Thaumatin-Like Protein (Pru av 2) Is a Cherry Allergen That Triggers Percutaneous Sensitization in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cherry Extract and Protein Estimation
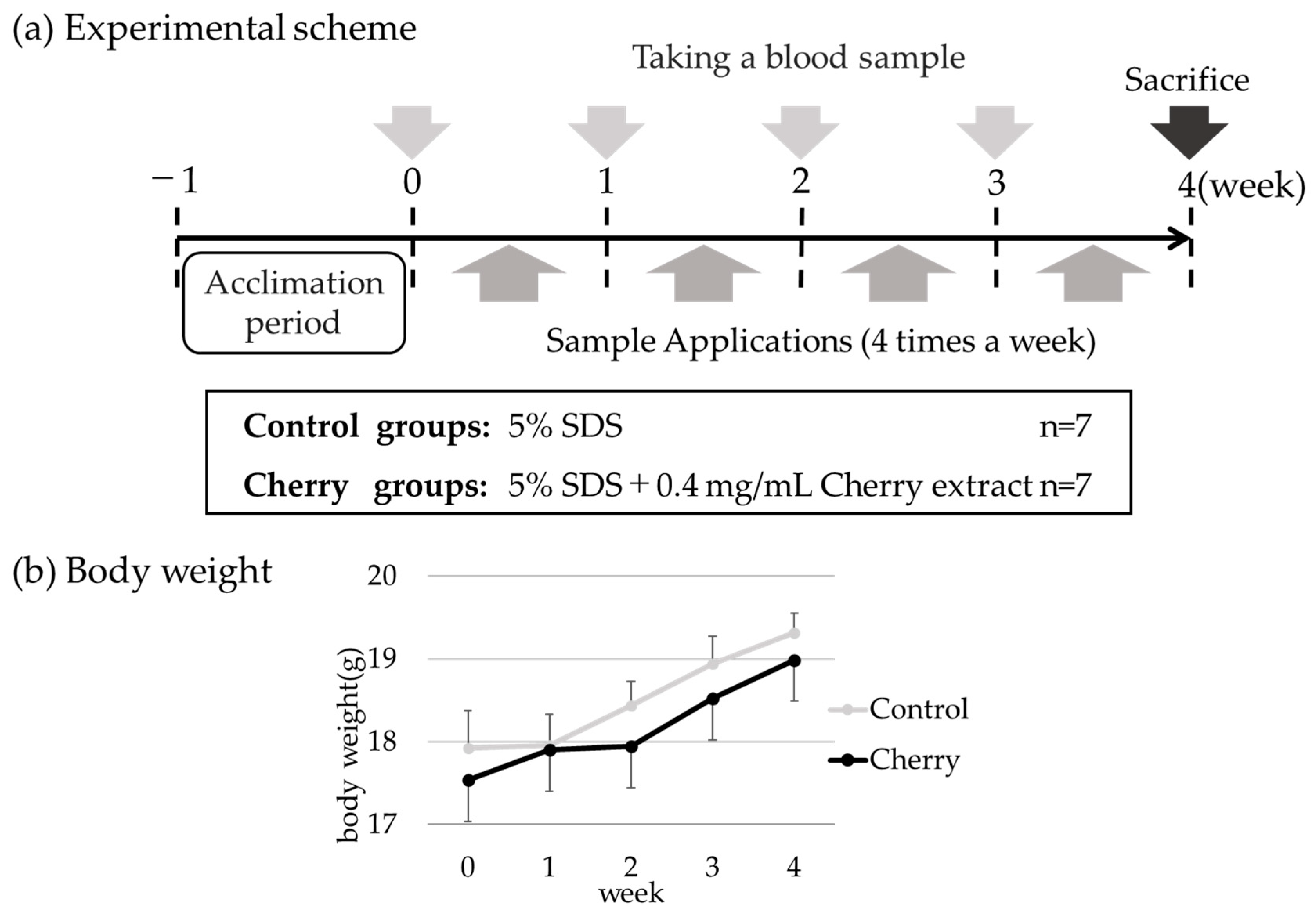
2.3. Animal Studies
2.4. Induction of Percutaneous Sensitization
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Electrophoresis and Immunoblotting
2.7. Purification and Identification of IgG1-Reactive Major Cherry Proteins
2.7.1. Ammonium Sulfate Precipitation
2.7.2. Ion-Exchange Chromatography
2.7.3. Gel-Filtration Chromatography
2.8. N-Terminal Amino Acid Sequence Analysis
2.9. Statistical Analysis
3. Results
3.1. Percutaneous Sensitization with Cherry Extract Did Not Affect Mouse Growth
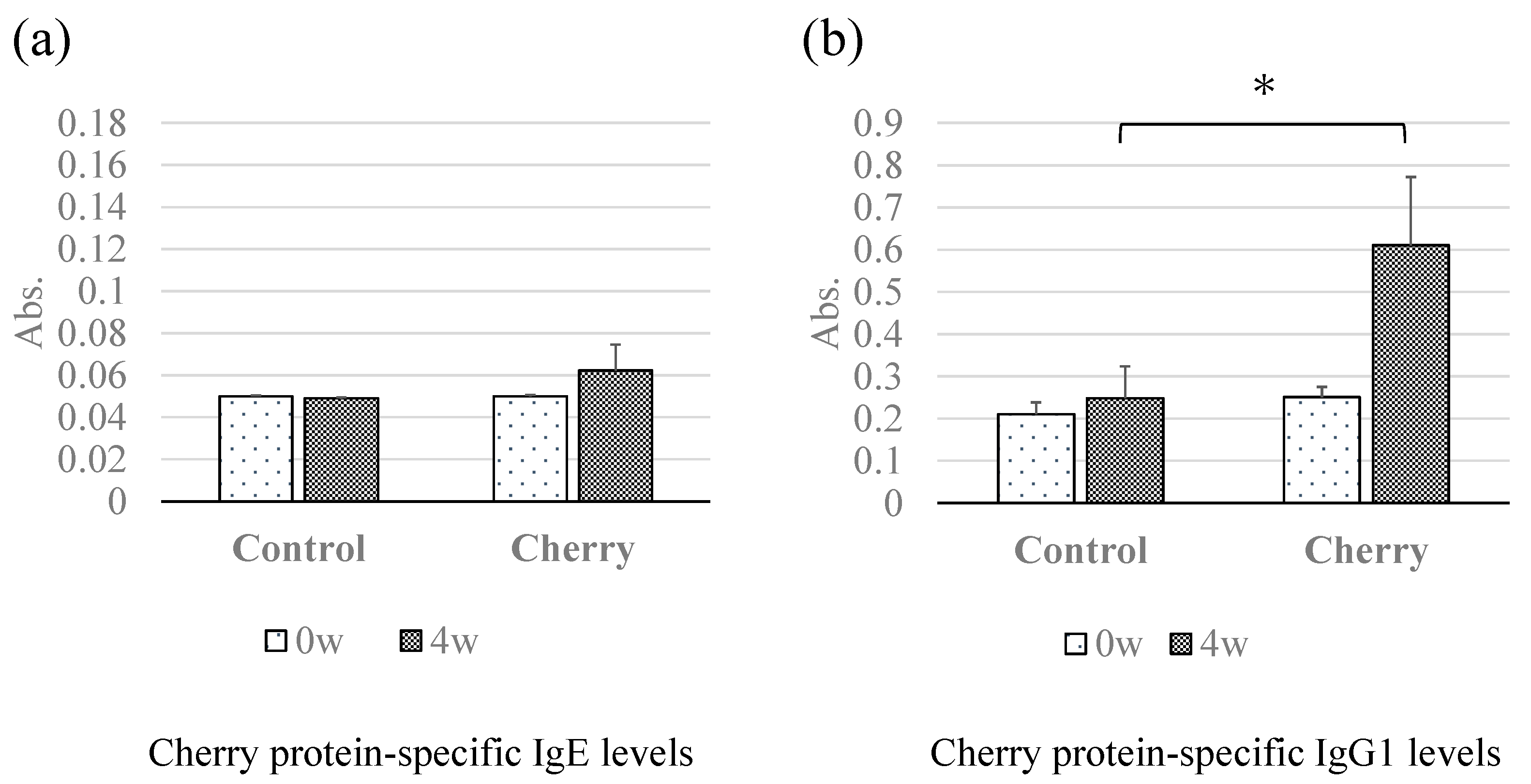
3.2. Changes in Cherry-Specific IgE and IgG1 Antibodies Determined Using ELISA
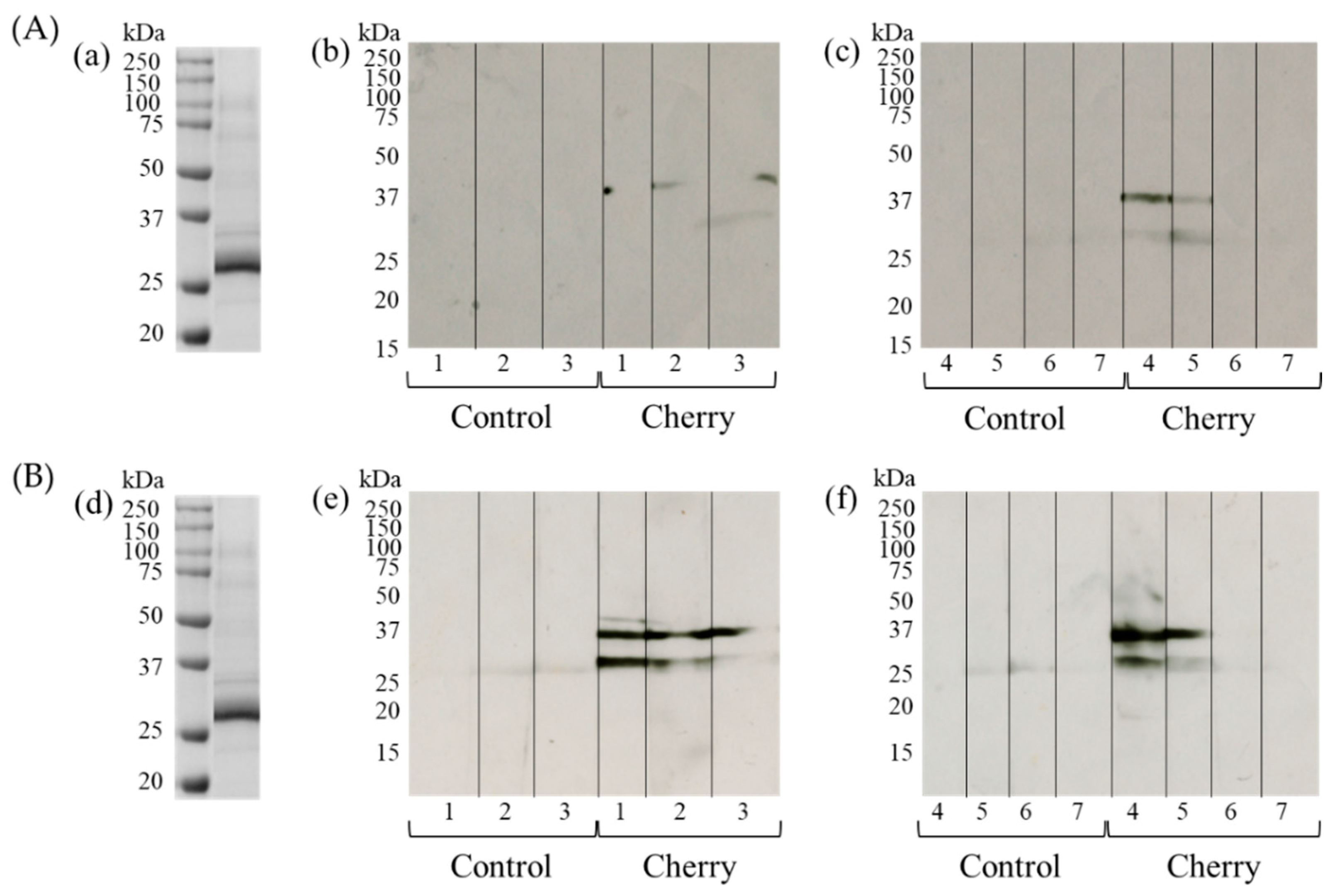
3.3. Detection of IgE- and IgG1-Binding Cherry Proteins Using Immunoblotting
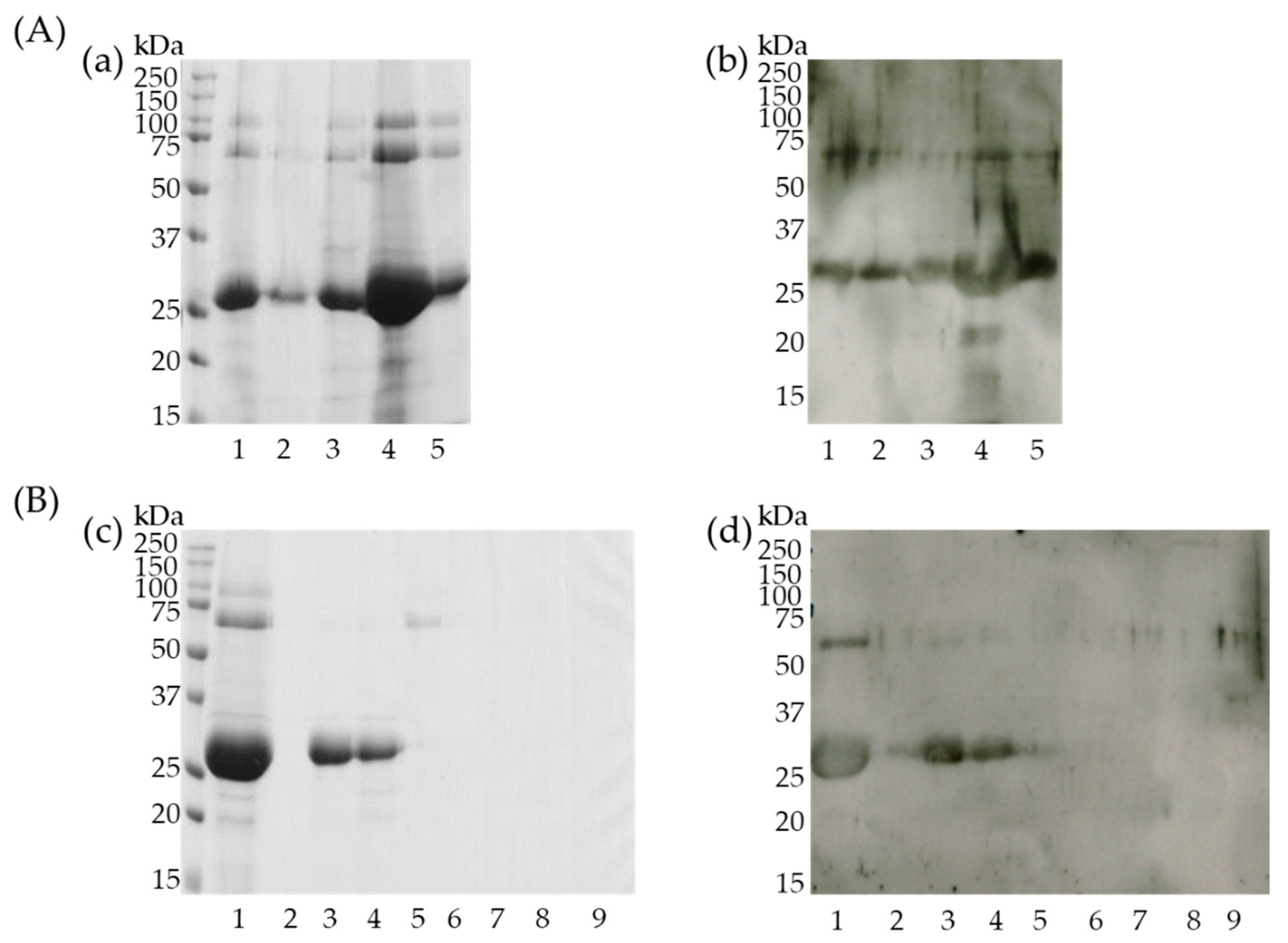
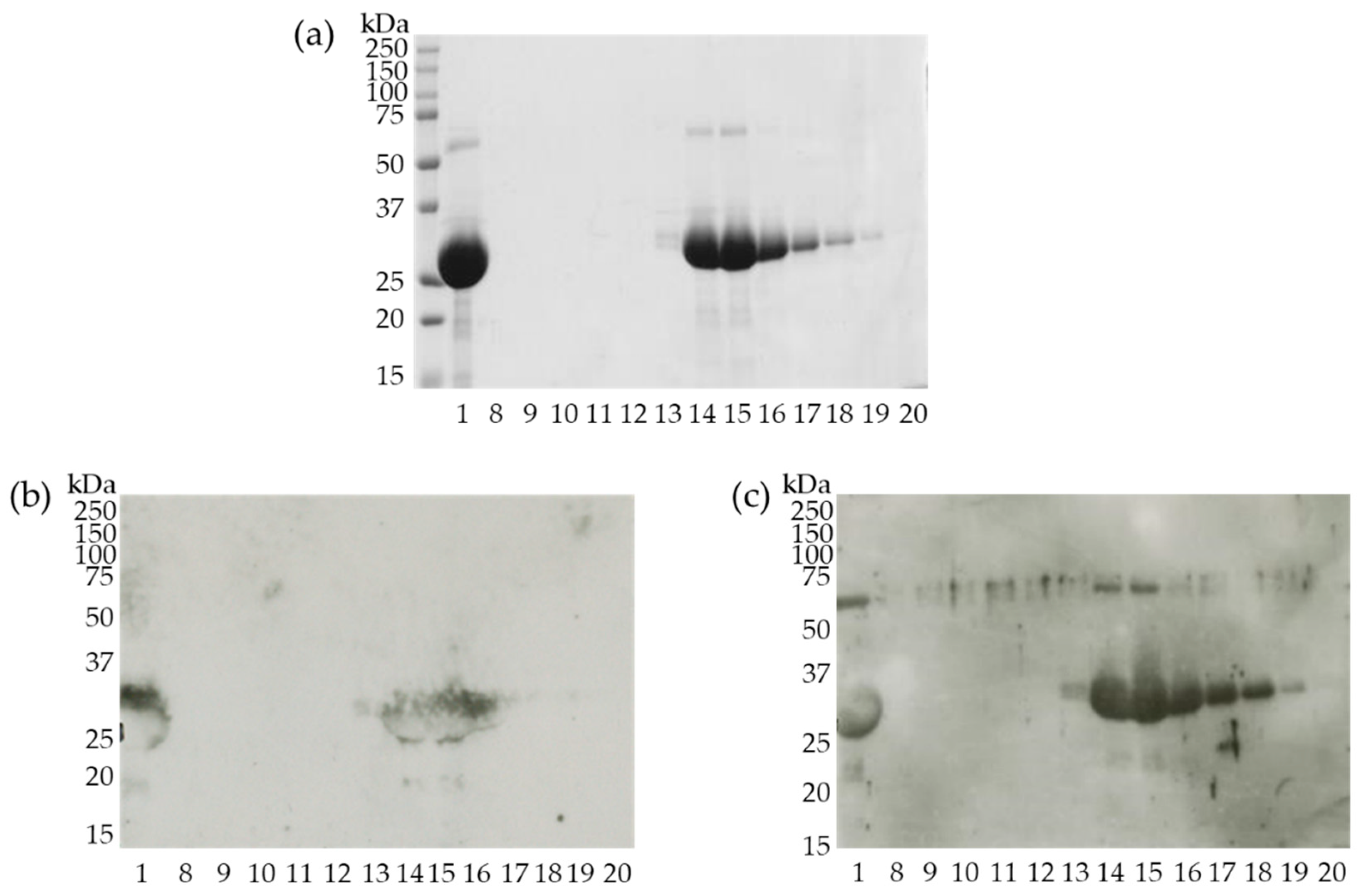
3.4. Identification of Cherry Antigens That Trigger Percutaneous Sensitization
3.5. Semi-Purified 27 kDa Protein Binds IgE
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sicherer, S.H.; Sampson, H.A. Food allergy: A review and update on epidemiology, pathogenesis, diagnosis, prevention, and management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pabst, O.; Mowat, A.M. Oral tolerance to food protein. Mucosal. Immunol. 2012, 5, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinuki, Y.; Kaneko, S.; Sakieda, K.; Murata, S.; Yoshida, Y.; Morita, E. Wheat-Dependent Exercise-Induced Anaphylaxis Sensitized with Hydrolyzed Wheat Protein in Soap. Allergol. Int. 2012, 61, 529–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukutomi, Y.; Itagaki, Y.; Taniguchi, M.; Saito, A.; Yasueda, H.; Nakazawa, T.; Hasegawa, M.; Nakamura, H.; Akiyama, K. Rhinoconjunctival sensitization to hydrolyzed wheat protein in facial soap can induce wheat-dependent exercise-induced anaphylaxis. J. Allergy Clin. Immunol. 2011, 127, 531–533. [Google Scholar] [CrossRef]
- Chinuki, Y.; Takahashi, H.; Dekio, I.; Kaneko, S.; Tokuda, R.; Nagao, M.; Fujisawa, T.; Morita, E. Higher allergenicity of high molecular weight hydrolysed wheat protein in cosmetics for percutaneous sensitization. Contact Dermat. 2013, 68, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Yagami, A.; Suzuki, K.; Nakamura, M.; Sano, A.; Iwata, Y.; Kobayashi, T.; Suzuki, M.; Hara, K.; Teshima, R.; Matsunaga, K. Case of anaphylactic reaction to soy following percutaneous sensitization by soy-based ingredients in cosmetic products. J. Dermatol. 2015, 42, 917–918. [Google Scholar] [CrossRef]
- Lack, G. Epidemiologic risks for food allergy. J. Allergy Clin. Immunol. 2008, 121, 1331–1336. [Google Scholar] [CrossRef]
- Matsumoto, K.; Saito, H. Epicutaneous immunity and onset of allergic diseases—per-“eczema”tous sensitization drives the allergy march. Allergol. Int. 2013, 62, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Inomata, N.; Nagashima, M.; Hakuta, A.; Aihara, M. Food allergy preceded by contact urticaria due to the same food: Involvement of epicutaneous sensitization in food allergy. Allergol. Int. 2015, 64, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Toit, G.D.; Katz, Y.; Sasieni, P.; Mesher, D.; Maleki, S.J.; Fisher, H.R.; Fox, A.T.; Turcanu, V.; Amir, T.; Zadik-Mnuhin, G.; et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008, 122, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.N.A.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.D.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Sporik, R.; Thorburn, J.; Hosking, C.S. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. J. Pediatr. 2000, 137, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Muto, T.; Fukuoka, A.; Kabashima, K.; Ziegler, S.F.; Nakanishi, K.; Matsushita, K.; Yoshimoto, T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int. Immunol. 2014, 26, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, A.E.; Sattentau, Q.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin–basophil axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399. [Google Scholar] [CrossRef] [Green Version]
- Murakami, H.; Ogawa, T.; Takafuta, A.; Yano, E.; Zaima, N.; Moriyama, T. Identification of the 7S and 11S globulins as percutaneously sensitizing soybean allergens as demonstrated through epidermal application of crude soybean extract. Biosci. Biotechnol. Biochem. 2018, 82, 1408–1416. [Google Scholar] [CrossRef] [Green Version]
- Murakami, H.; Ogawa, T.; Takafuta, A.; Yano, E.; Zaima, N.; Moriyama, T. Percutaneous sensitization to soybean proteins is attenuated by oral tolerance. J. Nutr. Sci. Vitaminol. 2018, 64, 683–686. [Google Scholar] [CrossRef]
- Scheurer, S.; Pastorello, E.A.; Wangorsch, A.; Kästnera, M.; Haustein, D.; Vieths, S. Recombinant allergens Pru av1 and Pru av 4 and a newly identified lipid transfer protein in the in vitro diagnosis of cherry allergy. J. Allergy Clin. Immunol. 2001, 107, 724–731. [Google Scholar] [CrossRef]
- Hartz, C.; Lauer, I.; Moncin, M.M.S.M.; Cistero-Bahima, A.; Foetisch, K.; Lidholm, J.; Vieths, S.; Scheurer, S. Comparison of IgE-binding capacity, cross-reactivity and biological potency of allergenic non-specific lipid transfer proteins from peach, cherry and hazelnut. Int. Arch. Allergy Immunol. 2010, 153, 335–346. [Google Scholar] [CrossRef]
- WHO/IUIS Allergen Nomenclature Home Page. Available online: http://www.allergen.org/search.php?allergensource=Prunus+avium&searchsource=Search (accessed on 27 November 2020).
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. J. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kyhse-Andersen, J. Electroblotting of multiple gels: A simple apparatus without buffer tank for rapid transfer of proteins from polycrylamide to nitrocellulose. J. Biochem. Biophys. Methods 1984, 10, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Edman, P.; Hogfeldt, E.; Sillen, L.G.; Kinell, P.-O. Method for determination of the amino acid sequence in peptides. Acta Chem. Scand. 1950, 4, 283–293. [Google Scholar] [CrossRef] [Green Version]
- Wel, H.; Loeve, K. Isolation and Characterization of Thaumatin I and II, the Sweet-Tasting Proteins from Thaumatococcus daniellii Benth. Eur. J. Biochem. 1972, 31, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sturrock, R.; Ekramoddoullah, A.K.M. The superfamily of thaumatin-like proteins: Its origin, evolution, and expression towards biological function. Plant. Cell Rep. 2010, 29, 419–436. [Google Scholar] [CrossRef]
- Breiteneder, H.; Radauer, C. A classification of plant food allergens. J. Allergy Clin. Immunol. 2004, 113, 821–830. [Google Scholar] [CrossRef]
- Inschlag, C.; Hoffmann-Sommergruber, K.; O’Riordain, G.; Ahorn, H.; Ebner, C.; Scheiner, O.; Breiteneder, H. Biochemical characterization of Pru a 2, a 23-kD thaumatin-like protein representing a potential major allergen in cherry (Prunus avium). Int. Arch. Allergy Immunol. 1998, 116, 22–28. [Google Scholar] [CrossRef]
- Gavrović-Jankulović, M.; Ćirković, T.; Vučković, O.; Atanasković-Marković, M.; Petersen, A.; Gojgić, G.; Burazer, L.; Jankov, R.M. Isolation and biochemical characterization of a thaumatin-like kiwi allergen. J. Allergy Clin. Immunol. 2002, 110, 805–810. [Google Scholar] [CrossRef]
- Krebitz, M.; Wagner, B.; Ferreira, F.; Peterbauer, C.; Campillo, N.; Witty, M.; Kolarich, D.; Steinkellner, H.; Scheiner, O.; Breiteneder, H. Plant-based heterologous expression of Mal d 2, a thaumatin-like protein and allergen of apple (Malus domestica), and its characterization as an antifungal protein. J. Mol. Biol. 2003, 329, 721–730. [Google Scholar] [CrossRef]
- Leone, P.; Menu-Bouaouiche, L.; Peumans, W.J.; Payan, F.; Barre, A.; Roussel, A.; Damme, E.J.M.V.; Rougé, P. Resolution of the structure of the allergenic and antifungal banana fruit thaumatin-like protein at 1.7-Å. Biochimie 2006, 88, 45–52. [Google Scholar] [CrossRef]
- Christensen, A.B.; Cho, B.H.; Næsby, M.; Gregersen, P.L.; Brandt, J.; Madriz-Ordeñana, K.; Collinge, D.B.; Thordal-Christensen, H. The molecular characterization of two barley proteins establishes the novel PR-17 family of pathogenesis-related proteins. Mol. Plant. Pathol. 2002, 3, 135–144. [Google Scholar] [CrossRef]
- Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trudel, J.; Grenier, J.; Potvin, C.; Asselin, A. Several thaumatinlike proteins bind to 1, 3-glucans. Plant. Physiol. 1998, 118, 1431–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grenier, J.; Potvin, C.; Trudel, J.; Asselin, A. Some thaumatin-like proteins hydrolyse polymeric b-1, 3-glucans. Plant. J. 1999, 19, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Potvin, C.; Asselin, A. Some fungi express b-1, 3- glucanases similar to thaumatin-like proteins. Mycologia 2000, 92, 841–848. [Google Scholar] [CrossRef]
- Fierens, E.; Gebruers, K.; Voet, A.R.D.; Maeyer, M.D.; Courtin, C.M.; Delcour, J.A. Biochemical and structural characterization of TLXI, the Triticum aestivum L. thaumatin-like xylanase inhibitor. J. Enzym. Inhib. Med. Chem. 2009, 24, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Smole, U.; Bublin, M.; Radauer, C.; Ebner, C.; Breiteneder, H. Mal d 2, the thaumatin-like allergen from apple, is highly resistant to gastrointestinal digestion and thermal processing. Int. Arch. Allergy Immunol. 2008, 147, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Midoro-Horiuti, T.; Goldblum, R.M.; Kurosky, A.; Wood, T.G.; Brooks, E.G. Variable expression of pathogenesis-related protein allergen in mountain cedar (Juniperus ashei) pollen. J. Immunol. 2000, 164, 188–2192. [Google Scholar] [CrossRef] [Green Version]
- Fujimura, T.; Futamura, N.; Midoro-Horiuti, T.; Togawa, A.; Goldblum, R.M.; Yasueda, H.; Saito, A.; Shinohara, K.; Masuda, K.; Kurata, K.; et al. Isolation and characterization of native Cry j 3 from Japanese cedar (Cryptomeria japonica) pollen. Allergy 2007, 62, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Palacín, A.; Rivas, L.A.; Gómez-Casado, C.; Aguirre, J.; Tordesillas, L.; Bartra, J.; Blanco, C.; Carrillo, T.; Cuesta-Herranz, J.; Bonny, J.A.; et al. The involvement of thaumatin-like proteins in plant food cross-reactivity: A multicenter study using a specific protein microarray. PLoS ONE 2012, 7, e44088. [Google Scholar] [CrossRef] [Green Version]

| Cycles | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Asp | 1.10 | 5.01 | 11.94 | 20.52 | 24.42 | 33.92 | 50.18 | 56.75 |
| Glu | 1.32 | 5.37 | 7.45 | 19.44 | 27.47 | 33.45 | 33.73 | 34.11 |
| Asn | 1.21 | 2.51 | 4.28 | 6.44 | 8.85 | 22.27 | 258.58 | 346.43 |
| Gln | 6.31 | 6.35 | 11.15 | 33.78 | 30.37 | 36.25 | 41.57 | 45.86 |
| Ser | 1.30 | 2.48 | 6.02 | 142.57 | 49.54 | 23.91 | 19.28 | 20.20 |
| Thr | 2.92 | 279.90 | 59.23 | 26.13 | 28.82 | 34.38 | 34.00 | 37.62 |
| His | 0.25 | 14.61 | 5.10 | 2.59 | 2.60 | 3.39 | 4.12 | 4.25 |
| Gly | 16.28 | 10.38 | 21.03 | 31.10 | 29.91 | 35.57 | 39.70 | 46.69 |
| Ala | 526.88 | 91.78 | 27.39 | 42.33 | 43.13 | 52.08 | 57.41 | 68.76 |
| Tyr | 4.70 | 5.72 | 5.05 | 7.92 | 13.42 | 17.06 | 18.24 | 20.99 |
| Arg | 1.44 | 2.27 | 2.58 | 23.59 | 8.40 | 6.50 | 6.89 | 6.45 |
| Met | 0.17 | 0.16 | 0.27 | 0.57 | 0.80 | 2.77 | 3.28 | 3.69 |
| Val | 1.76 | 3.03 | 11.25 | 22.41 | 28.31 | 38.54 | 42.67 | 45.15 |
| Pro | 1.18 | 9.73 | 29.07 | 46.34 | 56.17 | 69.89 | 77.27 | 86.14 |
| Trp | 1.23 | 0.71 | 0.73 | 0.48 | 2.00 | 1.07 | 2.55 | 2.17 |
| Phe | 0.36 | 4.26 | 14.78 | 22.84 | 392.79 | 182.05 | 85.35 | 66.37 |
| Lys | 0.05 | 0.93 | 1.73 | 3.29 | 8.57 | 353.62 | 151.10 | 70.93 |
| Ile | 1.85 | 6.72 | 491.51 | 118.35 | 22.74 | 12.88 | 9.12 | 14.75 |
| Leu | 0.50 | 5.85 | 19.65 | 21.70 | 21.72 | 32.54 | 32.84 | 38.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izumi, E.; Hidaka, S.; Hiroi, A.; Kinugasa, S.; Yano, E.; Zaima, N.; Moriyama, T. Thaumatin-Like Protein (Pru av 2) Is a Cherry Allergen That Triggers Percutaneous Sensitization in Mice. Foods 2021, 10, 134. https://doi.org/10.3390/foods10010134
Izumi E, Hidaka S, Hiroi A, Kinugasa S, Yano E, Zaima N, Moriyama T. Thaumatin-Like Protein (Pru av 2) Is a Cherry Allergen That Triggers Percutaneous Sensitization in Mice. Foods. 2021; 10(1):134. https://doi.org/10.3390/foods10010134
Chicago/Turabian StyleIzumi, Eri, Shota Hidaka, Ayako Hiroi, Serina Kinugasa, Erika Yano, Nobuhiro Zaima, and Tatsuya Moriyama. 2021. "Thaumatin-Like Protein (Pru av 2) Is a Cherry Allergen That Triggers Percutaneous Sensitization in Mice" Foods 10, no. 1: 134. https://doi.org/10.3390/foods10010134
APA StyleIzumi, E., Hidaka, S., Hiroi, A., Kinugasa, S., Yano, E., Zaima, N., & Moriyama, T. (2021). Thaumatin-Like Protein (Pru av 2) Is a Cherry Allergen That Triggers Percutaneous Sensitization in Mice. Foods, 10(1), 134. https://doi.org/10.3390/foods10010134






