Determination of Three Typical Metabolites of Pyrethroid Pesticides in Tea Using a Modified QuEChERS Sample Preparation by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry
Abstract
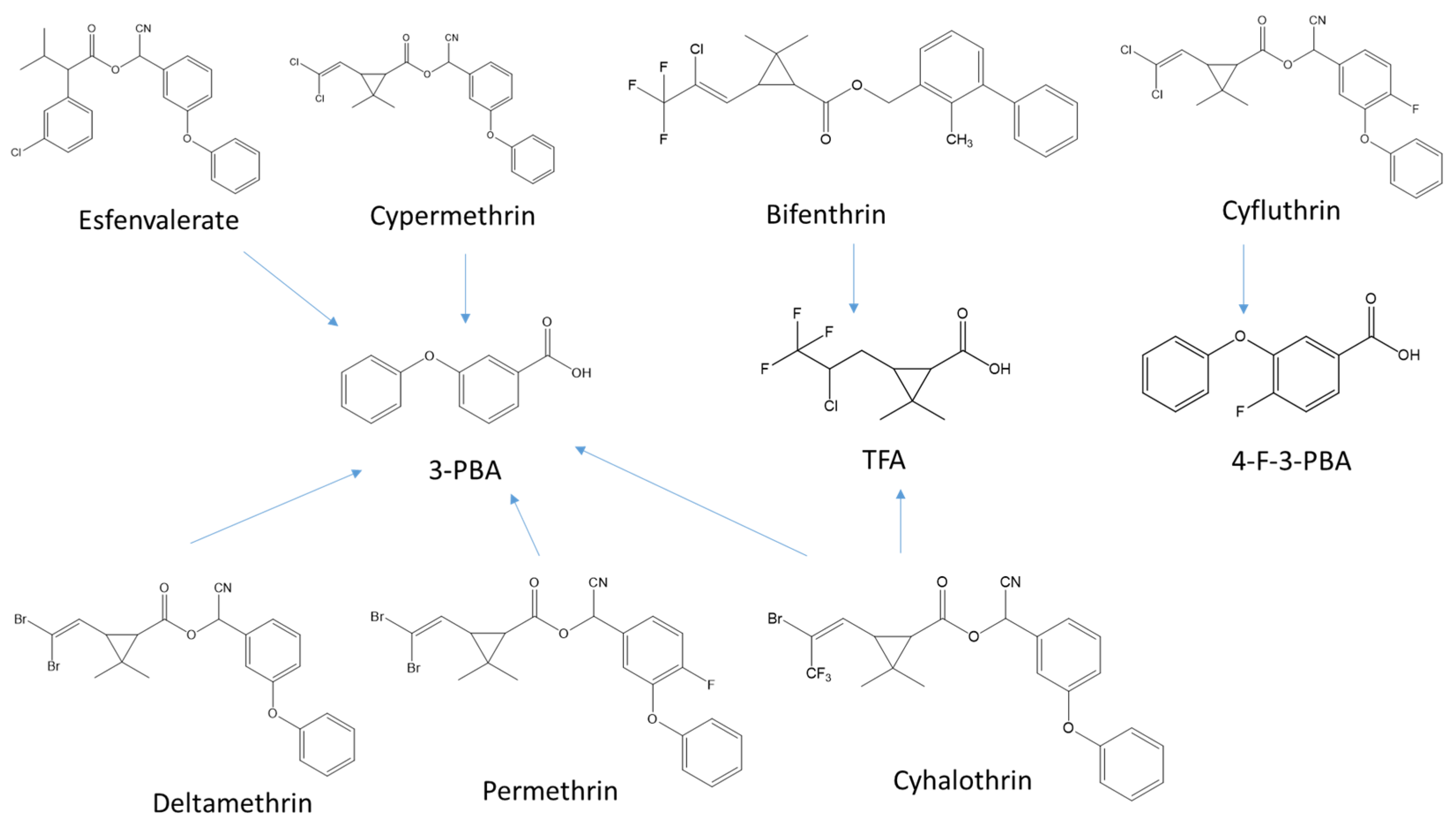
1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Sample Preparation
2.3. UHPLC-MS/MS Analysis
2.4. Validation Procedure
3. Results and Discussion
3.1. UHPLC-MS/MS Optimization
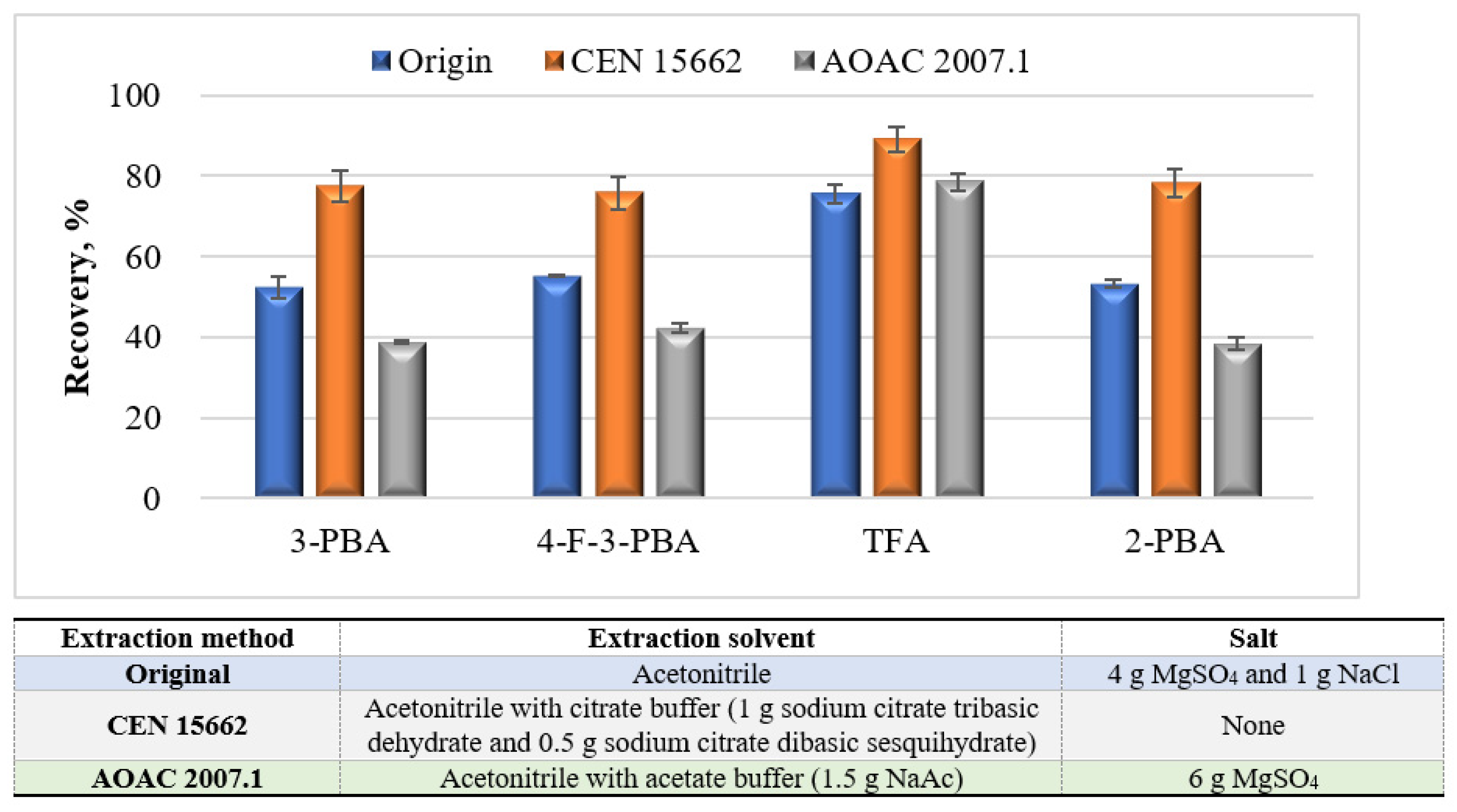
3.2. Modified QuEChERS Development
3.3. Method Performance
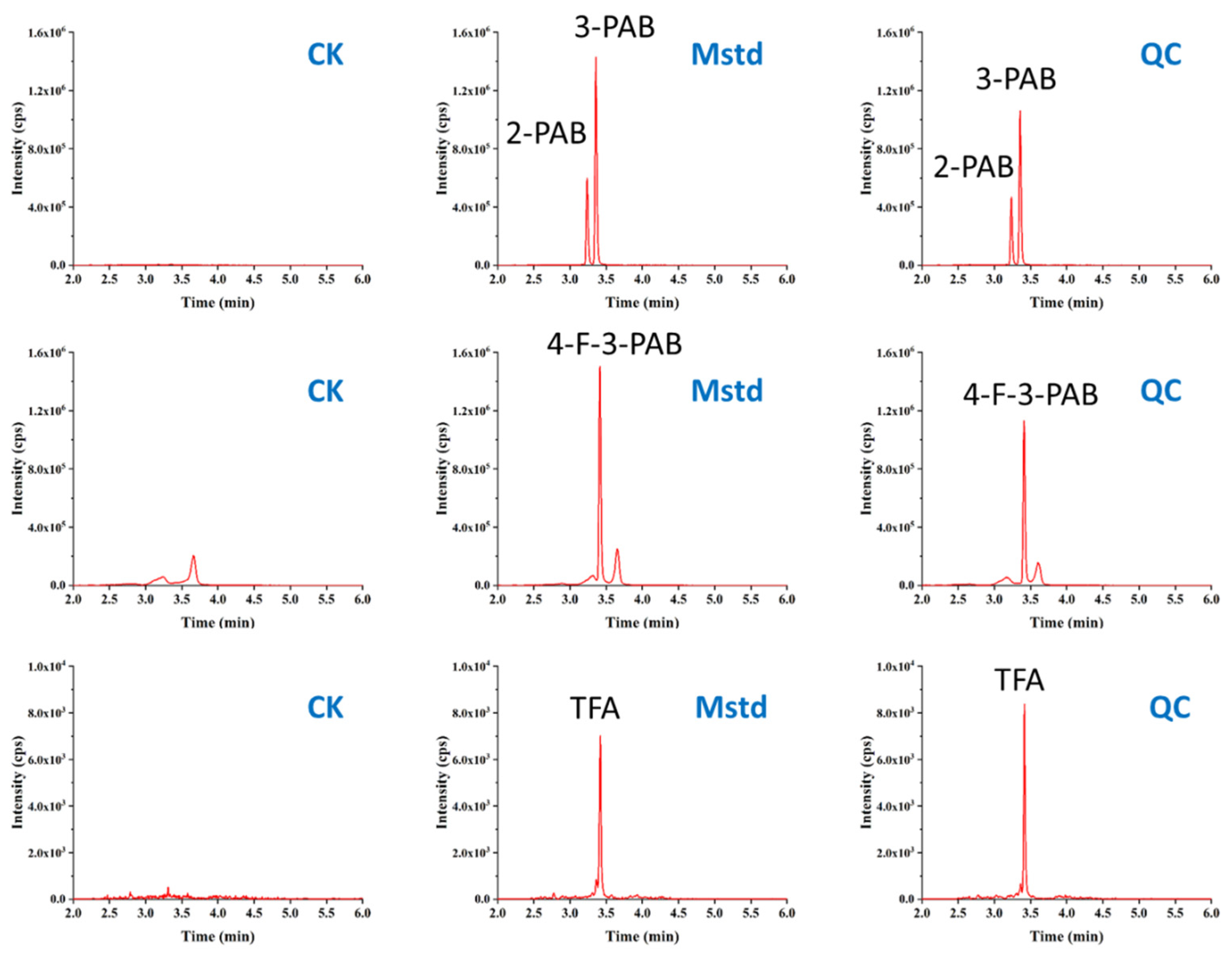
3.4. Analysis of Real Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US EPA. Pesticides Industry Sales and Usage—2006 and 2007 Market Estimates; EPA 733-R-11-001; Office of Chemical Safety and Pollution Prevention, US EPA: Washington, DC, USA, 2011.
- Fenner, K.; Canonica, S.; Wackett, L.P.; Elsner, M. Evaluating pesticide degradation in the environment: Blind spots and emerging opportunities. Science 2013, 341, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, Y.; Lydy, M.; You, J. Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: An overview. J. Hazard. Mater. 2017, 324, 258–271. [Google Scholar] [CrossRef] [PubMed]
- ICAMA (Institute for the Control of Agrochemicals, MOA of China). China Pesticide Information Network: Pesticide Market Review in 2015 and Outlook in 2016. Available online: http://www.chinapesticide.gov.cn/yqnq/1530.jhtml/ (accessed on 8 December 2015). (In Chinese)
- Li, Z.; Nie, J.; Lu, Z.; Xie, H.; Kang, L.; Chen, Q.; Li, A.; Zhao, X.; Xu, G.; Yan, Z. Cumulative risk assessement of the exposure to pyrethroids through fruits consumption in China—Based on a 3-year investigation. Food Chem. Toxicol. 2016, 96, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Miao, H.; Du, J.; Zou, J.; Zhang, G.; Zhao, Y.; Wu, Y. Organochlorine pesticides and pyrethroids in Chinese tea by screening and confirmatory detection using GC-NCI-MS and GC-MS/MS. J. Agric. Food Chem. 2014, 62, 7092–7100. [Google Scholar] [CrossRef]
- Morgan, M.K.; Macmillan, D.K.; Zehr, D.; Sobus, J.R. Pyrethroid insecticides and their environmental degradates in repeated duplicate-diet solid food samples of 50 adults. J. Expo. Sci. Environ. Epid. 2018, 28, 40–45. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Wang, C. Toxicity of the pyrethroid bifenthrin insecticide. Environ. Chem. Lett. 2018, 16, 1377–1391. [Google Scholar] [CrossRef]
- European Food Safety Authority. Modification of the existing MRLs for cypermethrin in various crops. EFSA J. 2011, 9, 2281–2310. [Google Scholar]
- Richards, J.; Lu, Z.; Fu, Q.; Schlenk, D.; Gan, J. Conversion of pyrethroid insecticides to 3-phenoxybenzoic acid on urban hard surfaces. Environ. Sci. Technol. Lett. 2017, 4, 546–550. [Google Scholar] [CrossRef]
- Mortimer, R.D.; Weber, D.F.; Sun, W.F. Analysis of total free and glucose-conjugated pyrethroid acid metabolites in tea infusions as hexafluoroisopropyl esters by gas chromatography with electron capture detection. J. AOAC Int. 1995, 78, 846–855. [Google Scholar] [CrossRef]
- Wang, X.; He, B.; Kong, B.; Wei, L.; Wang, R.; Zhou, C.; Shao, Y.; Lin, J.; Fu, Z. β-Cypermethrin and its metabolite 3-phenoxybenzoic acid exhibit immunotoxicity in murine macrophages. Acta Biochim. Biophys. Sin. 2017, 49, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Shen, O.; Sun, H.; Fei, J.; Lu, C.; Song, L.; Xia, Y.; Wang, S.; Wang, X. Assessing hormone receptor activities of pyrethroid insecticides and their metabolites in reporter gene assays. Toxicol. Sci. 2010, 116, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Schettgen, T.; Dewes, P.; Kraus, T. A method for the simultaneous quantification of eight metabolites of synthetic pyrethroids in urine of the general population using gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 5467–5478. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, D.; Ji, R.; Yao, K.; Deng, W.; Yuan, H.; Wu, Q.; Jia, Q.; Luo, P.; Zhou, K.; et al. Simultaneous determination of β-cypermethrin and its metabolite 3-phenoxybenzoic acid in microbial degradation systems by HPLC-UV. J. Chromatogr. Sci. 2016, 54, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, R.R.; Tsai, P.; Ponnusamy, V.K. A fast and sensitive analytical procedure for monitoring of synthetic pyrethroid pesticides’ metabolites in environmental water samples. Microchem. J. 2019, 148, 355–363. [Google Scholar] [CrossRef]
- López-García, M.; Romero-González, R.; Frenich, A.G. Monitoring of organophosphate and pyrethroid metabolites in human urine samples by an automated method (TurboFlowTM) coupled toultra-high performance liquid chromatography-Orbitrap mass spectrometry. J. Pharmaceut. Biomed. 2019, 173, 31–39. [Google Scholar] [CrossRef]
- Charisiadis, P.; Delplancke, T.; Makris, K.C. Cohort-friendly protocol for the determination of two urinary biomarkers of exposure to pyrethroids and neonicotinoids using gas chromatography-triple quadrupole mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 5013–5021. [Google Scholar] [CrossRef]
- Bragança, I.; Lemos, P.C.; Delerue-Matos, C.; Domingues, V.F. Pyrethroid pesticide metabolite, 3-PBA, in soils: Method development and application to real agricultural soils) and human urine and plasma. Environ. Sci. Pollut. Res. 2019, 26, 2987–2997. [Google Scholar]
- Behniwal, P.K.; She, J. Development of HPLC-MS/MS method for the simultaneous determination of metabolites of organophosphate pesticides, synthetic pyrethroids, herbicides and DEET in human urine. Intern. J. Environ. Anal. Chem. 2017, 97, 548–562. [Google Scholar] [CrossRef]
- Jeong, D.; Kang, J.S.; Kim, K.M.; Baek, S.; Choe, S.; Pyo, J. Simultaneous determination of pyrethroids and their metabolites in human plasma using liquid chromatography tandem mass spectrometry. Forensic. Sci. Int. 2019, 302, 109846–109852. [Google Scholar] [CrossRef]
- Rousis, N.I.; Zuccato, E.; Castiglioni, S. Monitoring population exposure to pesticides based on liquid chromatography-tandem mass spectrometry measurement of their urinary metabolites in urban wastewater: A novel biomonitoring approach. Sci. Total Environ. 2016, 571, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Morgan, M.K.; Graham, S.E.; Starr, J.M. Measurement of pyrethroids and their environmental degradation products in fresh fruits and vegetables using a modification of the quick easy cheap effective rugged safe (QuEChERS) method. Talanta 2016, 151, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Anastassiades, M.; Lehotay, S.J.; Štajnbajer, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produre. J. AOAC. Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [PubMed]
- British Standard. Foods of Plant Origin—Determination of Pesticide Residues Using GC–MS and/or LC–MS/MS Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE–Quechers–Method; EN 15662; British Standard: London, UK, 2008. [Google Scholar]
- AOAC. AOAC Official Method 2007.01, I AOAC Official Method 2007.01 pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate. Off. Methods Anal. AOAC Int. 2011, 90, 17–26. [Google Scholar]
- European Commision. Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; European Commision: Brussels, Belgium, 2017. [Google Scholar]
- Garí, M.; González-Quinteiro, Y.; Bravo, N.; Grimalt, J.O. Analysis of metabolites of organophosphate and pyrethroid pesticides in human urine from urban and agricultural populations (Catalonia and Galicia). Sci. Total Environ. 2018, 622–623, 526–533. [Google Scholar] [CrossRef]
- González-Ruiz, V.; Olives, A.I.; Martín, M.A. Core-shell particles lead the way to renewing high-performance liquid chromatography. TrAC Trends Anal. Chem. 2015, 64, 17–28. [Google Scholar] [CrossRef]
- Kaczyński, P. Large-scale multi-class herbicides analysis in oilseeds by rapid one-step QuEChERS-based extraction and cleanup method using liquid chromatography–tandem mass spectrometry. Food Chem. 2017, 230, 411–422. [Google Scholar] [CrossRef]
- Rutkowska, E.; Łozowicka, B.; Kaczyński, P. Modification of multiresidue QuEChERS protocol to minimize matrix effect and improve recoveries for determination of pesticide residues in dried herbs followed by GC-MS/MS. Food Anal. Method 2018, 11, 709–724. [Google Scholar] [CrossRef]
- Martínez-Domínguez, G.; Nieto-García, A.J.; Romero-González, R.; Frenich, A.G. Application of QuEChERS based method for the determination of pesticides in nutraceutical products (Camellia sinensis) by liquid chromatography coupled to triple quadrupole tandem mass spectrometry. Food Chem. 2015, 177, 182–190. [Google Scholar] [CrossRef]
- Chen, H.; Gao, G.; Liu, P.; Pan, M.; Chai, Y.; Liu, X.; Lu, C. Development and validation of an ultra performance liquid chromatography Q-Exactive Orbitrap mass spectrometry for the determination of fipronil and its metabolites in tea and chrysanthemum. Food Chem. 2018, 246, 328–334. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, L.; Jiang, Y.; Zhang, F.; Pan, C. Dispersive cleanup of acetonitrile extracts of tea samples by mixed multiwalled carbon nanotubes, primary secondary amine, and graphitized carbon black sorbents. J. Agric. Food Chem. 2012, 60, 4026–4033. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, S.; Feng, H.; Yang, Y.; Xiao, B.; Chen, D. An enhanced sensitivity and cleanup strategy for the nontargeted screening and targeted determination of pesticides in tea using modified dispersive solid-phase extraction and cold-induced acetonitrile aqueous two-phase systems coupled with liquid chromatography-high resolution mass spectrometry. Food Chem. 2019, 275, 530–538. [Google Scholar] [PubMed]
- Hu, Y.; Zheng, P.; He, Y.; Sheng, G. Response surface optimization for determination of pesticide multiresidues by matrix solid-phase dispersion and gas chromatography. J. Chromatogr. A 2005, 1098, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Dzuman, Z.; Zachariasova, M.; Veprikova, Z.; Godula, M.; Hajslova, J. Multi-analyte high performance liquid chromatography coupled to high resolution tandem mass spectrometry method for control of pesticide residues, mycotoxins, and pyrrolizidine alkaloids. Anal. Chim. Acta 2015, 863, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yin, P.; Wang, Q.; Jiang, Y.; Liu, X. A Modified QuEChERS Sample Preparation Method for the Analysis of 70 Pesticide Residues in Tea Using Gas Chromatography-Tandem Mass Spectrometry. Food Anal. Method 2014, 7, 1577–1587. [Google Scholar] [CrossRef]
- Hu, K.; Deng, W.; Zhu, Y.; Yao, K.; Li, J.; Liu, A.; Ao, X.; Zou, L.; Zhou, K.; He, L.; et al. Simultaneous degradation of β-cypermethrin and 3-phenoxybenzoic acid by Eurotium cristatum ET1, a novel “golden flower fungus” strain isolated from Fu Brick Tea. Microbiol. Open 2019, 8, e776. [Google Scholar] [CrossRef]
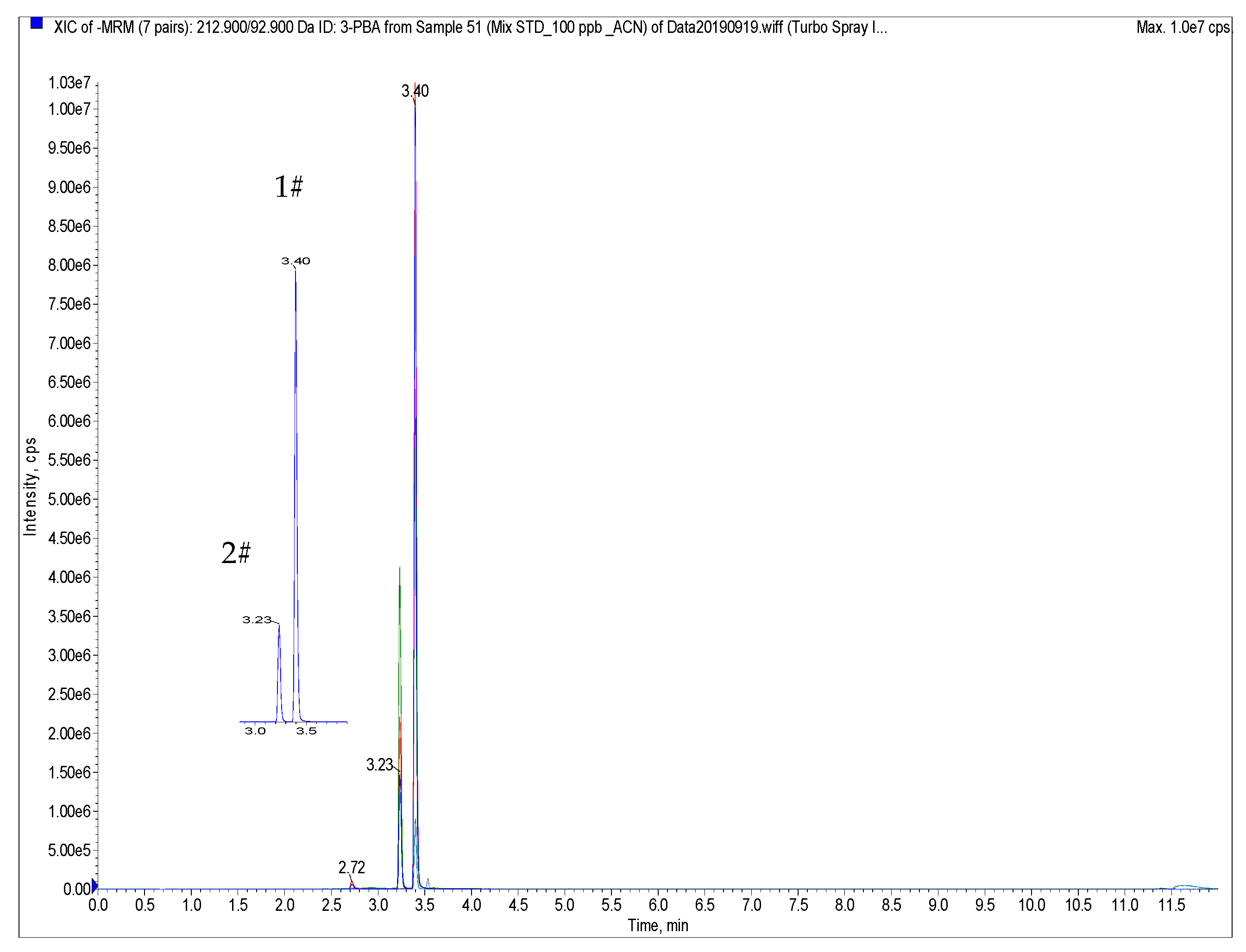

| Compound | Retention Time (min) | Precursor (m/z) | Declustering Potential DP (V) | Production (m/z) | Collision Energy CE (eV) |
|---|---|---|---|---|---|
| 3-PBA | 3.35 | 212.9 | −27 | 92.9 | −27 |
| 169.1 | −18 | ||||
| 4-F-3-PBA | 3.31 | 230.9 | −25 | 92.9 | −33 |
| 167.0 | −25 | ||||
| TFA | 3.450 | 240.9 | −30 | 35.0 | −15 |
| 121.0 | −15 | ||||
| 2-PBA | 3.19 | 212.9 | −27 | 92.9 | −27 |
| 169.1 | −18 |
| Adsorbents | Recoveries (%) | |||
|---|---|---|---|---|
| 3-PBA | 2-PBA | 4-F-3-PBA | TFA | |
| NH3-NTs | 103.0 ± 2.4 | 101.6 ± 1.3 | 102.3 ± 4.2 | 103.8 ± 1.6 |
| MWCNTs | 103.2 ± 2.3 | 108.3 ± 1.8 | 103.1 ± 5.1 | 102.0 ± 7.8 |
| GCB | 99.8 ± 2.9 | 104.9 ± 1.4 | 99.7 ± 5.0 | 92.6 ± 1.9 |
| C18 | 93.9 ± 3.0 | 103.6 ± 2.0 | 93.7 ± 6.8 | 93.8 ± 5.9 |
| PSA | 81.0 ± 3.7 | 55.7 ± 3.5 | 88.9 ± 2.8 | 111.9 ± 5.3 |
| PVPP | 86.4 ± 4.2 | 97.9 ± 0.9 | 88.4 ± 2.3 | 95.2 ± 2.5 |
| Florisil | 105.7 ± 3.9 | 98.4 ± 2.6 | 104.9 ± 2.7 | 104.9 ± 3.3 |
| ZrO | 94.0 ± 3.3 | 103.6 ± 0.7 | 94.1 ± 3.3 | 92.5 ± 4.4 |
| Adsorbents | Recoveries (%) | |||
|---|---|---|---|---|
| 3-PBA | 2-PBA | 4-F-3-PBA | TFA | |
| GCB (25 mg) + C18 + Florisil | 86.0 | 87.1 | 87.2 | 91.6 |
| GCB (50 mg) + C18 + Florisil | 84.2 | 86.0 | 86.8 | 97.6 |
| GCB (75 mg) + C18 + Florisil | 84.5 | 87.7 | 87.2 | 97.1 |
| GCB (100 mg) + C18 + Florisil | 85.6 | 87.5 | 89.0 | 98.8 |
| MWCNTs (25 mg) + C18 + Florisil | 82.0 | 82.4 | 82.9 | 92.0 |
| MWCNTs (50 mg) + C18 + Florisil | 78.1 | 76.4 | 79.7 | 91.9 |
| MWCNTs (75 mg) + C18 + Florisil | 75.7 | 78.8 | 76.7 | 99.0 |
| MWCNTs (100 mg) + C18 + Florisil | 69.4 | 75.8 | 74.5 | 92.0 |
| Compounds | Matrix | Linear Rang (ng mL−1) | Equation | R2 | MEs (%) | LOD (μg kg−1) | LOQ (μg kg−1) |
|---|---|---|---|---|---|---|---|
| 3-PBA | Green tea | 0.1–50 | Y = 2.341x + 0.533 | 0.9994 | −82.9 | 1.5 | 5 |
| Black tea | 0.1–50 | Y = 1.834x + 0.177 | 0.9999 | −83.7 | 2.2 | 5 | |
| 4-F-3-PBA | Green tea | 0.1–50 | Y = 5.426x + 0.628 | 0.9999 | −60.3 | 0.5 | 2 |
| Black tea | 0.1–50 | Y = 3.810x + 0.330 | 1.0000 | −63.6 | 0.5 | 2 | |
| TFA | Green tea | 1–100 | Y = 0.0263x + 0.0109 | 0.9993 | −53.2 | 5.2 | 10 |
| Black tea | 1–100 | Y = 0.0147x + 0.0105 | 0.9984 | −65.9 | 6.1 | 10 |
| Compounds | Teas | Recoveries, % | Intra-Day Precision (RSD, %, n = 5) | Inter-Day Precision (RSD, %, n = 3) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spiked Level (μg kg−1) | Spiked Level (μg kg−1) | Spiked Level (μg kg−1) | ||||||||
| 10 | 50 | 100 | 10 | 50 | 100 | 10 | 50 | 100 | ||
| 3-PBA | GT | 108.6 | 83.0 | 95.4 | 4.6 | 3.1 | 2.4 | 11.0 | 6.0 | 10.3 |
| BT | 101.0 | 100.1 | 99.7 | 13.2 | 3.1 | 5.4 | 7.9 | 3.5 | 3.1 | |
| 4-F-3-PBA | GT | 92.1 | 82.9 | 92.3 | 5.3 | 3.7 | 1.8 | 6.4 | 5.3 | 4.0 |
| BT | 88.0 | 88.3 | 85.1 | 9.2 | 3.9 | 2.6 | 6.1 | 4.3 | 11.1 | |
| TFA | GT | 110.4 | 113.4 | 109.7 | 3.6 | 1.2 | 4.9 | 8.0 | 4.0 | 6.1 |
| BT | 117.3 | 112.0 | 107.8 | 9.5 | 1.6 | 3.2 | 5.1 | 2.6 | 2.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, X.; Liu, P.; Jia, Q.; Han, H.; Jiang, C.; Qiu, J. Determination of Three Typical Metabolites of Pyrethroid Pesticides in Tea Using a Modified QuEChERS Sample Preparation by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Foods 2021, 10, 189. https://doi.org/10.3390/foods10010189
Chen H, Wang X, Liu P, Jia Q, Han H, Jiang C, Qiu J. Determination of Three Typical Metabolites of Pyrethroid Pesticides in Tea Using a Modified QuEChERS Sample Preparation by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Foods. 2021; 10(1):189. https://doi.org/10.3390/foods10010189
Chicago/Turabian StyleChen, Hongping, Xinlu Wang, Pingxiang Liu, Qi Jia, Haolei Han, Changling Jiang, and Jing Qiu. 2021. "Determination of Three Typical Metabolites of Pyrethroid Pesticides in Tea Using a Modified QuEChERS Sample Preparation by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry" Foods 10, no. 1: 189. https://doi.org/10.3390/foods10010189
APA StyleChen, H., Wang, X., Liu, P., Jia, Q., Han, H., Jiang, C., & Qiu, J. (2021). Determination of Three Typical Metabolites of Pyrethroid Pesticides in Tea Using a Modified QuEChERS Sample Preparation by Ultra-High Performance Liquid Chromatography Tandem Mass Spectrometry. Foods, 10(1), 189. https://doi.org/10.3390/foods10010189






