Efficient Reduction of Food Related Mould Spores on Surfaces by Hydrogen Peroxide Mist
Abstract
1. Introduction
2. Materials and Methods
2.1. Mould Strains
2.2. Preparation of Mould Spore Suspensions
2.3. Exposure of Mould Spores to H2O2 Mist
2.4. Effect of Food Soils on the H2O2 Mist Disinfection Effect
2.5. Fungicidal Suspension Test with Liquid H2O2
2.6. Calculations and Statistics
3. Results and Discussion
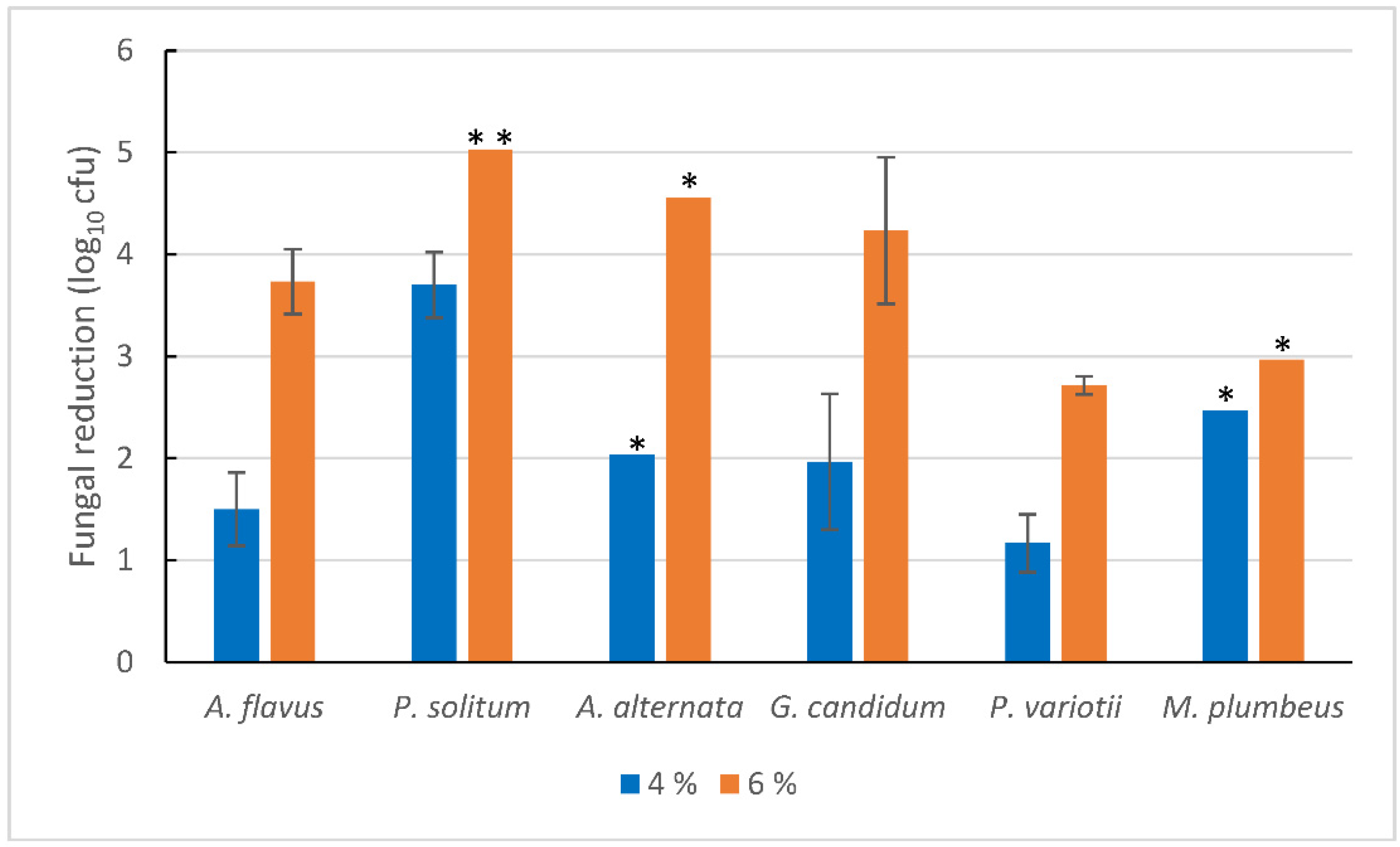
3.1. Reduction in Mould Spores after Exposure to H2O2 Mist
3.2. Influence of Food Soils on the Fungicidal Effect of H2O2 Mist Disinfection
3.3. Effect of Liquid H2O2 on Mould Spore Suspensions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taniwaki, M. Editorial overview: Food mycology. Curr. Opin. Food Sci. 2018, 23. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Piacentini, K.C.; Iwase, C.H.T.; Rocha, L.D.O. Toxigenic Alternaria species: Impact in cereals worldwide. Curr. Opin. Food Sci. 2018, 23, 57–63. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; Cbs Laboratory Manual Series; CBS-KNAW Fungal Bioderversity Centre: Utrecht, The Netherlands, 2010. [Google Scholar]
- Bärtschi, C.; Berthier, J.; Valla, G. Evolution of the Surface Fungal Flora of Reblochon Cheese. Lait 1994, 74, 105–114. [Google Scholar] [CrossRef][Green Version]
- Kure, C.F.; Skaar, I.; Brendehaug, J. Mould contamination in production of semi-hard cheese. Int. J. Food Microbiol. 2004, 93, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Marín, P.; Palmero, D.; Jurado, M. Occurrence of moulds associated with ovine raw milk and cheeses of the Spanish region of Castilla La Mancha. Int. J. Dairy Technol. 2015, 68, 565–572. [Google Scholar] [CrossRef]
- Kure, C.F.; Skaar, I. The fungal problem in cheese industry. Curr. Opin. Food Sci. 2019, 29, 14–19. [Google Scholar] [CrossRef]
- Asefa, D.T.; Kure, C.F.; Gjerde, R.O.; Omer, M.K.; Langsrud, S.; Nesbakken, T.; Skaar, I. Fungal growth pattern, sources and factors of mould contamination in a dry-cured meat production facility. Int. J. Food Microbiol. 2010, 140, 131–135. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, K.; Nielsen, P.V. Fungicidal effect of 15 disinfectants against 25 fungal contaminants commonly found in bread and cheese manufacturing. J. Food Prot. 1996, 59, 268–275. [Google Scholar] [CrossRef]
- Otter, J.A.; Yezli, S.; Perl, T.; Barbut, F.; French, G. The role of ‘no-touch’ automated room disinfection systems in infection prevention and control. J. Hosp. Infect. 2013, 83, 1–13. [Google Scholar] [CrossRef]
- Doll, M.; Morgan, D.J.; Anderson, D.; Bearman, G. Touchless Technologies for Decontamination in the Hospital: A Review of Hydrogen Peroxide and UV Devices. Curr. Infect. Dis. Rep. 2015, 17, 44. [Google Scholar] [CrossRef]
- Falagas, M.E.; Thomaidis, P.; Kotsantis, I.; Sgouros, K.; Samonis, G.; Karageorgopoulos, D. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: A systematic review. J. Hosp. Infect. 2011, 78, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Møretrø, T.; Fanebust, H.; Fagerlund, A.; Langsrud, S. Whole room disinfection with hydrogen peroxide mist to control Listeria monocytogenes in food industry related environments. Int. J. Food Microbiol. 2019, 292, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Masotti, F.; Vallone, L.; Ranzini, S.; Silvetti, T.; Morandi, S.; Brasca, M. Effectiveness of air disinfection by ozonation or hydrogen peroxide aerosolization in dairy environments. Food Control. 2019, 97, 32–38. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Mansour, M.; Sorenson, D. Performance of fogged disinfectants to inactivate conidia of Penicillium digitatum within citrus degreening rooms. Postharvest Biol. Technol. 2014, 91, 134–140. [Google Scholar] [CrossRef]
- Feliziani, E.; Lichter, A.; Smilanick, J.L.; Ippolito, A. Disinfecting agents for controlling fruit and vegetable diseases after harvest. Postharvest Biol. Technol. 2016, 122, 53–69. [Google Scholar] [CrossRef]
- Forney, C.F.; Rij, R.E.; Denis-Arrue, R.; Smilanick, J.L. Vapor Phase Hydrogen Peroxide Inhibits Postharvest Decay of Table Grapes. HortScience 1991, 26, 1512–1514. [Google Scholar] [CrossRef]
- Smilanick, J.L.; Goates, B.J.; Denisarrue, R.; Simmons, G.F.; Peterson, G.L.; Henson, D.J.; Rij, R.E. Germinability of Tilletia spp Teliospores after Hydrogen-Peroxide Treatment. Plant Dis. 1994, 78, 861–865. [Google Scholar] [CrossRef]
- Begum, M.; Hocking, A.; Miskelly, D. Inactivation of food spoilage fungi by ultra violet (UVC) irradiation. Int. J. Food Microbiol. 2009, 129, 74–77. [Google Scholar] [CrossRef]
- CEN. En 1276. Chemical Disinfectants and Antiseptics—Quantitative Suspension Test for the Evaluation of Bactericidal Activity of Chemical Disinfectants and Antiseptics for Use in Food, Industrial, Domestic and Institutional Areas—Test Methods and Requirements (Phase 2—Step 1); CEN (European Comitee for standardization): Brussels, Belgium, 1997. [Google Scholar]
- CEN. En 13697. Chemical Disinfectants and Antiseptics—Quantitative Non-Porous Surface Test for the Evaluation of Bactericidal and/or Fungicidal Activity of Chemical Disinfectants and Antiseptics for Use in Food, Industrial, Domestic and Institutional Areas—Test Methods and Requirements (Phase 2—Step 2); CEN (European Comitee for Standardization): Brussels, Belgium, 2002. [Google Scholar]
- Dijksterhuis, J. The fungal spore and food spoilage. Curr. Opin. Food Sci. 2017, 17, 68–74. [Google Scholar] [CrossRef]
- Dijksterhuis, J. Fungal spores: Highly variable and stress-resistant vehicles for distribution and spoilage. Food Microbiol. 2019, 81, 2–11. [Google Scholar] [CrossRef]
- Scaramuzza, N.; Cigarini, M.; Mutti, P.; Berni, E.; Massimo, C. Sanitization of packaging and machineries in the food industry: Effect of hydrogen peroxide on ascospores and conidia of filamentous fungi. Int. J. Food Microbiol. 2020, 316, 108421. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, K.; Arima, T.-H.; Iwashita, K.; Yamada, O.; Gomi, K.; Akita, O. Aspergillus oryzae atfB encodes a transcription factor required for stress tolerance in conidia. Fungal Genet. Biol. 2008, 45, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.O.; Stefanello, A.; Garcia, M.V.; Parussolo, G.; Stefanello, R.F.; Moro, C.B.; Copetti, M.V. Efficacy of commercial sanitizers against fungi of concern in the food industry. LWT 2018, 97, 25–30. [Google Scholar] [CrossRef]
- Bernardi, A.O.; Stefanello, A.; Lemos, J.G.; Garcia, M.V.; Copetti, M.V. Antifungal activity of commercial sanitizers against strains of Penicillium roqueforti, Penicillium paneum, Hyphopichia burtonii, and Aspergillus pseudoglaucus: Bakery spoilage fungi. Food Microbiol. 2019, 83, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Buchen, S.Y.; Marth, E.H. Sporicidal Action of Hydrogen Peroxide on Conidia from Toxigenic Strains of Aspergillus flavus and Aspergillus parasiticus: Ii. Effects of Ph, Sucrose, Glucose, and Sodium Chloride. J. Food Protec. 1977, 40, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Vardar, C.; Ilhan, K.; Karabulut, O.A. The application of various disinfectants by fogging for decreasing postharvest diseases of strawberry. Postharvest Biol. Technol. 2012, 66, 30–34. [Google Scholar] [CrossRef]
- Delgado, D.A.; de Souza Sant’Ana, A.; Granato, D.; de Massaguer, P.R. Inactivation of Neosartorya fischeri and Paecilomyces variotii on Paperboard Packaging Material by Hydrogen Peroxide and Heat. Food Control 2012, 23, 165–170. [Google Scholar] [CrossRef]
| Mould Species | Nofima Strain Collection Number | Origin |
|---|---|---|
| Alternaria alternata | MF07134 | Wheat |
| Aspergillus flavus | MF04921 | Food waste |
| Geotrichum candidum | MF04935 | Horse skin |
| Mucor plumbeus | MF07127 | Cheese |
| Paecilomyces variotii | MF04901 | Food |
| Penicillium solitum | MF07110 | Production environment, dry cured meat |
| Mould § | Log Reduction | |||||
|---|---|---|---|---|---|---|
| 2 h * | 4 h * | |||||
| Replicate 1 | Replicate 2 | Replicate 3 | Replicate 1 | Replicate 2 | Replicate 3 | |
| Alternaria alternata | >3.0 †,‡ | >3.0 | >3.0 | >3.0 | >3.0 | >3.0 |
| Geotrichum candidum | 2.1 | 2.8 | >3.0 | >3.0 | >3.0 | >3.0 |
| Paecilomyces variotii | >3.0 | 2.4 | −0.4 | >3.0 | >3.0 | 0.2 |
| Aspergillus flavus | 0.1 | >3.0 | >3.0 | 0.64 | >3.0 | >3.0 |
| Penicillium solitum | >3.0 | >3.0 | >3.0 | >3.0 | >3.0 | >3.0 |
| Mucor plumbeus | 2.8 | >3.0 | >3.0 | >3.0 | >3.0 | >3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kure, C.F.; Langsrud, S.; Møretrø, T. Efficient Reduction of Food Related Mould Spores on Surfaces by Hydrogen Peroxide Mist. Foods 2021, 10, 55. https://doi.org/10.3390/foods10010055
Kure CF, Langsrud S, Møretrø T. Efficient Reduction of Food Related Mould Spores on Surfaces by Hydrogen Peroxide Mist. Foods. 2021; 10(1):55. https://doi.org/10.3390/foods10010055
Chicago/Turabian StyleKure, Cathrine Finne, Solveig Langsrud, and Trond Møretrø. 2021. "Efficient Reduction of Food Related Mould Spores on Surfaces by Hydrogen Peroxide Mist" Foods 10, no. 1: 55. https://doi.org/10.3390/foods10010055
APA StyleKure, C. F., Langsrud, S., & Møretrø, T. (2021). Efficient Reduction of Food Related Mould Spores on Surfaces by Hydrogen Peroxide Mist. Foods, 10(1), 55. https://doi.org/10.3390/foods10010055





