Application of FTIR-PAS in Rapid Assessment of Rice Quality under Climate Change Conditions
Abstract
1. Introduction
2. Materials and Methods
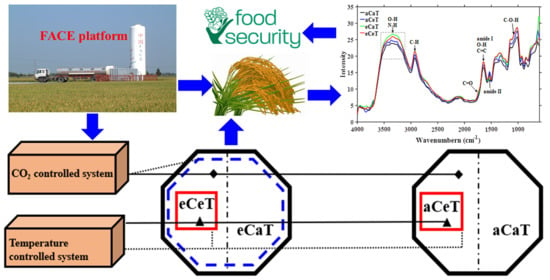
2.1. Experimental Site
2.2. T-FACE Facility
2.3. Crop Cultivation and Experimental Treatment
2.4. Spectra Recording
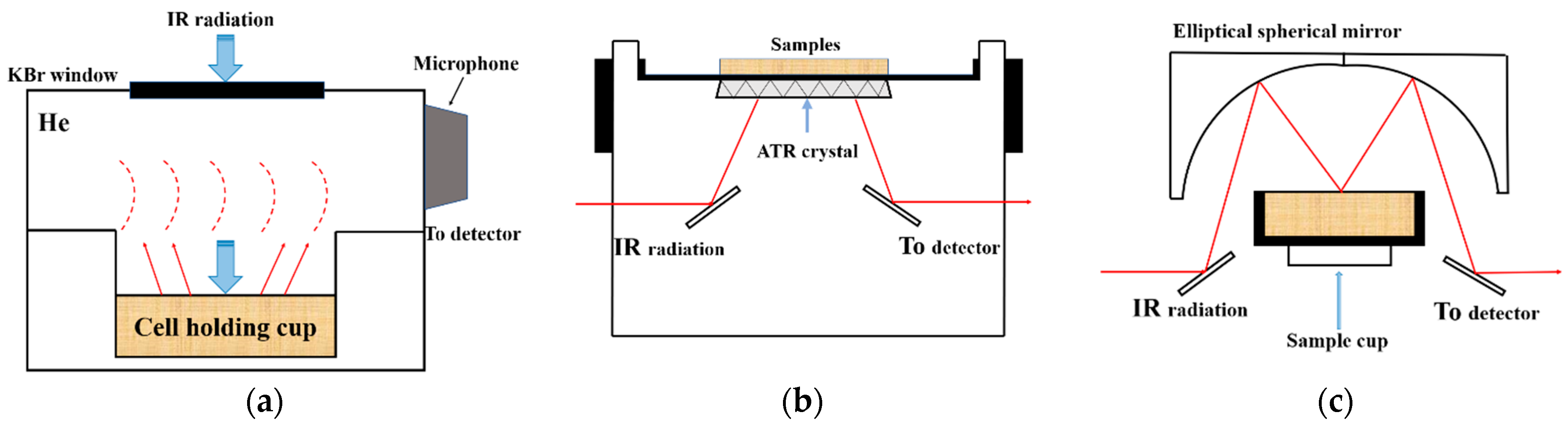
2.4.1. FTIR-PAS Spectra Acquisition
2.4.2. Attenuated Total Reflectance Fourier-Transform Infrared Spectroscopy
2.4.3. Diffuse Reflectance Fourier-Transform Infrared Spectroscopy
2.5. Chemical Methods
2.6. Statistical Analysis
3. Results and Discussion
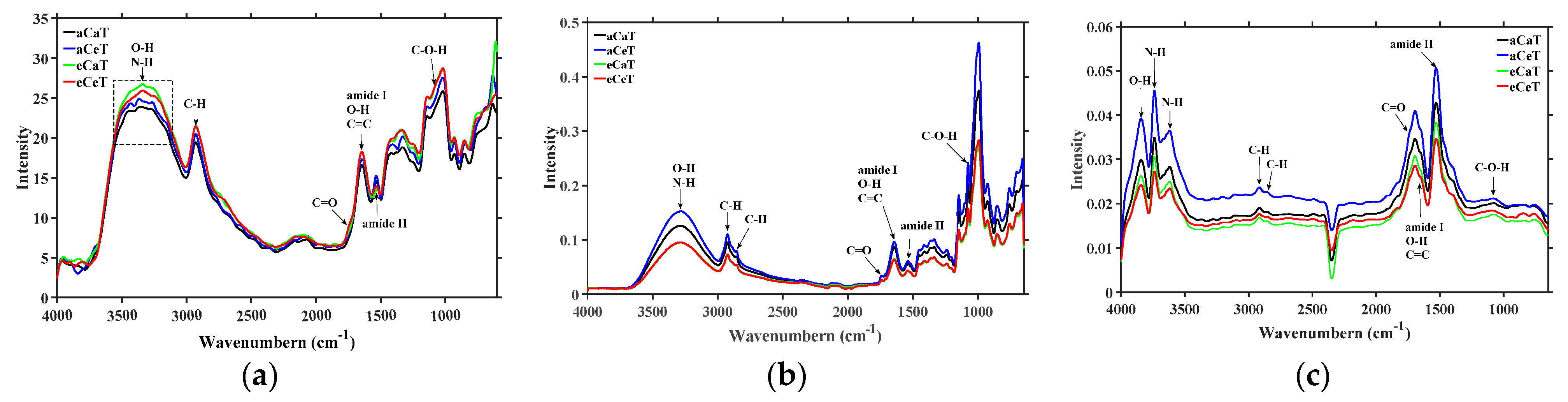
3.1. Spectral Characterization
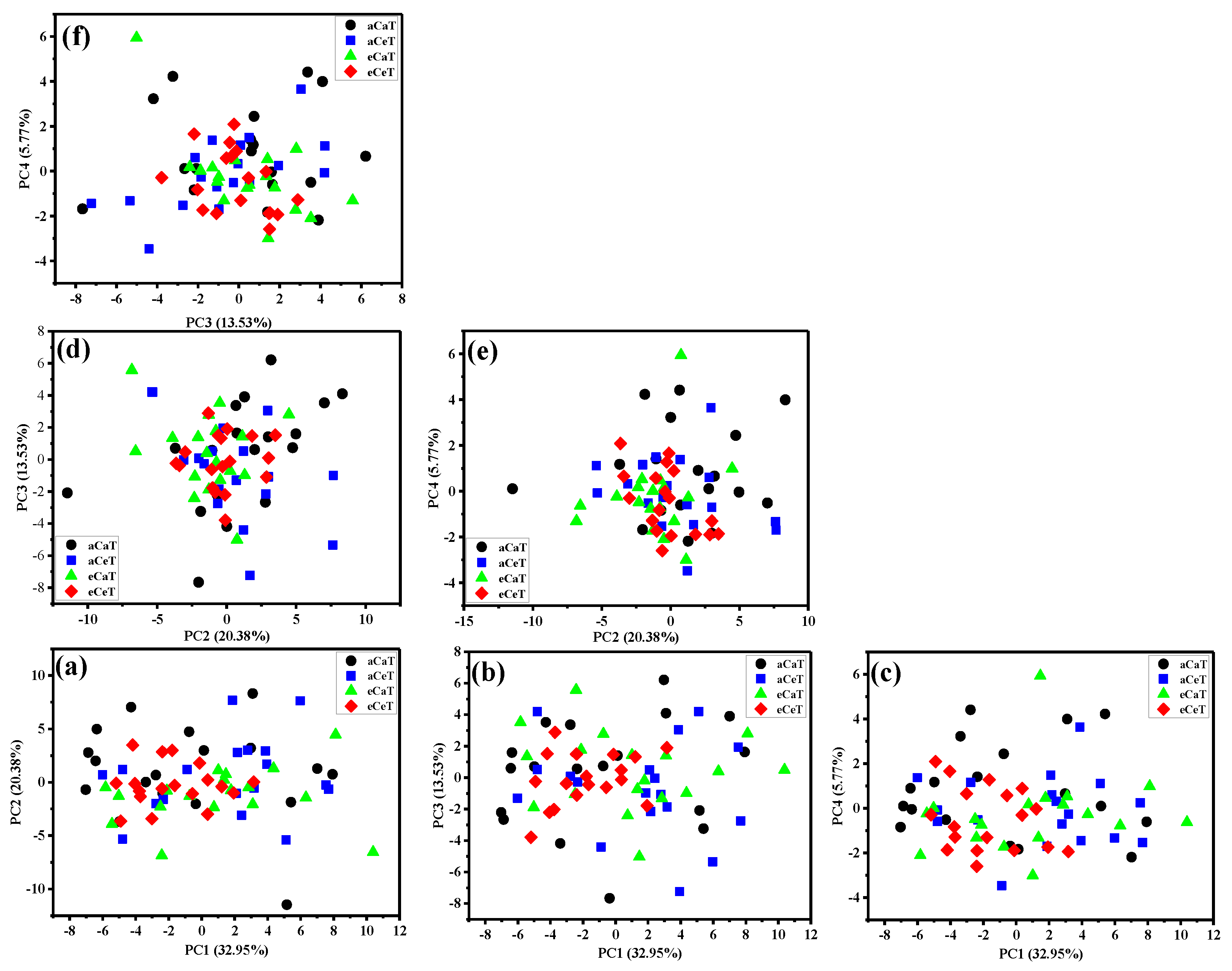
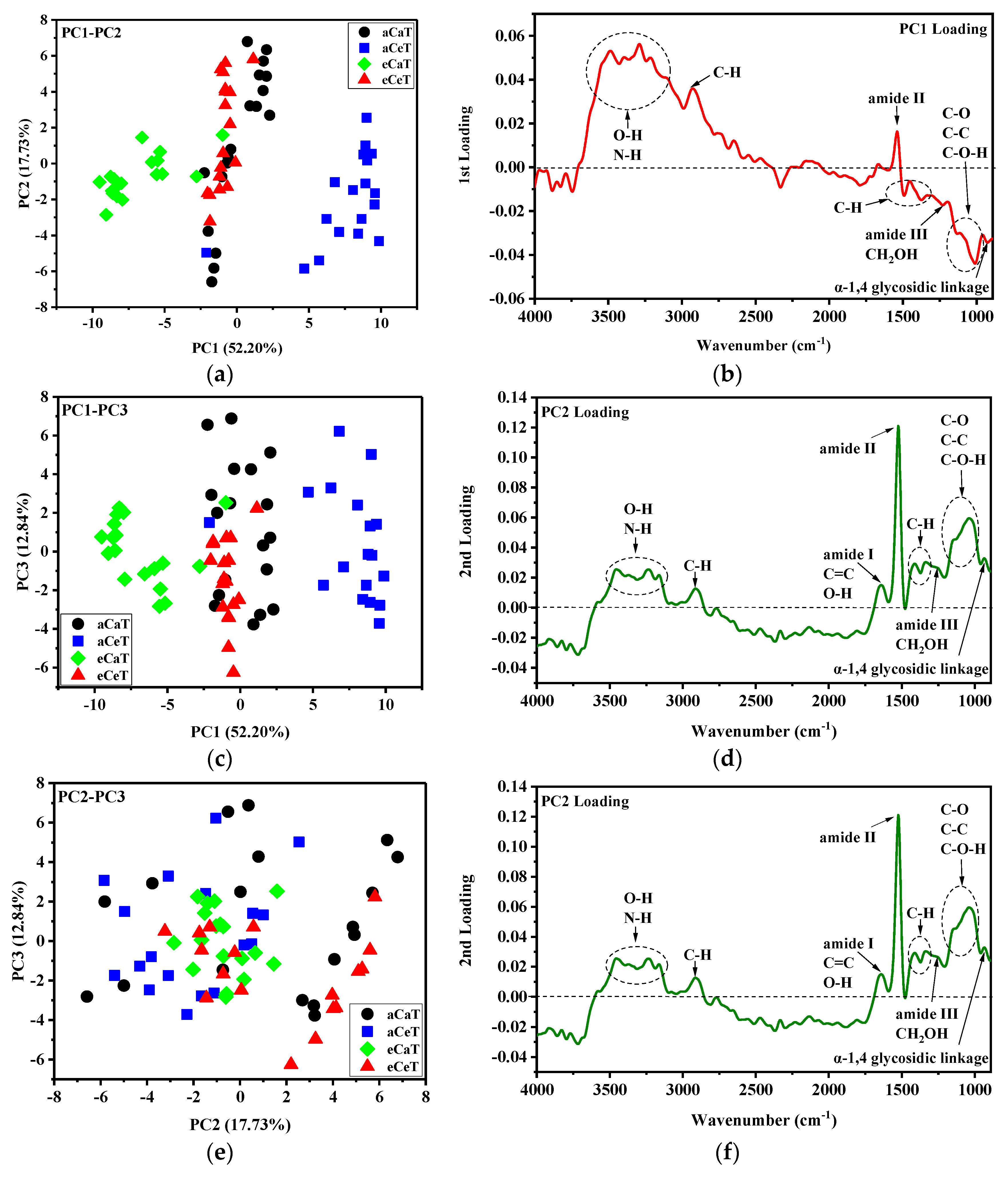
3.2. Principal Component Analysis (PCA)
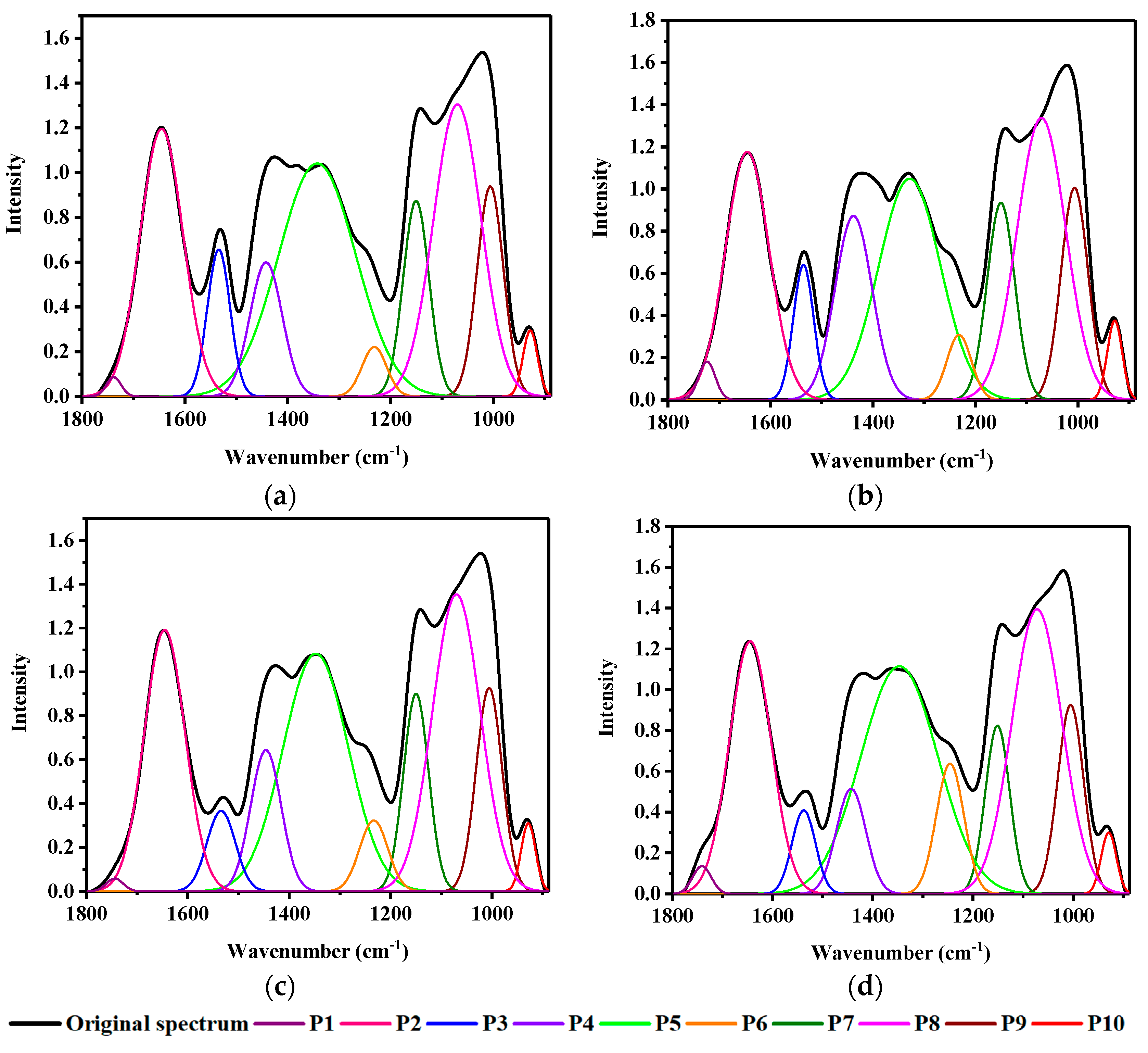
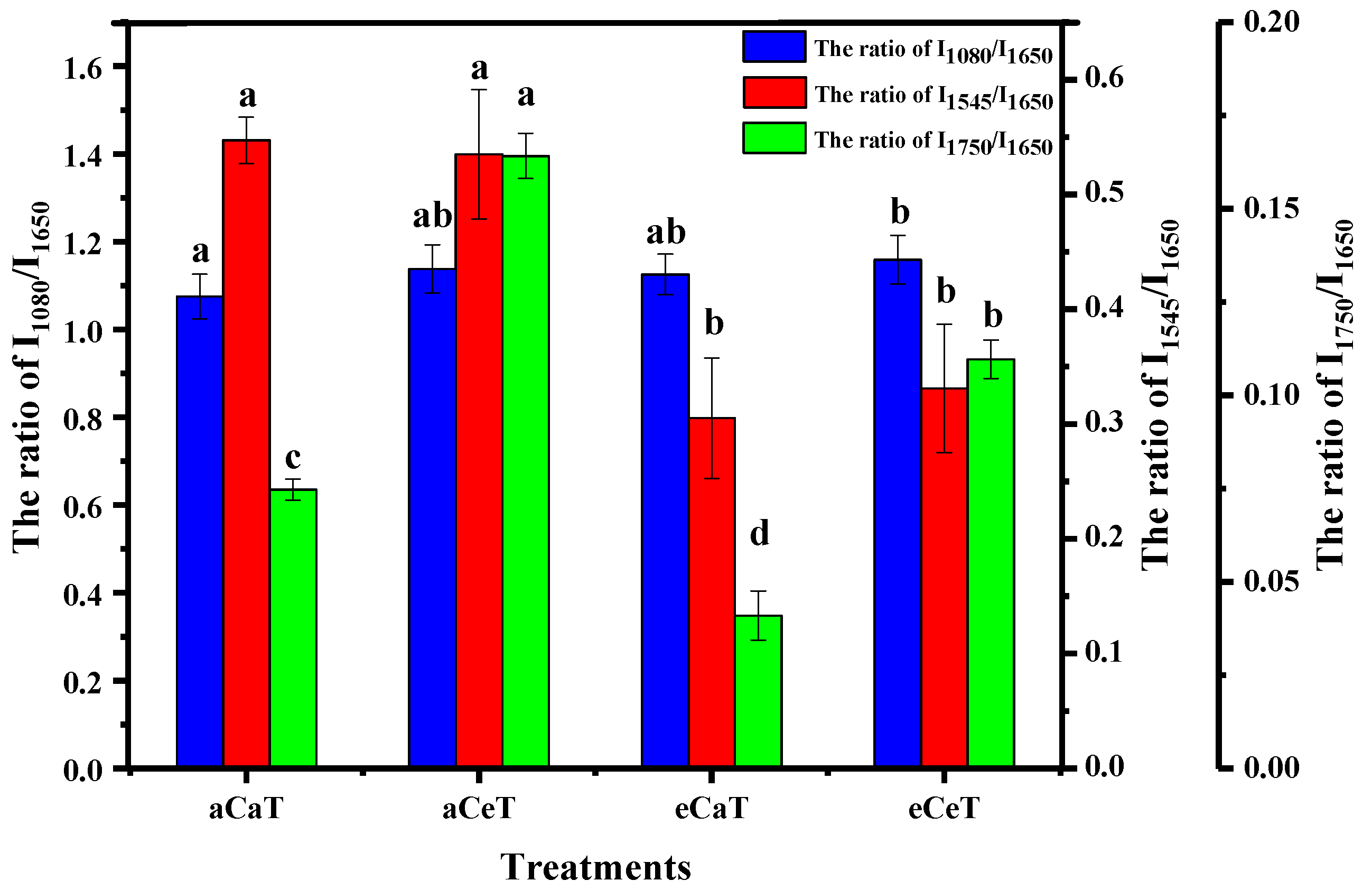
3.3. Curve Fitting through Deconvolution
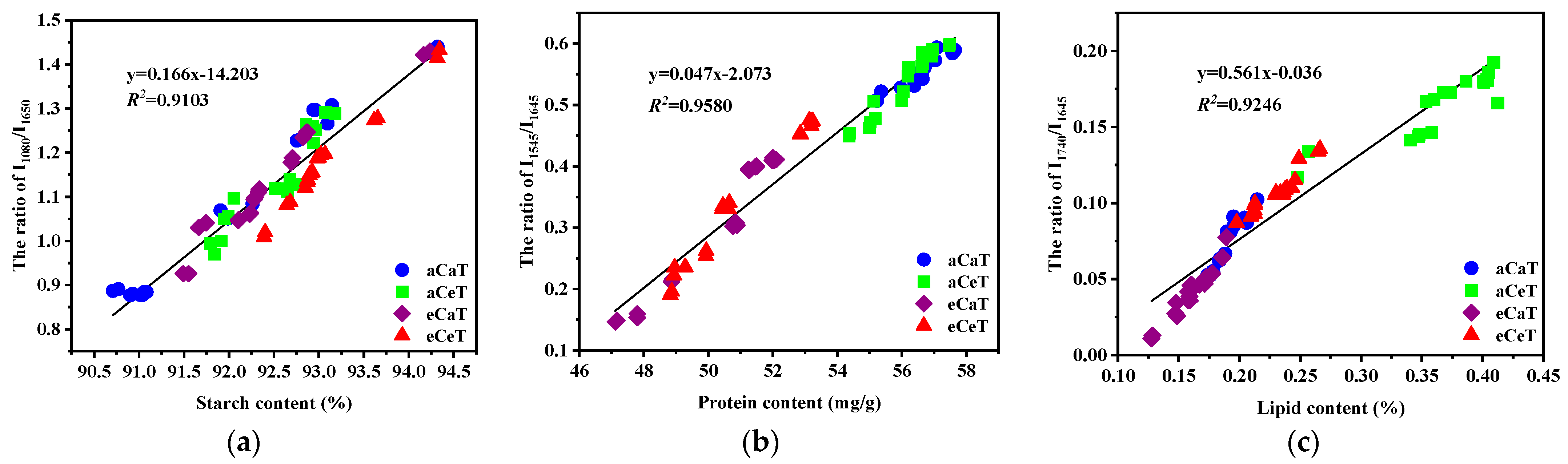
3.4. Detection of Rice Nutritional Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziska, L.H.; Manalo, P.A.; Ordonez, R.A. Intraspecific variation in the response of rice (Oryza sativa L.) to increased CO2 and temperature: Growth and yield response of 17 cultivars. J. Exp. Bot. 1996, 47, 1353–1359. [Google Scholar] [CrossRef]
- Bailey, R.L.; West, K.P., Jr.; Black, R.E. The epidemiology of global micronutrient deficiencies. Ann. Nutr. Metab. 2015, 66, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Kobayashi, K.; Loladze, I.; Zhu, J.; Jiang, Q.; Xu, X.; Liu, G.; Seneweera, S.; Ebi, K.L.; Drewnowski, A.; et al. Carbon dioxide (CO2) levels this century will alter the protein, micronutrients, and vitamin content of rice grains with potential health consequences for the poorest rice-dependent countries. Sci. Adv. 2018, 4, 1012. [Google Scholar] [CrossRef] [PubMed]
- DellaPenna, D. Nutritional genomics: Manipulating plant micronutrients to improve human health. Science 1999, 285, 375–379. [Google Scholar] [CrossRef]
- Rizza, W.; Veronese, N.; Fontana, L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res. Rev. 2014, 13, 38–45. [Google Scholar] [CrossRef]
- Taub, D.R.; Miller, B.; Allen, H. Effects of elevated CO2 on the protein concentration of food crops: A meta-analysis. Glob. Chang. Biol. 2008, 14, 565–575. [Google Scholar] [CrossRef]
- Usui, Y.; Sakai, H.; Tokida, T.; Nakamura, H.; Nakagawa, H.; Hasegawa, T. Rice grain yield and quality responses to free-air CO2 enrichment combined with soil and water warming. Glob. Chang. Biol. 2016, 22, 1256–1270. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Dong, G.; Gu, H.; Huang, J.; Zhu, J.; Yang, H.; Liu, G.; Han, Y. The impact of free-air CO2 enrichment (FACE) and nitrogen supply on grain quality of rice. Field Crops Res. 2007, 102, 128–140. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Bahuguna, R.N.; Pal, M.; Shah, D.; Maurya, S.; Jagadish, K.S.V. Elevated CO2 and heat stress interactions affect grain yield, quality and mineral nutrient composition in rice under field conditions. Field Crops Res. 2017, 206, 149–157. [Google Scholar] [CrossRef]
- Terao, T.; Miura, S.; Yanagihara, T.; Hirose, T.; Nagata, K.; Tabuchi, H.; Kim, H.Y.; Lieffering, M.; Okada, M.; Kobayashi, K. Influence of free-air CO2 enrichment (FACE) on the eating quality of rice. J. Sci. Food Agric. 2005, 85, 1861–1868. [Google Scholar] [CrossRef]
- Wang, J.; Hasegawa, T.; Li, L.; Lam, S.K.; Zhang, X.; Liu, X.; Pan, G. Changes in grain protein and amino acids composition of wheat and rice under short-term increased [CO2] and temperature of canopy air in a paddy from East China. New Phytol. 2019, 222, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Madan, P.; Jagadish, S.V.K.; Craufurd, P.Q.; Fitzgerald, M.; Lafarge, T.; Wheeler, T.R. Effect of elevated CO2 and high temperature on seed-set and grain quality of rice. J. Exp. Bot. 2012, 63, 3843–3852. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.Z.; Pan, P.; Wand, F.B.; Wei, K.S.; Li, Z.W.; Shi, C.H.; Wei, G.; Chen, F.M. Effect of high temperature on the expressions of genes encoding starch synthesis enzymes in developing rice endosperms. J. Integr. Agric. 2015, 14, 642–659. [Google Scholar] [CrossRef]
- AACC International. Total Starch Assay Procedure (Amyloglucosidase/α-Amylase Method). In AACC Approved Methods of Analysis, 11th ed.; 76-13.01; AACC International: St. Paul, MN, USA, 2018. [Google Scholar]
- Jung, S.D.; Rickert, A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. J. Am. Oil Chem. Soc. 2003, 80, 1169–1173. [Google Scholar] [CrossRef]
- Official Methods of Analysis, 17th ed.; Method 979.09; AOAC International: Gaithersburg, MD, USA, 2000.
- Matsler, A.L.; Siebenmorgen, T.J. Evaluation of operating conditions for surface lipid extraction from rice using a Soxtec system. Cereal Chem. 2005, 82, 282–286. [Google Scholar] [CrossRef]
- Robinson, J.E.; Singh, R.S.; Kays, E. Evaluation of an automated hydrolysis and extraction method for quantification of total fat, lipid classes and trans fat in cereal products. Food Chem. 2008, 107, 1144–1150. [Google Scholar] [CrossRef]
- Fazeli, N.; Amir, B.; Afkari, H.; Mahdi, S. Prediction of amylose content, protein content, breakdown, and setback viscosity of Kadus rice and its flour by near-infrared spectroscopy (NIRS) analysis. J. Food Process. Preserv. 2020, e15069. [Google Scholar] [CrossRef]
- Chen, H.; Tan, C.; Lin, Z. Authenticity detection of black rice by near-infrared spectroscopy and support vector data description. Int. J. Anal. Chem. 2018, 1–8. [Google Scholar] [CrossRef]
- Siriphollakul, P.; Kanlayanarat, S.; Rittiron, R.; Wanitchang, J.; Suwonsichon, T.; Boonyaritthongchai, P.; Nakano, K. Pasting properties by near-infrared reflectance analysis of whole grain paddy rice samples. Innov. Opt. Health Sci. 2015, 8, 1–8. [Google Scholar] [CrossRef]
- Barnaby, J.Y.; Huggins, T.D.; Lee, H.; Mcclung, A.M.; Pinson, S.R.M.; Oh, M.; Bauchan, G.R.; Tarpley, L.; Lee, K.J.; Kim, M.S.; et al. Vis/NIR hyperspectral imaging production environment, and physicochemical grain properties in rice. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J. Analysis of potato chips using FTIR photoacoustic spectroscopy. J. Sci. Food Agric. 2000, 80, 1805–1810. [Google Scholar] [CrossRef]
- Irudayaraj, J.; Sivakesava, S.; Kamath, S.; Yang, H. Monitoring chemical changes in some foods using Fourier transform photoacoustic spectroscopy. J. Food Sci. 2001, 66, 1416–1421. [Google Scholar] [CrossRef]
- Lu, Y.; Du, C.; Yu, C.; Zhou, J. Fast and nondestructive determination of protein content in rapeseeds (Brassica napus L.) using Fourier transform infrared photoacoustic spectroscopy (FTIR-PAS). J. Sci. Food Agric. 2014, 94, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Bruun, S.; Peltre, C.; Houot, S.; Jensen, L.S. FTIR-PAS: A powerful tool for characterising the chemical composition and predicting the labile C fraction of various organic waste products. Waste Manag. 2015, 39, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome, N.D.F.; Calderon, J. Soil chemical insights provided through vibrational spectroscopy. Adv. Agron. 2014, 126, 1–148. [Google Scholar]
- Ma, F.; Du, C.W.; Zhou, J.M.; Shen, Y.Z. Investigation of soil properties using different techniques of mid-infrared spectroscopy. Eur. J. Soil Sci. 2019, 70, 96–106. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Attenuated total reflectance Fourier transform infrared analysis of fly ash geopolymer gel aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.S.; Fogarty, W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef]
- Cai, C.; Li, G.; Yang, H.; Yang, J.; Liu, H.; Struik, P.C.; Luo, W.; Yin, X.; Di, L.; Guo, X.; et al. Do all leaf photosynthesis parameters of rice acclimate to elevated CO2, elevated temperature, and their combination, in FACE environments? Glob. Chang. Biol. 2018, 24, 1685–1707. [Google Scholar] [CrossRef]
- Wang, W.; Cai, C.; Lam, S.K.; Liu, G.; Zhu, J. Elevated CO2 cannot compensate for japonica grain yield losses under increasing air temperature because of the decrease in spikelet density. Eur. J. Agron. 2018, 99, 21–29. [Google Scholar] [CrossRef]
- Wang, W.; Cai, C.; He, J.; Gu, J.; Zhu, G.; Zhang, W.; Zhu, J.; Liu, G. Yield, dry matter distribution and photosynthetic characteristics of rice under elevated CO2 and increased temperature conditions. Field Crops Res. 2020, 248, 107605. [Google Scholar] [CrossRef]
- IPCC. Summary for policymakers. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- National Bureau of Statistics of China (NBS). China Statistical Yearbook; China Statistics Press: Beijing, China, 2018.
- Pucher, G.W.; Leavenworth, C.S.; Vickery, H.B. Determination of starch in plant tissues. Anal. Chem. 1948, 20, 850–853. [Google Scholar] [CrossRef]
- Yang, J.C.; Zhang, J.H.; Wang, Z.Q.; Zhu, Q.S.; Wang, W. Remobilization of carbon reserves in response to water deficit during grain filling of rice. Field Crops Res. 2001, 71, 47–55. [Google Scholar] [CrossRef]
- Fan, D.; Ma, W.; Wang, L.; Huang, J.; Zhao, J.; Zhang, H.; Chen, W. Determination of structural changes in microwaved rice starch using Fourier transform infrared and Raman spectroscopy. Starch-Starke 2012, 64, 598–606. [Google Scholar] [CrossRef]
- Bedoya, A.; Gordillo-Delgado, F.; Cruz-Santillana, Y.E.; Plazas, J.; Marin, E. Thermal effusivity measurement of conventional and organic coffee oils via photopyroelectric technique. Food Res. Int. 2017, 102, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Du, C.; Yu, C.; Zhou, J. Use of FTIR-PAS combined with chemometrics to quantify nutritional information in rapeseeds (Brassica napus). J. Plant Nutr. Soil Sci. 2014, 177, 927–933. [Google Scholar]
- Yang, H.; Irudayaraj, J. Characterization of semisolid fats and edible oils by Fourier transform infrared photoacoustic spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 291–295. [Google Scholar] [CrossRef]
- Sujka, K.; Koczon, P.; Ceglińska, A.C.; Reder, M.; Ciemniewska-Zytkiewicz, H. The application of FT-IR spectroscopy for quality control of flours obtained from polish producers. J. Anal. Methods Chem. 2017, 2017, 4315678. [Google Scholar] [CrossRef]
- Delval, F.; Crini, G.; Bertini, S.; Morin-Crini, N.; Badot, P.M.; Vebrel, J.; Torri, G. Characterization of crosslinked starch materials with spectroscopic techniques. J. Appl. Polym. Sci. 2004, 93, 2650–2663. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef]
- Bekiaris, G.; Jensen, L.S.; Engelsen, S.B.; Magid, J.; Bruun, S. Three different Fourier-transform mid-infrared sampling techniques to characterize bio-organic samples. J. Environ. Qual. 2020, 49, 1310–1321. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Du, C.; Ma, F.; Zhou, J. Diagnosis of nitrogen status in Chinese cabbage (Brassica rapa chinensis) using the ratio of amide II to amide I in leaves based on mid-infrared photoacoustic spectroscopy. J. Plant Nutr. Soil Sci. 2015, 178, 888–895. [Google Scholar] [CrossRef]
- Wu, K.; Du, C.; Ma, F.; Shen, Y.; Liang, D.; Zhou, J. Rapid diagnosis of nitrogen status in rice based on Fourier transform infrared photoacoustic spectroscopy (FTIR-PAS). Plant Methods 2019, 15, 94. [Google Scholar] [CrossRef]
- Dean, A.P.; Sigee, D.C.; Estrada, B.; Pittman, J.K. Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour. Technol. 2010, 101, 4499–4507. [Google Scholar] [CrossRef]
- Uprety, D.C.; Sen, S.; Dwivedi, N. Rising atmospheric carbon dioxide on grain quality in crop plants. Physiol. Mol. Biol. Plants 2010, 16, 215–227. [Google Scholar] [CrossRef]
- Sasaki, H.; Hara, T.; Ito, S.; Uehara, N.; Kim, H.Y.; Lieffering, M.; Okada, M.; Kobayashi, K. Effect of free-air CO2 enrichment on the storage of carbohydrate fixed at different stages in rice (Oryza sativa L.). Field Crop. Res. 2007, 100, 24–31. [Google Scholar] [CrossRef]
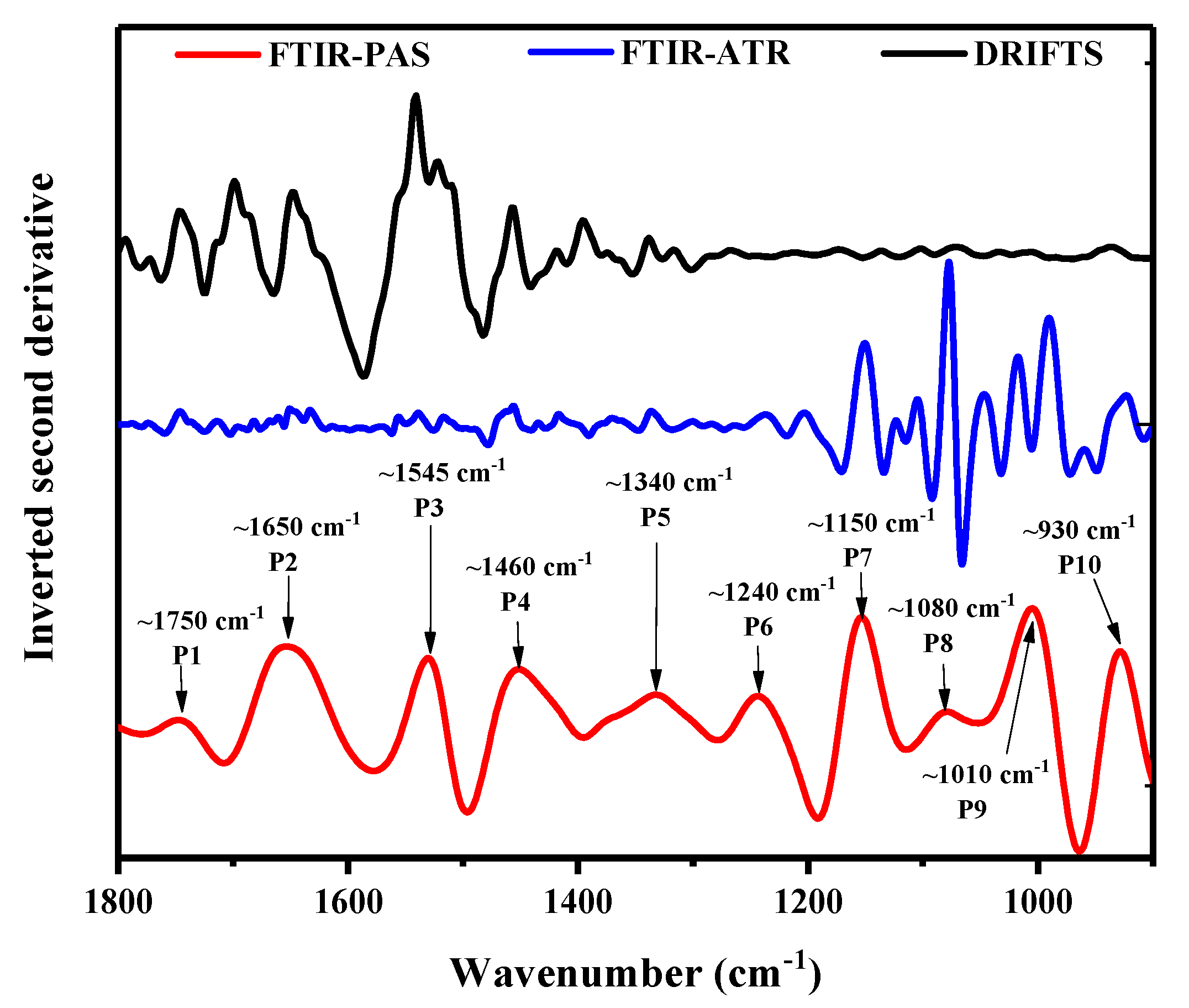
| FTIR-PAS | FTIR-ATR | DRIFT | ||||
|---|---|---|---|---|---|---|
| Wavelength (cm−1) | Assignment | Profiling Depth (μm) | Wavelength (cm−1) | Assignment | Wavelength (cm−1) | Assignment |
| 3600–3000 | -O-H/-N-H | 5.26–5.76 | 3600–3000 | -O-H/-N-H | 3843 | -O-H |
| 2920 | -C-H | 5.84 | 2920 | -C-H | 3735 | -N-H |
| 1750 | -C=O | 7.54 | 2850 | -C-H | 3618 | -N-H |
| 1650 | amide I/-O-H/-C=C | 7.77 | 1750 | -C=O | 2920 | -C-H |
| 1545 | amide II | 8.07 | 1650 | amide I/-O-H/-C=C | 2850 | -C-H |
| 1460 | -C-O-O/-C-H | 8.26 | 1530 | amide II | 1750 | -C=O |
| 1340 | -C-H | 8.62 | 1460 | -C-O-O/-C-H | 1650 | amide I/-O-H/-C=C |
| 1240 | amideIII/-CH2OH | 8.96 | 1340 | -C-H | 1530 | amide II |
| 1150 | -C-O/-C-C | 9.30 | 1240 | amideIII/-CH2OH | 1460 | -C-O-O/-C-H |
| 1080 | -C-O-H | 9.60 | 1150 | -C-O/-C-C | 1080 | -C-O-H |
| 1010 | -C-O-C/-C-O | 9.93 | 1080 | -C-O-H | 930 | α-1,4 glycosidic linkage |
| 930 | α-1,4 glycosidic linkage | 10.34 | 1010 | -C-O-C/-C-O | ||
| 930 | α-1,4 glycosidic linkage | |||||
| Coefficient of Variation | FTIR-PAS | FTIR-ATR | DRIFT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I1080 | I1545 | I1650 | I1750 | I1080 | I1530 | I1650 | I1750 | I1080 | I1530 | I1650 | I1750 | ||
| Within group | aCaT | 0.032 | 0.058 | 0.041 | 0.057 | 0.075 | 0.112 | 0.035 | 0.143 | 0.111 | 0.218 | 0.157 | 0.140 |
| aCeT | 0.051 | 0.067 | 0.043 | 0.066 | 0.094 | 0.121 | 0.034 | 0.037 | 0.089 | 0.437 | 0.245 | 0.065 | |
| eCaT | 0.067 | 0.099 | 0.091 | 0.051 | 0.125 | 0.154 | 0.050 | 0.310 | 0.127 | 0.555 | 0.349 | 0.152 | |
| eCeT | 0.057 | 0.100 | 0.113 | 0.061 | 0.150 | 0.167 | 0.161 | 0.152 | 0.097 | 0.337 | 0.307 | 0.113 | |
| Among groups | 0.135 | 0.143 | 0.137 | 0.072 | 0.192 | 0.154 | 0.181 | 0.144 | 0.128 | 0.759 | 0.163 | 0.764 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, L.; Ma, F.; Du, C. Application of FTIR-PAS in Rapid Assessment of Rice Quality under Climate Change Conditions. Foods 2021, 10, 159. https://doi.org/10.3390/foods10010159
Wei L, Ma F, Du C. Application of FTIR-PAS in Rapid Assessment of Rice Quality under Climate Change Conditions. Foods. 2021; 10(1):159. https://doi.org/10.3390/foods10010159
Chicago/Turabian StyleWei, Lianlian, Fei Ma, and Changwen Du. 2021. "Application of FTIR-PAS in Rapid Assessment of Rice Quality under Climate Change Conditions" Foods 10, no. 1: 159. https://doi.org/10.3390/foods10010159
APA StyleWei, L., Ma, F., & Du, C. (2021). Application of FTIR-PAS in Rapid Assessment of Rice Quality under Climate Change Conditions. Foods, 10(1), 159. https://doi.org/10.3390/foods10010159