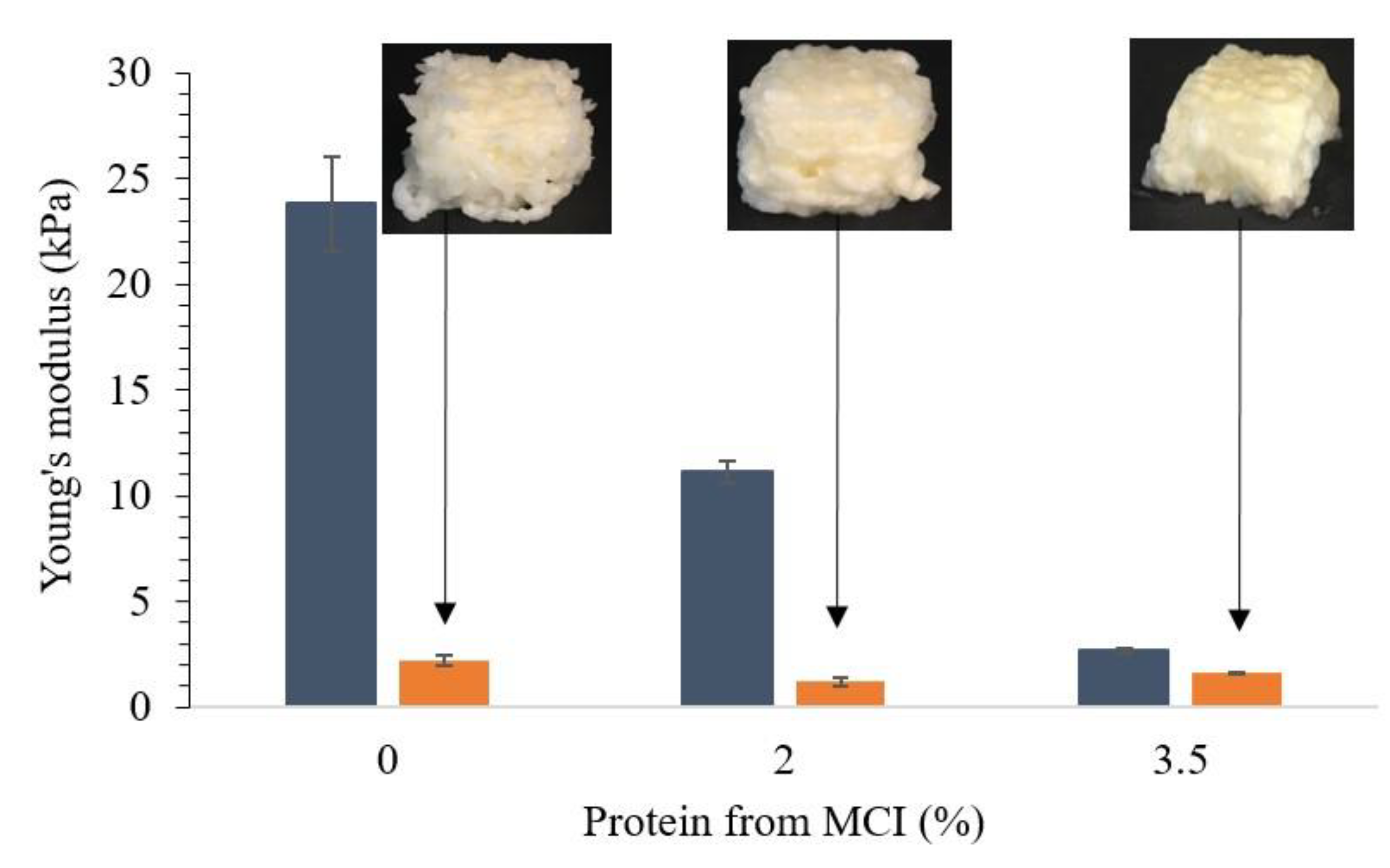
3.3. Effect of Addition of MWP or MCI on Printability of WPI Emulsion Gels
3.4. Creep–Recovery Behaviour of WPI Emulsion Gels Substituted with MWP and MCI
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Zhang, M.; Bhandari, B.; Wang, Y. 3D printing: Printing precision and application in food sector. Trends Food Sci. Technol. 2017, 69, 83–94. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, D.; Wei, G.; Ma, Y.; Bhandari, B.; Zhou, P. 3D printed milk protein food simulant: Improving the printing performance of milk protein concentration by incorporating whey protein isolate. Innov. Food Sci. Emerg. Technol. 2018, 49, 116–126. [Google Scholar] [CrossRef]
- Liu, Z.; Bhandari, B.; Prakash, S.; Mantihal, S.; Zhang, M. Linking rheology and printability of a multicomponent gel system of carrageenan-xanthan-starch in extrusion based additive manufacturing. Food Hydrocoll. 2019, 87, 413–424. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Bhandari, B.; Yang, C. Investigation on fish surimi gel as promising food material for 3D printing. J. Food Eng. 2018, 220, 101–108. [Google Scholar] [CrossRef]
- Hamilton, C.A.; Alici, G.; in het Panhuis, M. 3D printing Vegemite and Marmite: Redefining “breadboards”. J. Food Eng. 2018, 220, 83–88. [Google Scholar] [CrossRef]
- Yang, F.; Guo, C.; Zhang, M.; Bhandari, B.; Liu, Y. Improving 3D printing process of lemon juice gel based on fluid flow numerical simulation. LWT 2019, 102, 89–99. [Google Scholar] [CrossRef]
- Kouzani, A.Z.; Adams, S.; Oliver, R.; Nguwi, Y.Y.; Hemsley, B.; Balandin, S. 3D printing of a pavlova. In Proceedings of the IEEE Region 10 Annual International Conference (TENCON), Penang, Malaysia, 5–8 November 2017; pp. 2281–2285. [Google Scholar]
- Severini, C.; Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A. Printing a blend of fruit and vegetables. New advances on critical variables and shelf life of 3D edible objects. J. Food Eng. 2018, 220, 89–100. [Google Scholar] [CrossRef]
- Keerthana, K.; Anukiruthika, T.; Moses, J.A.; Anandharamakrishnan, C. Development of fiber-enriched 3D printed snacks from alternative foods: A study on button mushroom. J. Food Eng. 2020, 287, 110116. [Google Scholar] [CrossRef]
- Kim, H.W.; Bae, H.; Park, H.J. Classification of the printability of selected food for 3D printing: Development of an assessment method using hydrocolloids as reference material. J. Food Eng. 2017, 215, 23–32. [Google Scholar] [CrossRef]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3D printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Vancauwenberghe, V.; Katalagarianakis, L.; Wang, Z.; Meerts, M.; Hertog, M.; Verboven, P.; Moldenaers, P.; Hendrickx, M.E.; Lammertyn, J.; Nicolaï, B. Pectin based food-ink formulations for 3-D printing of customizable porous food simulants. Innov. Food Sci. Emerg. Technol. 2017, 42, 138–150. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, Y.; Liu, C.; Regenstein, J.M.; Liu, X.; Zhou, P. Rheological and mechanical behavior of milk protein composite gel for extrusion-based 3D food printing. LWT 2019, 102, 338–346. [Google Scholar] [CrossRef]
- Lee, C.P.; Karyappa, R.; Hashimoto, M. 3D printing of milk-based product. RSC Adv. 2020, 10, 29821–29828. [Google Scholar] [CrossRef]
- Le Tohic, C.; O’Sullivan, J.J.; Drapala, K.P.; Chartrin, V.; Chan, T.; Morrison, A.P.; Kerry, J.P.; Kelly, A.L. Effect of 3D printing on the structure and textural properties of processed cheese. J. Food Eng. 2018, 220, 56–64. [Google Scholar] [CrossRef]
- Cao, Y.; Mezzenga, R. Design principles of food gels. Nat. Food 2020, 1, 106–118. [Google Scholar] [CrossRef]
- Godoi, F.C.; Prakash, S.; Bhandari, B.R. 3D printing technologies applied for food design: Status and prospects. J. Food Eng. 2016, 179, 44–54. [Google Scholar] [CrossRef]
- Monahan, F.J.; German, J.B.; Kinsella, J.E. Effect of pH and temperature on protein unfolding and thiol/ disulfide interchange reactions during heat-induced gelation of whey proteins. J. Agric. Food Chem. 1995, 43, 46–52. [Google Scholar] [CrossRef]
- Van der Linden, E.; Foegeding, E.A. Gelation. Principles, Models and Applications to Proteins. Gelation: Principles, Models and Applications to Proteins. In Modern Biopolymer Science; Kasapis, S., Norton, I.T., Ubbink, J.B., Eds.; Academic Press: Cambridge, MA, USA, 2009; pp. 29–91. [Google Scholar]
- Hussain, R.; Gaiani, C.; Jeandel, C.; Ghanbaja, J.; Scher, J. Combined effect of heat treatment and ionic strength on the functionality of whey proteins. J. Dairy Sci. 2012, 95, 6260–6273. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Kessler, H.-G. Properties of mixed and filled-type dairy gels. J. Food Eng. 1989, 54, 1213–1217. [Google Scholar] [CrossRef]
- Renard, D.; Lavenant, L.; Sanchez, C.; Hemar, Y.; Horne, D. Heat-induced flocculation of microparticulated whey proteins ( MWP ); consequences for mixed gels made of MWP and b –lactoglobulin. Colloids Surf. B Biointerfaces 2002, 24, 73–85. [Google Scholar] [CrossRef]
- Horne, D.S. Casein Micelle Structure and Stability. In Milk Proteins, 3rd ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 133–162. [Google Scholar]
- Liu, G.; Jæger, T.C.; Nielsen, S.B.; Ray, C.A.; Ipsen, R. Physicochemical properties of milk protein ingredients and their acid gelation behaviour in different ionic environments. Int. Dairy J. 2018, 85, 16–20. [Google Scholar] [CrossRef]
- Shimada, K.; Cheftel, J.C. Sulfhydryl group/disulfide bond interchange reactions during heat-induced gelation of whey protein isolate. J. Agric. Food Chem. 1989, 37, 161–168. [Google Scholar] [CrossRef]
- Çakir, E.; Daubert, C.R.; Drake, M.A.; Vinyard, C.J.; Essick, G.; Foegeding, E.A. The effect of microstructure on the sensory perception and textural characteristics of whey protein/κ-carrageenan mixed gels. Food Hydrocoll. 2012, 26, 33–43. [Google Scholar] [CrossRef]
- Wilson, S.A.; Cross, L.M.; Peak, C.W.; Gaharwar, A.K. Shear-thinning and thermo-reversible nanoengineered inks for 3D bioprinting. ACS Appl. Mater. Interfaces 2017, 9, 43449–43458. [Google Scholar] [CrossRef]
- Spotti, M.J.; Tarhan, Ö.; Schaffter, S.; Corvalan, C.; Campanella, O.H. Whey protein gelation induced by enzymatic hydrolysis and heat treatment: Comparison of creep and recovery behavior. Food Hydrocoll. 2017, 63, 696–704. [Google Scholar] [CrossRef]
- Ako, K.; Nicolai, T.; Durand, D.; Brotons, G. Micro-phase separation explains the abrupt structural change of denatured globular protein gels on varying the ionic strength or the pH. Soft Matter 2009, 5, 4033–4041. [Google Scholar] [CrossRef]
- Langton, M.; Hermansson, A.M. Fine-stranded and particulate gels of β-lactoglobulin and whey protein at varying pH. Food Hydrocoll. 1992, 5, 523–539. [Google Scholar] [CrossRef]
- Urbonaite, V.; van der Kaaij, S.; de Jongh, H.H.J.; Scholten, E.; Ako, K.; van der Linden, E.; Pouvreau, L. Relation between gel stiffness and water holding for coarse and fine-stranded protein gels. Food Hydrocoll. 2016, 56, 334–343. [Google Scholar] [CrossRef]
- Boye, J.I.; Alli, I.; Ismail, A.A.; Gibbs, B.F.; Konishi, Y. Factors affecting molecular characteristics of whey protein gelation. Int. Dairy J. 1995, 5, 337–353. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Schrader, K. A comparative study of the gelation properties of whey protein concentrate and whey protein isolate. Le Lait 2006, 86, 259–271. [Google Scholar] [CrossRef]
- van Vliet, T. Rheological properties of filled gels. Influence of filler matrix interaction. Colloid Polym. Sci. 1988, 266, 518–524. [Google Scholar] [CrossRef]
- Dickinson, E. Emulsion gels: The structuring of soft solids with protein-stabilized oil droplets. Food Hydrocoll. 2012, 28, 224–241. [Google Scholar] [CrossRef]
- Boutin, C.; Giroux, H.J.; Paquin, P.; Britten, M. Characterization and acid-induced gelation of butter oil emulsions produced from heated whey protein dispersions. Int. Dairy J. 2007, 17, 696–703. [Google Scholar] [CrossRef]
- van Vliet, T. Rheology and fracture mechanics of foods. In Rheology and Fracture Mechanics of Foods; CRC Press: Boca Roton, FL, USA, 2013. [Google Scholar]
- Hossain, M.K.; Krah, A.; Hensel, O.; Diakite, M. Microparticulation of whey protein concentrates using the hot extrusion process: The influence of protein concentrations and other parameters. J. Food Process Technol. 2019, 10, 5. [Google Scholar]
- Torres, I.C.; Mutaf, G.; Larsen, F.H.; Ipsen, R. Effect of hydration of microparticulated whey protein ingredients on their gelling behaviour in a non-fat milk system. J. Food Eng. 2016, 184, 31–37. [Google Scholar] [CrossRef]
- Chung, C.; Degner, B.; McClements, D.J. Development of reduced-calorie foods: Microparticulated whey proteins as fat mimetics in semi-solid food emulsions. Food Res. Int. 2014, 56, 136–145. [Google Scholar] [CrossRef]
- Sturaro, A.; Penasa, M.; Cassandro, M.; Varotto, A.; De Marchi, M. Effect of microparticulated whey proteins on milk coagulation properties. J. Dairy Sci. 2014, 97, 6729–6736. [Google Scholar] [CrossRef]
- Ipsen, R. Microparticulated whey proteins for improving dairy product texture. Int. Dairy J. 2017, 67, 73–79. [Google Scholar] [CrossRef]
- Renard, D.; Robert, P.; Faucheron, S.; Sanchez, C. Rheological properties of mixed gels made of microparticulated whey proteins and β-lactoglobulin. Colloids Surf. B Biointerfaces 1999, 12, 113–121. [Google Scholar] [CrossRef]
- Felix da Silva, D.; Ahrné, L.; Ipsen, R.; Hougaard, A.B. Casein-based powders: Characteristics and rehydration properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 240–254. [Google Scholar] [CrossRef]
- Gaiani, C.; Scher, J.; Schuck, P.; Hardy, J.; Desobry, S.; Banon, S. The dissolution behaviour of native phosphocaseinate as a function of concentration and temperature using a rheological approach. Int. Dairy J. 2006, 16, 1427–1434. [Google Scholar] [CrossRef]
- de Faria, J.T.; Minim, V.P.R.; Minim, L.A. Evaluating the effect of protein composition on gelation and viscoelastic characteristics of acid-induced whey protein gels. Food Hydrocoll. 2013, 32, 64–71. [Google Scholar] [CrossRef]
- Qian, Y.; Kawashima, S. Use of creep recovery protocol to measure static yield stress and structural rebuilding of fresh cement pastes. Cem. Concr. Res. 2016, 90, 73–79. [Google Scholar] [CrossRef]
| Protein Source | |||
|---|---|---|---|
| Sample Code | WPI (%) | MWP (%) | MCI (%) |
| WPI(10) | 10 | 0 | 0 |
| MWP(2) | 8 | 2 | 0 |
| MWP(3.5) | 6.5 | 3.5 | 0 |
| MWP(5) | 5 | 5 | 0 |
| MCI(2) | 8 | 0 | 2 |
| MCI(3.5) | 6.5 | 0 | 3.5 |
| MCI(5) | 5 | 0 | 5 |
| Yield Stress (Pa) | Storage Modulus, G’ (Pa) | Loss Modulus, G’’ (Pa) | |
|---|---|---|---|
| WPI Gels without Fat | |||
| WPI(10)Fat(0) | 139 ± 16 | 106 ± 12 | 29 ± 2 |
| WPI(15)Fat(0) | * | 12,404 ± 135 | 1480 ± 30 |
| WPI(20)Fat(0) | * | 58,707 ± 1829 | 8079 ± 67 |
| Emulsion Gels with 10% Fat | |||
| WPI(10) | 826 ± 106 | 2734 ± 711 | 399 ± 94 |
| WPI(15) | * | 41,001 ± 858 | 5633 ± 195 |
| WPI(20) | * | 82,886 ± 698 | 11,503 ± 39 |
| MWP(2) | 382 ± 100 | 414 ± 42 | 61 ± 2 |
| MWP(3.5) | 525 ± 70 | 201 ± 37 | 37 ± 2 |
| MCI(2) | 275 ± 9 | 747 ± 53 | 120 ± 18 |
| MCI(3.5) | 293 ± 58 | 215 ± 38 | 46 ± 11 |
| Creep 1 | Creep 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters of the Model | Parameters of the Model | |||||||||
| RS% | α | λ1σ0 | λ2σ0 | λ1–λ2 | RS% | α | λ1σ0 | λ2σ0 | λ1–λ2 | |
| WPI(10) | 100.0 | 0.099 ± 0.009 | 0.035 ± 0.001 | 0.042 ± 0.002 | −0.008 | 100.0 | 0.112 ± 0.000 | 0.025 ± 0.007 | 0.039 ± 0.001 | −0.014 |
| MWP(2) | 70.2 | 0.134 ± 0.010 | 0.376 ± 0.013 | 0.307 ± 0.010 | 0.069 | 67.8 | 0.083 ± 0.006 | 0.451 ± 0.041 | 0.319 ± 0.022 | 0.132 |
| MWP(3.5) | 80.5 | 0.155 ± 0.004 | 0.660 ± 0.137 | 0.595 ± 0.141 | 0.065 | 74.8 | 0.116 ± 0.010 | 0.673 ± 0.121 | 0.531 ± 0.120 | 0.135 |
| MCI(2) | 92.9 | 0.108 ± 0.008 | 0.144 ± 0.019 | 0.144 ± 0.000 | 0.001 | 92.9 | 0.100 ± 0.016 | 0.145 ± 0.043 | 0.141 ± 0.008 | 0.004 |
| MCI(3.5) | 77.8 | 0.185 ± 0.029 | 0.214 ± 0.020 | 0.194 ± 0.069 | 0.020 | 64.6 | 0.134 ± 0.026 | 0.234 ± 0.058 | 0.167 ± 0.049 | 0.067 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sager, V.F.; Munk, M.B.; Hansen, M.S.; Bredie, W.L.P.; Ahrné, L. Formulation of Heat-Induced Whey Protein Gels for Extrusion-Based 3D Printing. Foods 2021, 10, 8. https://doi.org/10.3390/foods10010008
Sager VF, Munk MB, Hansen MS, Bredie WLP, Ahrné L. Formulation of Heat-Induced Whey Protein Gels for Extrusion-Based 3D Printing. Foods. 2021; 10(1):8. https://doi.org/10.3390/foods10010008
Chicago/Turabian StyleSager, Valeska F., Merete B. Munk, Mikka Stenholdt Hansen, Wender L. P. Bredie, and Lilia Ahrné. 2021. "Formulation of Heat-Induced Whey Protein Gels for Extrusion-Based 3D Printing" Foods 10, no. 1: 8. https://doi.org/10.3390/foods10010008
APA StyleSager, V. F., Munk, M. B., Hansen, M. S., Bredie, W. L. P., & Ahrné, L. (2021). Formulation of Heat-Induced Whey Protein Gels for Extrusion-Based 3D Printing. Foods, 10(1), 8. https://doi.org/10.3390/foods10010008






