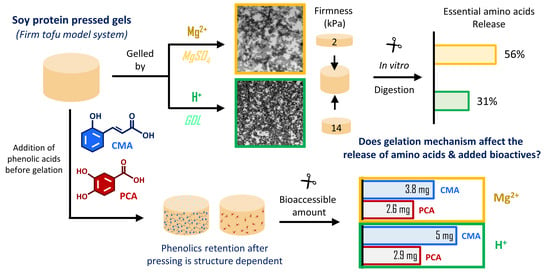
Soy Protein Pressed Gels: Gelation Mechanism Affects the In Vitro Proteolysis and Bioaccessibility of Added Phenolic Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Soy Protein Gels
2.1.1. Soy Protein Isolate Composition
2.1.2. Pressed Soy Protein Gels (Firm Tofu Model System)
2.2. Characterisation of Gels
2.2.1. Composition
2.2.2. Retention of Phenolic Acids in the Curd
2.2.3. HPLC Conditions
2.2.4. Texture Profile Analysis
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. Transmission Electron Microscopy (TEM)
2.3. In Vitro Digestion Static Protocol
2.3.1. Oral Phase
2.3.2. Gastric Phase
2.3.3. Intestinal Phase
2.3.4. Sample Collection
2.4. Digesta Analysis
2.4.1. Degree of Protein Hydrolysis
2.4.2. Free Amino Acid Determination
2.4.3. Total Content of Free Amino Acids
2.4.4. Phenolic Acids Release
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physical Properties of Control and Fortified Gels
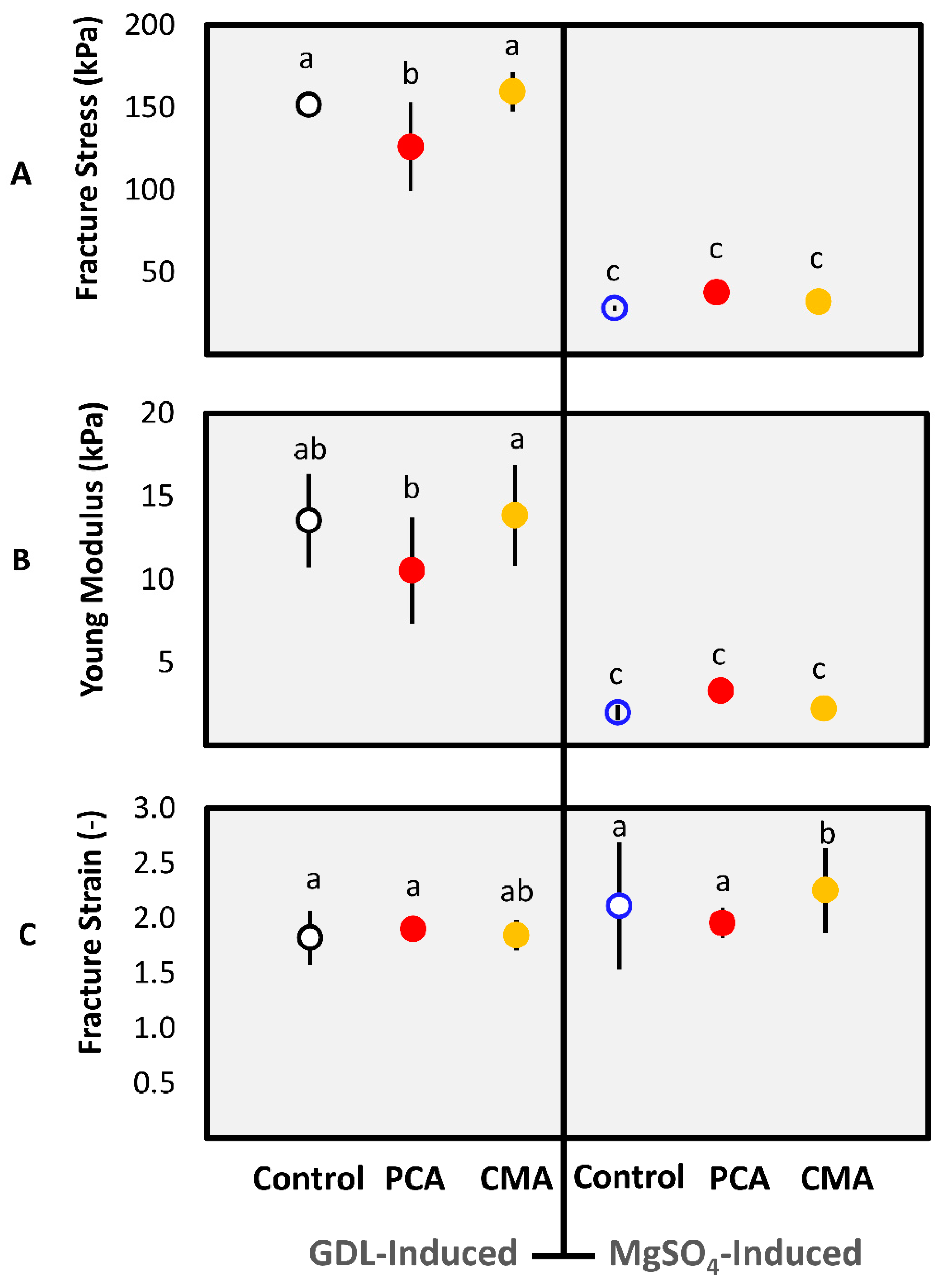
3.1.1. Gel Characteristics
3.1.2. Textural Properties
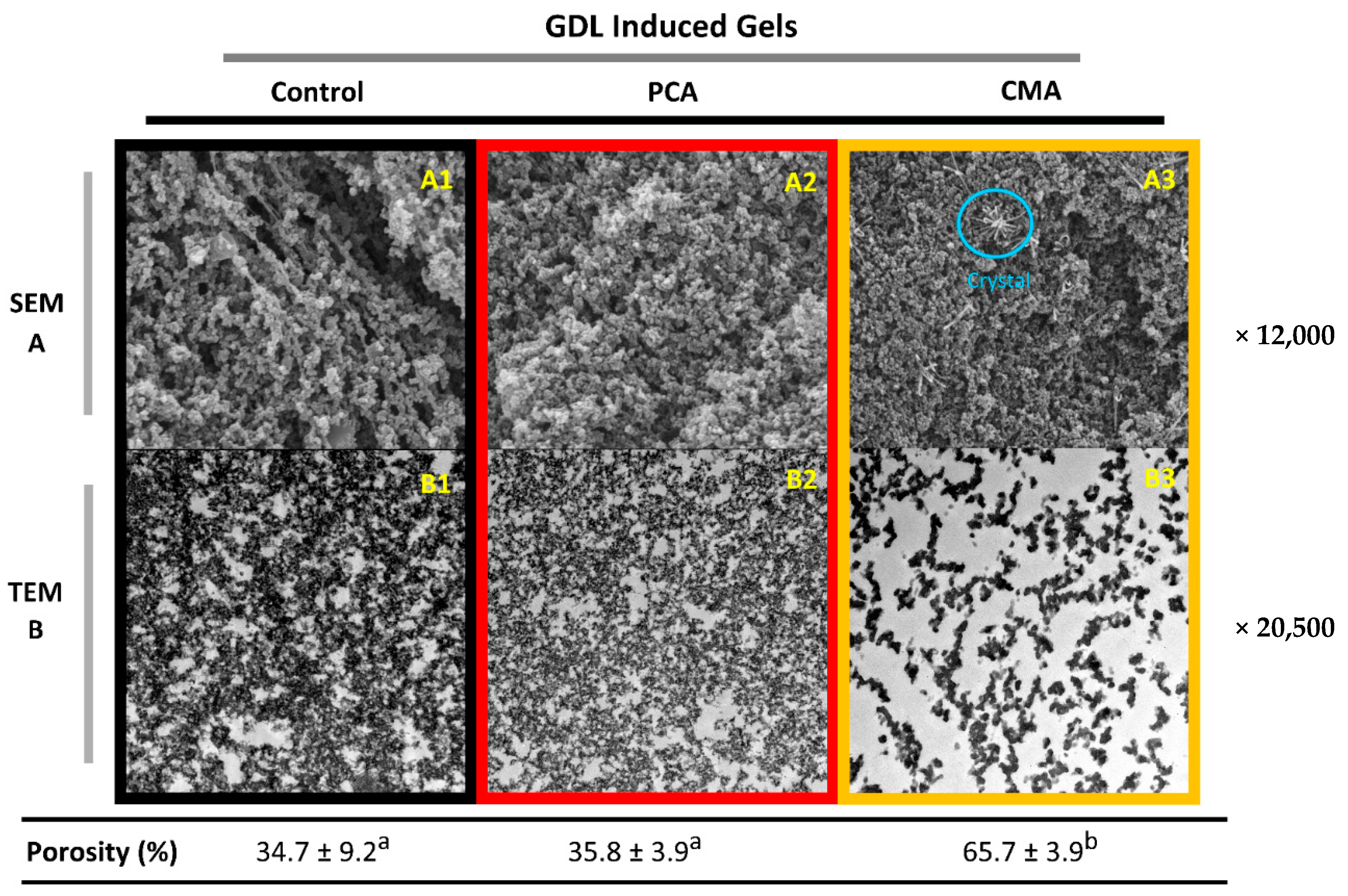
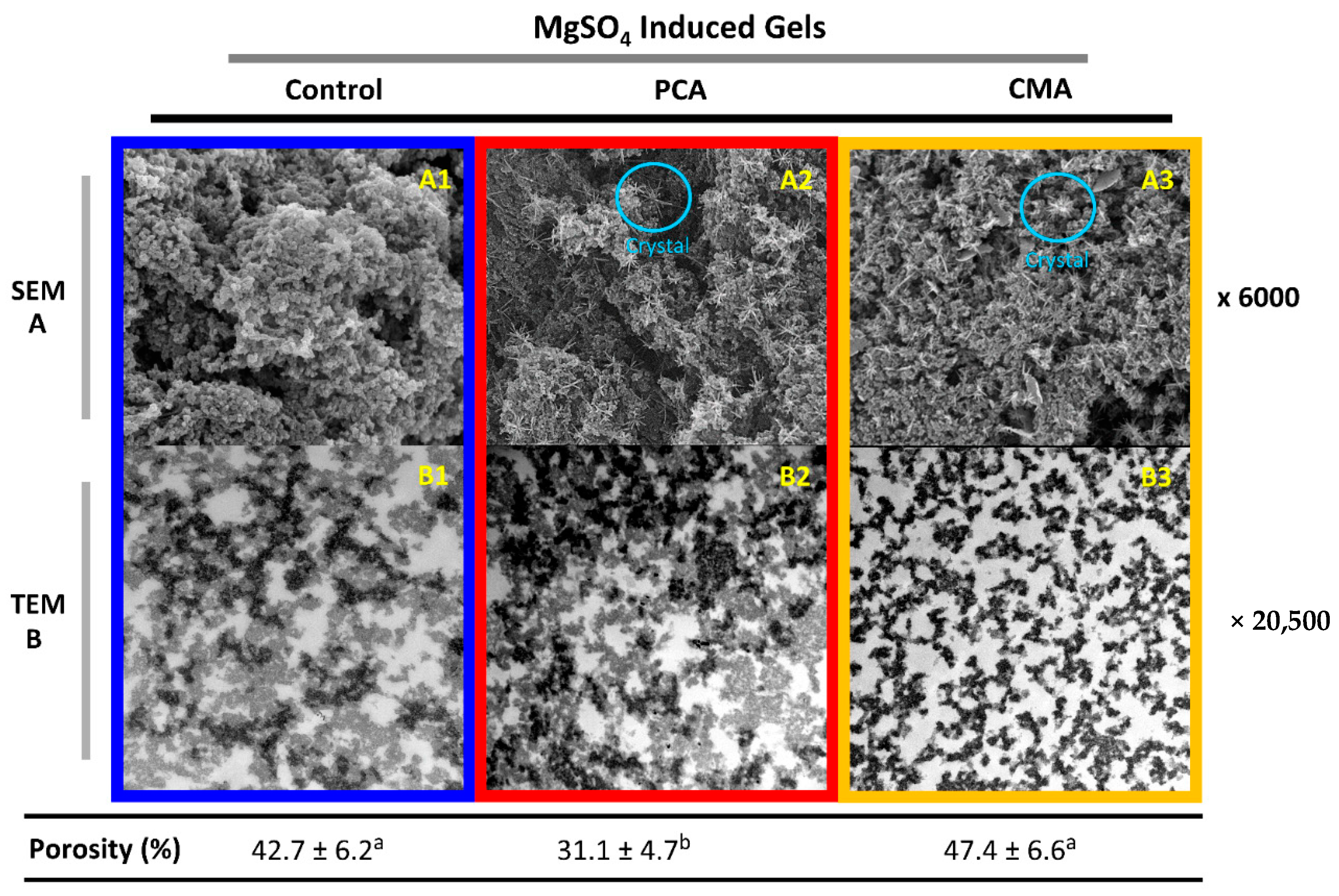
3.1.3. Microstructural Characteristics of Gels
3.2. Digesta Characterisation
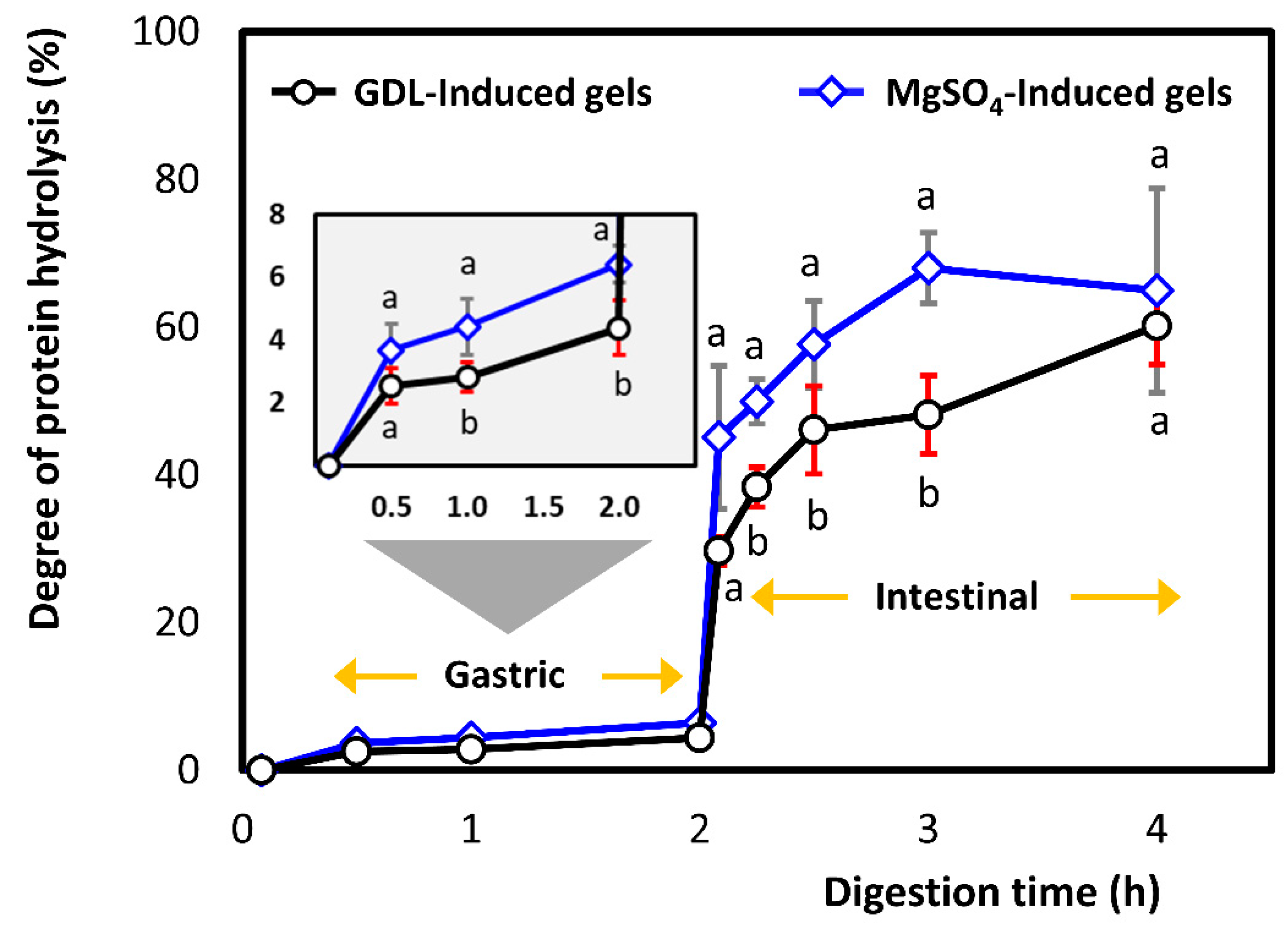
3.2.1. Degree of Proteolysis
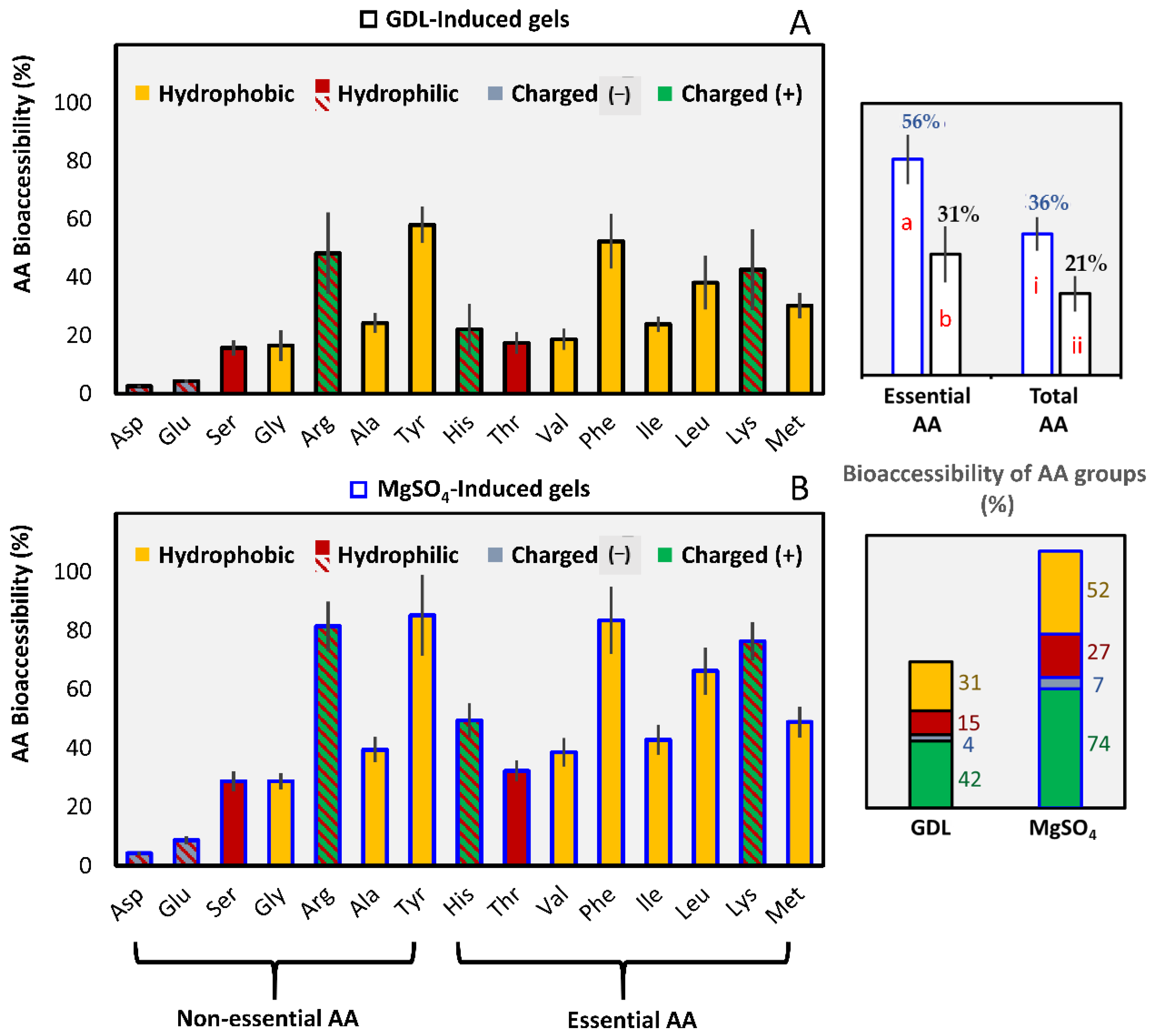
3.2.2. Amino Acids Bioaccessibility
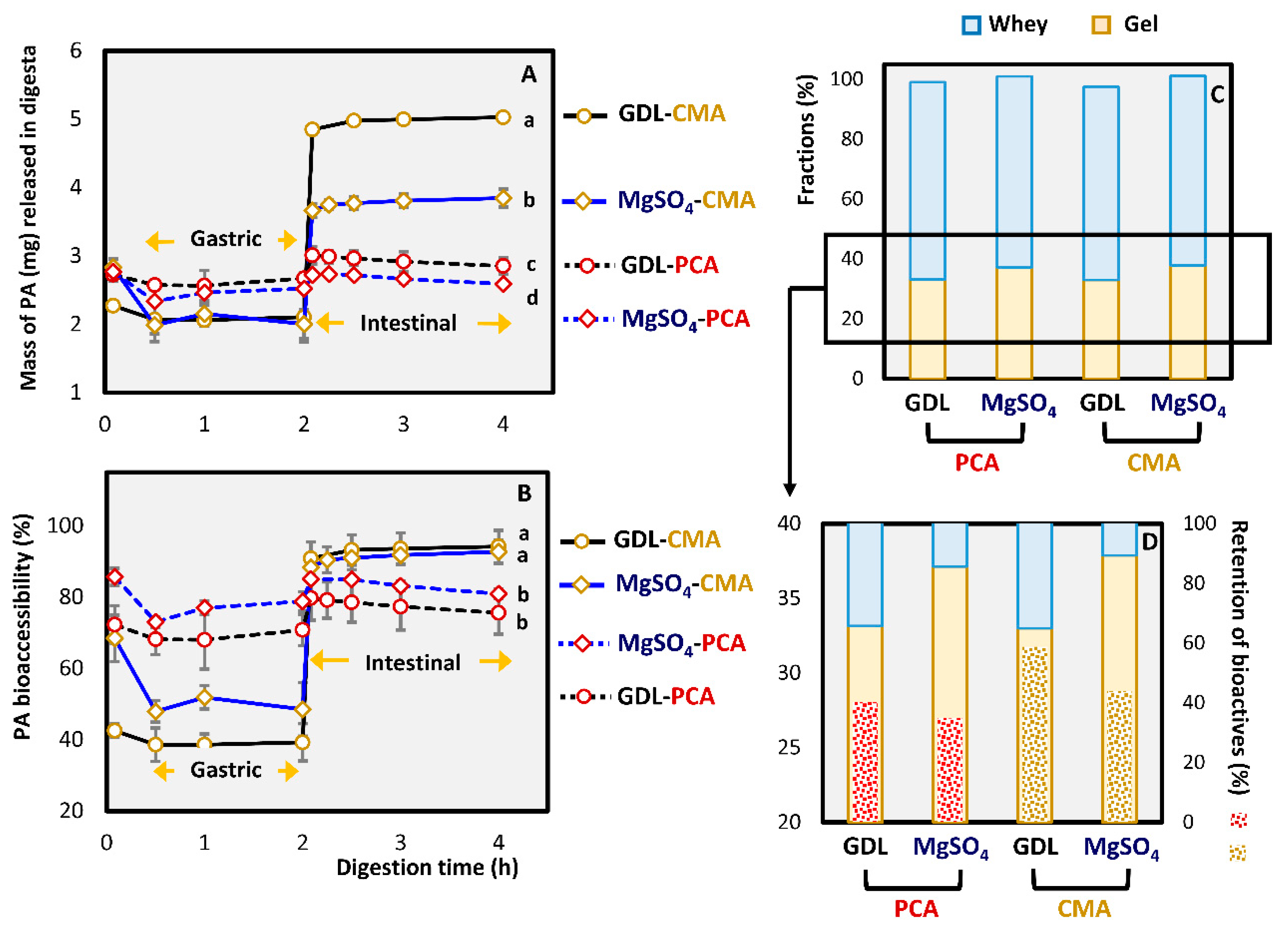
3.2.3. In Vitro Bioaccessibility of Phenolic Acids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar] [CrossRef]
- Raccach, M. The antimicrobial activity of phenolic antioxidants in foods: A review. J. Food Saf. 1984, 6, 141–170. [Google Scholar] [CrossRef]
- Bellik, Y.; Boukraâ, L.; Alzahrani, H.A.; Bakhotmah, B.A.; Abdellah, F.; Hammoudi, S.M.; Iguer-Ouada, M. Molecular Mechanism Underlying Anti-Inflammatory and Anti-Allergic Activities of Phytochemicals: An Update. Molecules 2013, 18, 322–353. [Google Scholar] [CrossRef]
- Wahle, K.W.; Brown, I.; Rotondo, D.; Heys, S.D. Plant phenolics in the prevention and treatment of cancer. Adv. Exp. Med. Biol. 2010, 698, 36–51. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.; Golding, M.; Singh, H. Food Structures, Digestion and Health; Elsevier Science: Amsterdam, The Netherlands, 2014. [Google Scholar]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef] [PubMed]
- Lamothe, S.; Azimy, N.; Bazinet, L.; Couillard, C.; Britten, M. Interaction of green tea polyphenols with dairy matrices in a simulated gastrointestinal environment. Food Funct. 2014, 5, 2621–2631. [Google Scholar] [CrossRef]
- Moser, S.; Chegeni, M.; Jones, O.G.; Liceaga, A.; Ferruzzi, M.G. The effect of milk proteins on the bioaccessibility of green tea flavan-3-ols. Food Res. Int. 2014, 66, 297–305. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT—Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Georgakouli, K.; Mpesios, A.; Kouretas, D.; Petrotos, K.; Mitsagga, C.; Giavasis, I.; Jamurtas, A.Z. The Effects of an Olive Fruit Polyphenol-Enriched Yogurt on Body Composition, Blood Redox Status, Physiological and Metabolic Parameters and Yogurt Microflora. Nutrients 2016, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT—Food Sci. Technol. 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT—Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Petrotos, K.; Karkanta, F.; Gkoutsidis, P.E.; Giavasis, I.; Papatheodorou, K.; Ntontos, A.C. Production of Novel Bioactive Yogurt Enriched with Olive Fruit Polyphenols. World Acad. Sci. Eng. Technol. 2012, 6, 170–175. [Google Scholar]
- Giroux, H.J.; De Grandpré, G.; Fustier, P.; Champagne, C.P.; St-Gelais, D.; Lacroix, M.; Britten, M. Production and characterization of Cheddar-type cheese enriched with green tea extract. Dairy Sci. Technol. 2013, 93, 241–254. [Google Scholar] [CrossRef]
- Han, J.; Britten, M.; St-Gelais, D.; Champagne, C.P.; Fustier, P.; Salmieri, S.; Lacroix, M. Effect of polyphenolic ingredients on physical characteristics of cheese. Food Res. Int. 2011, 44, 494–497. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Gastro-pancreatic release of phenolic compounds incorporated in a polyphenols-enriched cheese-curd. LWT—Food Sci. Technol. 2015, 60, 957–963. [Google Scholar] [CrossRef]
- Lamothe, S.; Langlois, A.; Bazinet, L.; Couillard, C.; Britten, M. Antioxidant activity and nutrient release from polyphenol-enriched cheese in a simulated gastrointestinal environment. Food Funct. 2016, 7, 1634–1644. [Google Scholar] [CrossRef] [PubMed]
- Çam, M.; İçyer, N.C.; Erdoğan, F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT—Food Sci. Technol. 2014, 55, 117–123. [Google Scholar] [CrossRef]
- Maltais, A.; Remondetto, G.E.; Subirade, M. Soy protein cold-set hydrogels as controlled delivery devices for nutraceutical compounds. Food Hydrocoll. 2009, 23, 1647–1653. [Google Scholar] [CrossRef]
- Maltais, A.; Remondetto, G.E.; Subirade, M. Tabletted soy protein cold-set hydrogels as carriers of nutraceutical substances. Food Hydrocoll. 2010, 24, 518–524. [Google Scholar] [CrossRef]
- Kalman, D.S. Amino Acid Composition of an Organic Brown Rice Protein Concentrate and Isolate Compared to Soy and Whey Concentrates and Isolates. Foods 2014, 3, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Baroni, L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients 2018, 10, 43. [Google Scholar] [CrossRef]
- Liu, Z.-M.; Ho, S.C.; Chen, Y.-M.; Ho, S.; To, K.; Tomlinson, B.; Woo, J. Whole soy, but not purified daidzein, had a favorable effect on improvement of cardiovascular risks: A 6-month randomized, double-blind, and placebo-controlled trial in equol-producing postmenopausal women. Mol. Nutr. Food Res. 2014, 58, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Montville, J.B.; Ahuja, J.K.C.; Martin, C.L.; Heendeniya, K.Y.; Omolewa-Tomobi, G.; Steinfeldt, L.C.; Anand, J.; Adler, M.E.; LaComb, R.P.; Moshfegh, A. USDA Food and Nutrient Database for Dietary Studies (FNDDS), 5.0. Procedia Food Sci. 2013, 2, 99–112. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. The Book of Tofu: Food for Mankind; Autumn Press: Brookline, MA, USA, 1975. [Google Scholar]
- Prabhakaran, M.P.; Perera, C.O.; Valiyaveettil, S. Effect of different coagulants on the isoflavone levels and physical properties of prepared firm tofu. Food Chem. 2006, 99, 492–499. [Google Scholar] [CrossRef]
- Rui, X.; Fu, Y.; Zhang, Q.; Li, W.; Zare, F.; Chen, X.; Jiang, M.; Dong, M. A comparison study of bioaccessibility of soy protein gel induced by magnesiumchloride, glucono-δ-lactone and microbial transglutaminase. LWT—Food Sci. Technol. 2016, 71, 234–242. [Google Scholar] [CrossRef]
- Kozu, H.; Nakata, Y.; Nakajima, M.; Neves, M.A.; Uemura, K.; Sato, S.; Kobayashi, I.; Ichikawa, S. Development of a Human Gastric Digestion Simulator Equipped with Peristalsis Function for the Direct Observation and Analysis of the Food Digestion Process. Food Sci. Technol. Res. 2014, 20, 225–233. [Google Scholar] [CrossRef]
- Reynaud, Y.; Lopez, M.; Riaublanc, A.; Souchon, I.; Dupont, D. Hydrolysis of plant proteins at the molecular and supra-molecular scales during in vitro digestion. Food Res. Int. 2020, 134, 109204. [Google Scholar] [CrossRef]
- Helrich, K. Official Methods of Analysis of the AOAC. Volume 2. Food Composition, Additives, Natural Contaminants; Association of Official Analytical Chemists Inc.: Arlington, TX, USA, 1990; pp. 685–1298. [Google Scholar]
- Wen, D.; Li, C.; Di, H.; Liao, Y.; Liu, H. A universal HPLC method for the determination of phenolic acids in compound herbal medicines. J. Agric. Food Chem. 2005, 53, 6624–6629. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: Eat Lansing, MI, USA, 1996. [Google Scholar]
- Havea, P.; Watkinson, P.; Kuhn-Sherlock, B. Heat-Induced Whey Protein Gels: Protein−Protein Interactions and Functional Properties. J. Agric. Food Chem. 2009, 57, 1506–1512. [Google Scholar] [CrossRef]
- Venable, J.H.; Coggeshall, R. A simplified lead citrate stain for use in electron microscopy. J. Cell Biol. 1965, 25, 407–408. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.V.C.; Legland, D.; Cauty, C.; Kolotuev, I.; Floury, J. Characterization of the microstructure of dairy systems using automated image analysis. Food Hydrocoll. 2015, 44, 360–371. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Lorieau, L.; Halabi, A.; Ligneul, A.; Hazart, E.; Dupont, D.; Floury, J. Impact of the dairy product structure and protein nature on the proteolysis and amino acid bioaccessiblity during in vitro digestion. Food Hydrocoll. 2018, 82, 399–411. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Schasteen, C.S.; Wu, J.; Schulz, M.G.; Parsons, C.M. Correlation of an immobilized digestive enzyme assay with poultry true amino acid digestibility for soybean meal. Poult. Sci. 2007, 86, 343–348. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants—Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Liu, H. Measurement of Blood Plasma Amino Acids in Ultrafiltrates by High-Performance Liquid Chromatography with Automatic Precolumn O-Phthaldialdehyde Derivatization. In Amino Acid Analysis Protocols; Cooper, C., Packer, N., Williams, K., Eds.; Humana Press: Totowa, NJ, USA, 2000; pp. 123–140. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Gruppen, H.; van Vliet, T. Influence of pH and Ionic Strength on Heat-Induced Formation and Rheological Properties of Soy Protein Gels in Relation to Denaturation and Their Protein Compositions. J. Agric. Food Chem. 2002, 50, 6064–6071. [Google Scholar] [CrossRef]
- Hermansson, A.M. Physico-chemical aspects of soy proteins structure formation. J. Texture Stud. 1978, 9, 33–58. [Google Scholar] [CrossRef]
- Lee, K.H.; Ryu, H.S.; Rhee, K.C. Protein solubility characteristics of commercial soy protein products. J. Am. Oil Chem. Soc. 2003, 80, 85–90. [Google Scholar] [CrossRef]
- McMurry, J. Organic Chemistry with Biological Applications; Brooks/Cole Cengage Learning: Boston, MA, USA, 2011. [Google Scholar]
- Hsia, S.-Y.; Hsiao, Y.-H.; Li, W.-T.; Hsieh, J.-F. Aggregation of soy protein-isoflavone complexes and gel formation induced by glucono-δ-lactone in soymilk. Sci. Rep. 2016, 6, 35718. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Rioux, L.-E.; Labrie, S.; Turgeon, S.L. Commercial cheeses with different texture have different disintegration and protein/peptide release rates during simulated in vitro digestion. Int. Dairy J. 2016, 56, 169–178. [Google Scholar] [CrossRef]
- Bornhorst, G.M.; Ferrua, M.J.; Singh, R.P. A Proposed Food Breakdown Classification System to Predict Food Behavior during Gastric Digestion. J. Food Sci. 2015, 80, R924–R934. [Google Scholar] [CrossRef] [PubMed]
- Kohyama, K.; Sasaki, T.; Hayakawa, F. Characterization of Food Physical Properties by the Mastication Parameters Measured by Electromyography of the Jaw-Closing Muscles and Mandibular Kinematics in Young Adults. Biosci. Biotechnol. Biochem. 2008, 72, 1690–1695. [Google Scholar] [CrossRef]
- Walstra, P. Physical Chemistry of Foods; Taylor & Francis: New York, NY, USA, 2002. [Google Scholar]
- Urbonaite, V.; de Jongh, H.H.J.; van der Linden, E.; Pouvreau, L. Origin of Water Loss from Soy Protein Gels. J. Agric. Food Chem. 2014, 62, 7550–7558. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Cao, F.-H.; Li, X.-J.; Mu, D.-D.; Zhong, X.-Y.; Jiang, S.-T.; Zheng, Z.; Luo, S.-Z. Effects of different salts on the gelation behaviour and mechanical properties of citric acid-induced tofu. Int. J. Food Sci. Technol. 2020, 55, 785–794. [Google Scholar] [CrossRef]
- Tay, S.L.; Tan, H.Y.; Perera, C.O. The Coagulating Effects of Cations and Anions on Soy Protein. Int. J. Food Prop. 2006, 9, 317–323. [Google Scholar] [CrossRef]
- Kohyama, K.; Sano, Y.; Doi, E. Rheological Characteristics and Gelation Mechanism of Tofu (Soybean Curd). J. Agric. Food Chem. 1995, 43, 1808–1812. [Google Scholar] [CrossRef]
- Deman, J.M. Texture and microstructure of soybean curd (tofu) as affected by different coagulants. Food Struct. 1986, 5, 83–89. [Google Scholar]
- Lu, J.Y.; Carter, E.; Chung, R.A. Use of calcium salts for soybean curd preparation. J. Food Sci. 1980, 45, 32–34. [Google Scholar] [CrossRef]
- Church, F.C.; Porter, D.H.; Catignani, G.L.; Swaisgood, H.E. An o-phthalaldehyde spectrophotometric assay for proteinases. Anal. Biochem. 1985, 146, 343–348. [Google Scholar] [CrossRef]
- Goodman, B.E. Insights into digestion and absorption of major nutrients in humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.J.C.; Morton, H.; Coad, J.; Pedley, K.C. In vitro digestion for assessing micronutrient bioavailability: The importance of digestion duration. Int. J. Food Sci. Nutr. 2019, 70, 71–77. [Google Scholar] [CrossRef]
- Qiao, Y.; Gumpertz, M.; Van Kempen, T. Stability of pepsin (EC 3.4.23.1) during in vitro protein digestibility assay. J. Food Biochem. 2002, 26, 355–375. [Google Scholar] [CrossRef]
- Qiao, Y.; Gumpertz, M.; Van Kempen, T. Stability of a pancreatic enzyme coctail during in vitro protein digestibility assays. J. Food Biochem. 2005, 29, 205–220. [Google Scholar] [CrossRef]
- Petsko, G.A.; Ringe, D. From sequence to structure. Protein Struct. Funct. 2004, 1, 49. [Google Scholar]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry, 4th ed.; Taylor & Francis: Boca Raton, FL, USA, 2007. [Google Scholar]
- Gray, G.M.; Cooper, H.L. Protein Digestion and Absorption. Gastroenterology 1971, 61, 535–544. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.-P.; Motilva, M.-J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Vallejo, F.; Gil-Izquierdo, A.; Pérez-Vicente, A.; García-Viguera, C. In Vitro Gastrointestinal Digestion Study of Broccoli Inflorescence Phenolic Compounds, Glucosinolates, and Vitamin C. J. Agric. Food Chem. 2004, 52, 135–138. [Google Scholar] [CrossRef] [PubMed]
| GDL-INDUCED GELS | MgSO4-INDUCED GELS | |||||
|---|---|---|---|---|---|---|
| Properties | Control | PCA | CMA | Control | PCA | CMA |
| Moisture (%) | 83.6 ± 0.43 ab | 84.2 ± 0.80 abc | 83.4 ± 0.34 a | 86.2 ± 0.74 bd | 85.2 ± 0.38 cd | 85.7 ± 0.11 cd |
| Yield (%) | 31.9 ± 2.17 a | 33.1 ± 5.15 a | 34.1 ± 3.51 a | 38.7 ± 5.18 a | 37.1 ± 3.12 a | 37.9 ± 3.30 a |
| Protein in curd (%) | 15.1 ± 0.83 a | 14.7 ± 0.48 ab | 15.4 ± 0.38 a | 13.2 ± 1.05 bc | 13.2 ± 0.40 bc | 12.3 ± 0.54 c |
| Soluble protein in whey (%) | 0.13 ± 0.02 a | 0.13 ± 0.02 a | 0.12 ± 0.00 a | 0.36 ± 0.03 b | 0.35 ± 0.05 b | 0.30 ± 0.05 b |
| Whey (%) | 67.0 ± 2.97 a | 65.9 ± 4.23 a | 64.5 ± 0.83 a | 60.9 ± 4.66 a | 63.9 ± 2.57 a | 63.3 ± 2.93 a |
| pH of whey (-) | 4.7 ± 0.08 a | 4.7 ± 0.11 a | 4.8 ± 0.06 a | 6.4 ± 0.03 b | 6.1 ± 0.04 c | 6.1 ± 0.07 c |
| Zeta potential of whey (mV) | −3.4 ± 0.21 a | −2.0 ± 0.05 b | −2.7 ± 0.04 cd | −2.5 ± 0.07 d | −2.6 ± 0.03 d | −3.1 ± 0.11 ac |
| Retention of PA in curd (%) | n/a | 40.2 ± 6.62 a | 58.8 ± 3.59 b | n/a | 35.0 ± 6.72 a | 43.8 ± 6.17 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinea, M.; Ellis, A.; Golding, M.; Loveday, S.M. Soy Protein Pressed Gels: Gelation Mechanism Affects the In Vitro Proteolysis and Bioaccessibility of Added Phenolic Acids. Foods 2021, 10, 154. https://doi.org/10.3390/foods10010154
Marinea M, Ellis A, Golding M, Loveday SM. Soy Protein Pressed Gels: Gelation Mechanism Affects the In Vitro Proteolysis and Bioaccessibility of Added Phenolic Acids. Foods. 2021; 10(1):154. https://doi.org/10.3390/foods10010154
Chicago/Turabian StyleMarinea, Marina, Ashling Ellis, Matt Golding, and Simon M. Loveday. 2021. "Soy Protein Pressed Gels: Gelation Mechanism Affects the In Vitro Proteolysis and Bioaccessibility of Added Phenolic Acids" Foods 10, no. 1: 154. https://doi.org/10.3390/foods10010154
APA StyleMarinea, M., Ellis, A., Golding, M., & Loveday, S. M. (2021). Soy Protein Pressed Gels: Gelation Mechanism Affects the In Vitro Proteolysis and Bioaccessibility of Added Phenolic Acids. Foods, 10(1), 154. https://doi.org/10.3390/foods10010154