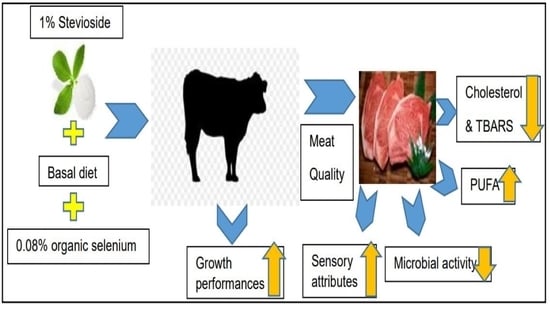
Sensory Attributes, Microbial Activity, Fatty Acid Composition and Meat Quality Traits of Hanwoo Cattle Fed a Diet Supplemented with Stevioside and Organic Selenium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Management of the Animals, Diets, and Experimental Design
2.3. Growth Performances and Slaughtering
2.4. Proximate Composition, Carcass Traits, and Cholesterol Analysis
2.5. Meat Quality Analysis (Meat Color, Drip Loss, Cooking Loss, Water Holding Capacity (WHC), and Shear Force)
2.6. pH Value and Oxidative Stability of Meat
2.7. Fatty Acid Analysis
2.8. Meat Microbial Analysis and Sensory Evaluation
2.9. Statistical Analysis
3. Results
3.1. Growth Performance, Proximate Analysis, and Cholesterol Content
3.2. Carcass Traits
3.3. Meat Quality Analysis (Meat Color, Drip Loss, Cooking Loss, WHC, and Shear Force)
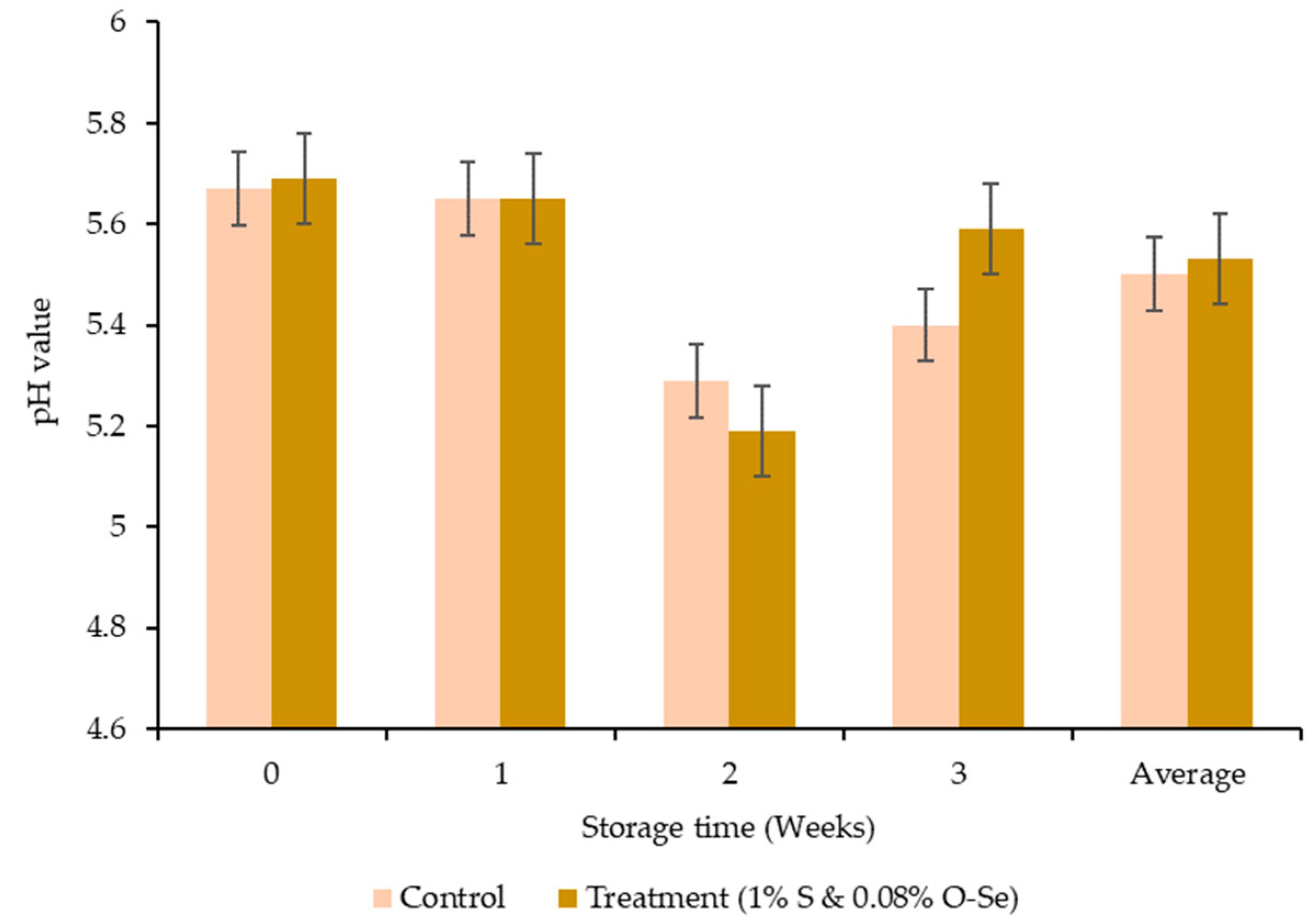
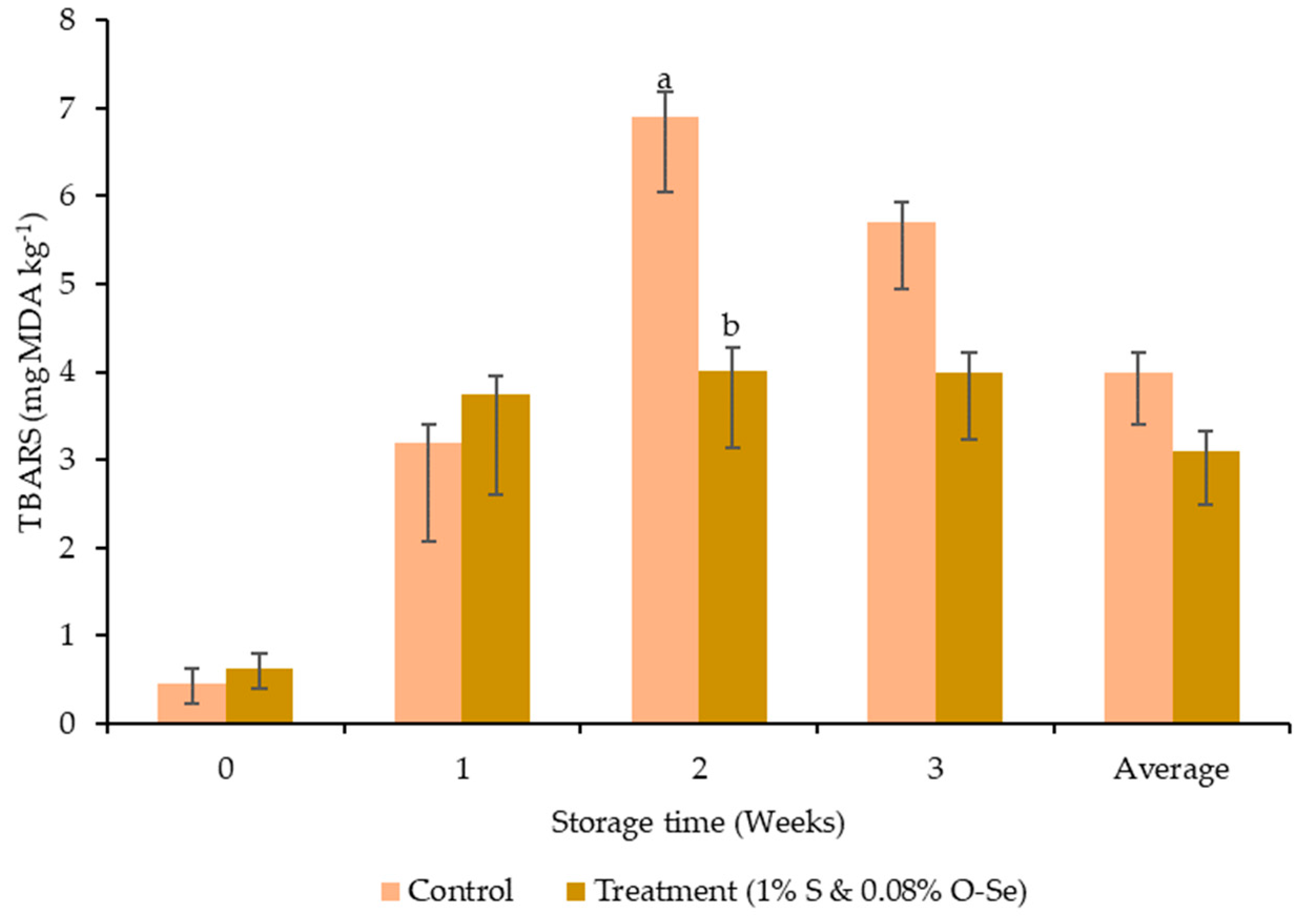
3.4. pH Value and Oxidative Stability of Meat
3.5. Fatty Acid Analysis
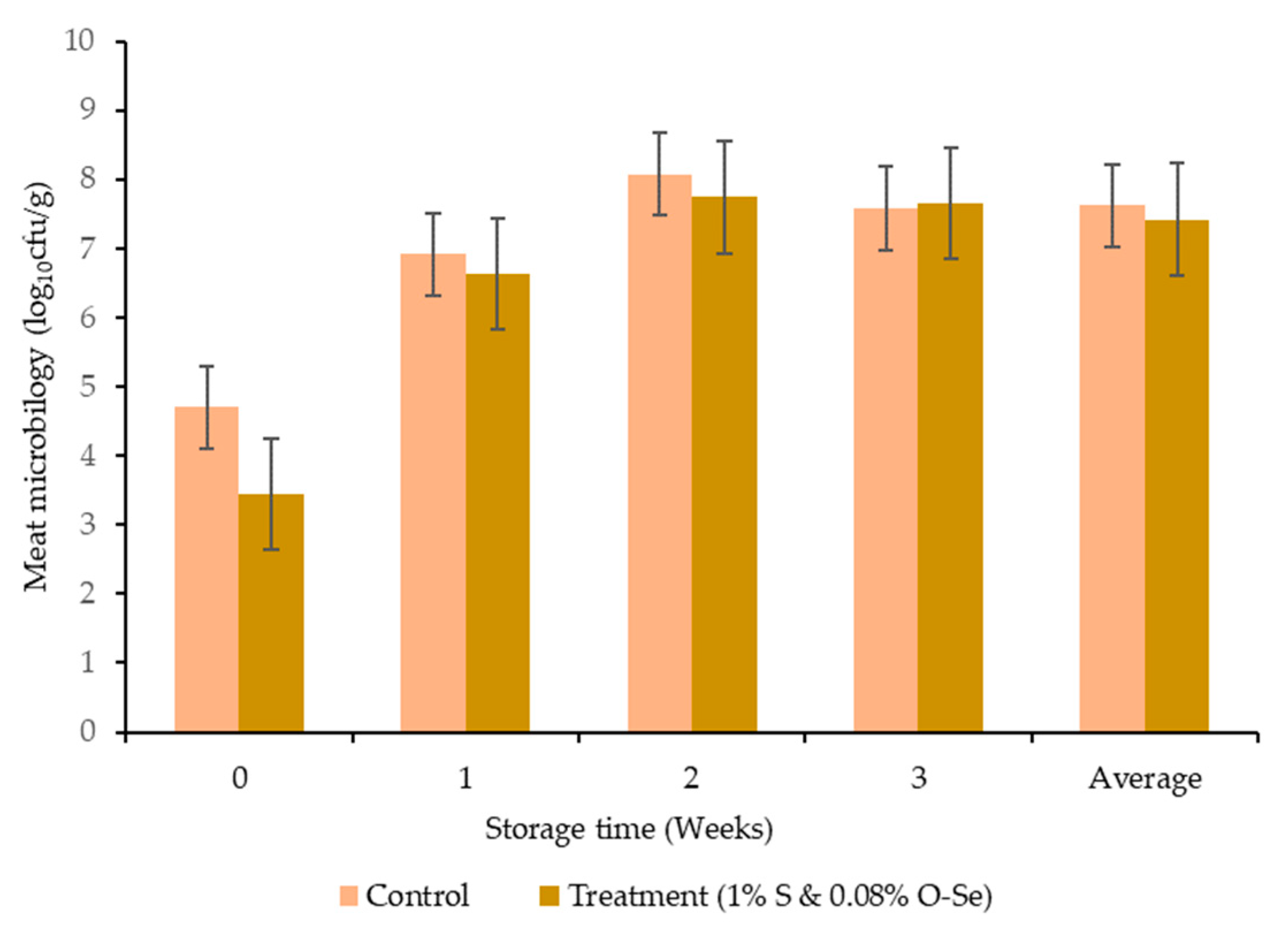
3.6. Microbial Analysis and Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hwang, Y.H.; Kim, G.-D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Cho, S.H.; Chang, J.; Nam, K.C. Keys to production and processing of Hanwoo beef: A perspective of tradition and science. Anim. Front. 2012, 2, 32–38. [Google Scholar] [CrossRef]
- Kamel, C. Natural Plant Extracts: Classical Medies Bring Modern Animal Production Solutions. Available online: https://agris.fao.org/agris-search/search.do?recordID=QC2001600008 (accessed on 28 November 2008).
- Suresh, G.; Das, R.K.; Brar, S.K.; Rouissi, T.; Ramirez, A.; Chorfi, Y.; Godbout, S. Alternatives to antibiotics in poultry feed: Molecular perspectives. Crit. Rev. Microbiol. 2017, 44, 318–355. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Hossain, M.E.; Kim, G.M.; Hwang, J.A.; Ji, H.; Yang, C.J. Effects of Resveratrol and Essential Oils on Growth Performance, Immunity, Digestibility and Fecal Microbial Shedding in Challenged Piglets. Asian Australas. J. Anim. Sci. 2013, 26, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [ Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Tiwari, A.K. Downstream processing of stevioside and its potential applications. Biotechnol. Adv. 2011, 29, 781–791. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Kozuka, M.; Tokuda, H.; Takayasu, J.; Nishino, H.; Miyakoshi, M.; Mizutani, K.; Lee, K.-H. Cancer preventive agents. Part 8: Chemopreventive effects of stevioside and related compounds. Bioorganic Med. Chem. 2009, 17, 600–605. [Google Scholar] [CrossRef]
- Pariwat, P.; Homvisasevongsa, S.; Muanprasat, C.; Chatsudthipong, V. A Natural Plant-Derived Dihydroisosteviol Prevents Cholera Toxin-Induced Intestinal Fluid Secretion. J. Pharmacol. Exp. Ther. 2008, 324, 798–805. [Google Scholar] [CrossRef] [Green Version]
- Abou-Arab, A.E.; Abou-Arab, A.A.; Abu-Salem, M.F. Physico-chemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana Bertoni plant. Afr. J. Food Sci. 2010, 4, 269–281. [Google Scholar] [CrossRef]
- Muanda, F.N.; Soulimani, R.; Diop, B.; Dicko, A. Study on chemical composition and biological activities of essential oil and extracts from Stevia rebaudiana Bertoni leaves. LWT Food Sci. Technol. 2011, 44, 1865–1872. [Google Scholar] [CrossRef]
- Shukla, S.; Mehta, A.; Bajpai, V.K.; Shukla, S. In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chem. Toxicol. 2009, 47, 2338–2343. [Google Scholar] [CrossRef] [PubMed]
- Tadhani, M.B.; Patel, V.H.; Subhash, R. In vitro antioxidant activities of Stevia rebaudiana leaves and callus. J. Food Compos. Anal. 2007, 20, 323–329. [Google Scholar] [CrossRef]
- Munro, P.J.; Lirette, A.; Anderson, D.M.; Ju, H.Y. Effects of a new sweetener, Stevia, on performance of newly weaned pigs. Can. J. Anim. Sci. 2000, 80, 529–531. [Google Scholar] [CrossRef]
- Paredes-López, D.; Robles-Huaynate, R.; Carrión-Molina, M. Effect of Stevia rebaudiana Bertonni Leaves powder on Lipid Profiles and Productive Parameters of Laying Hens. Sci. Agropecu 2019, 10, 275–282. [Google Scholar] [CrossRef]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.-R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Albanes, D.; Till, C.; Klein, E.A.; Goodman, P.J.; Mondul, A.M.; Weinstein, S.J.; Taylor, P.R.; Parnes, H.L.; Gaziano, J.M.; Song, X.; et al. Plasma Tocopherols and Risk of Prostate Cancer in the Selenium and Vitamin E Cancer Prevention Trial (SELECT). Cancer Prev. Res. 2014, 7, 886–895. [Google Scholar] [CrossRef] [Green Version]
- Speckmann, B.; Grune, T. Epigenetic effects of selenium and their implications for health. Epigenetics 2015, 10, 179–190. [Google Scholar] [CrossRef]
- Schrauzer, G.N. The nutritional significance, metabolism and toxicology of selenomethionine. Adv. Food Nutr. Res. 2003, 47, 73–112. [Google Scholar] [CrossRef]
- Taylor, J.B.; Marchello, M.J.; Finley, J.W.; Neville, T.L.; Combs, G.F.; Caton, J.S. Nutritive value and display-life attributes of selenium-enriched beef-muscle foods. J. Food Compos. Anal. 2008, 21, 183–186. [Google Scholar] [CrossRef]
- Cozzi, G.; Prevedello, P.; Stefani, A.L.; Piron, A.; Contiero, B.; Lante, A.; Gottardo, F.; Chevaux, E. Effect of dietary supplementation with different sources of selenium on growth response, selenium blood levels and meat quality of intensively finished Charolais young bulls. Animal 2011, 5, 1531–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svoboda, M.; Saláková, A.; Fajt, Z.; Ficek, R.; Buchtová, H.; Drábek, J. Selenium from Se-enriched lactic acid bacteria as a new Se source for growing-finishing pigs. Pol. J. Vet. Sci. 2009, 12, 355–361. [Google Scholar] [PubMed]
- Zhan, X.; Wang, M.; Zhao, R.; Li, W.; Xu, Z. Effects of different selenium source on selenium distribution, loin quality and antioxidant status in finishing pigs. Anim. Feed Sci. Technol. 2007, 132, 202–211. [Google Scholar] [CrossRef]
- Biazik, E.; Straková, E.; Suchý, P. Effects of Organic Selenium in Broiler Feed on the Content of Selenium and Fatty Acid Profile in Lipids of Thigh Muscle Tissue. Acta Vet. Brno 2013, 82, 277–282. [Google Scholar] [CrossRef] [Green Version]
- Rubayet Bostami, A.B.M.; Mun, H.S.; Yang, C.J. Loin eye muscle physico-chemical attributes, sensory evaluation and proximate composition in Korean Hanwoo cattle subjected to slaughtering along with stunning with or without pithing. Meat Sci. 2018, 145, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- KAPE 2012. ‘The beef carcass grading.’ (Korea Institute for Animal Products Quality Evaluation, Gunposi, Gyungki-do, South Korea). Available online: http://www.ekape.or.kr/view/eng/system/beef.asp (accessed on 15 May 2012).
- King, A.J.; Paniangvait, P.; Jones, A.D.; German, J.B. Rapid Method for Quantification of Cholesterol in Turkey Meat and Products. J. Food Sci. 1998, 63, 382–385. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Grau, R.; Hamm, G. A simple method for determining water binding in muscles. Diet Nat. 1953, 40, 29–30. [Google Scholar]
- Witte, V.C.; Krause, G.F.; Bailey, M.E. A new extraction method for determining 2-thiobarbituric acid values of pork and beef during storage. J. Food Sci. 1970, 35, 582–585. [Google Scholar] [CrossRef]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [Green Version]
- Meilgaard, M.C.; Carr, B.T.; Civille, G.V. Sensory Evaluation Techniques; CRC Press: Boca Raton, FL, USA, 2006; pp. 25–28. ISBN 978-1-00-304072-9. [Google Scholar]
- Geetha, V.; Chakravarthula, S.N. Chemical composition and anti-inflammatory activity of Boswellia ovalifoliolata essential oils from leaf and bark. J. For. Res. 2018, 29, 373–381. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Sana Ullah, K.R.; Alagawany, M.; Farag, M.R.; et al. Medicinal and therapeutic potential of herbs and plant metabolites/extracts countering viral pathogens-Current knowledge and future prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Golestan, I. Phytogenics as new class of feed additive in poultry industry. J. Anim. Vet. Adv. 2010, 9, 2295–2304. [Google Scholar]
- Krause, D.O.; House, J.D.; Nyachoti, C.M. Alternatives to antibiotics in swine diets: A molecular approach. Proc. Manit. Swine Sem. 2005, 19, 57–66. [Google Scholar]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Velichko, O.A. Selenium in Poultry Nutrition: From Sodium Selenite to Organic Selenium Sources. Jpn. Poult. Sci. 2018, 55, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Hellfritsch, C.; Brockhoff, A.; Stähler, F.; Meyerhof, W.; Hofmann, T. Human Psychometric and Taste Receptor Responses to Steviol Glycosides. J. Agric. Food Chem. 2012, 60, 6782–6793. [Google Scholar] [CrossRef]
- Ahmed, J.; Preissner, S.; Dunkel, M.; Worth, C.L.; Eckert, A.; Preissner, R. Super Sweet—A resource on natural and artificial sweetening agents. Nucleic Acids Res. 2010, 14, 39. [Google Scholar]
- Abudula, R.; Matchkov, V.V.; Jeppesen, P.B.; Nilsson, H.; Aalkjaer, C.; Hermansen, K. Rebaudioside A directly stimulates insulin secretion from pancreatic beta cells: A glucose-dependent action via inhibition of ATP-sensitive K + -channels*. Diabetes Obes. Metab. 2008, 10, 1074–1085. [Google Scholar] [CrossRef]
- Han, X.; Chen, C.; Zhang, X.; Wei, Y.; Tang, S.; Wang, J.; Tan, Z.; Xu, L. Effects of Dietary Stevioside Supplementation on Feed Intake, Digestion, Ruminal Fermentation, and Blood Metabolites of Goats. Animals 2019, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Cho, S.; Mbiriri, D.T.; Shim, K.; Lee, A.L.; Oh, S.J.; Yang, J.; Ryu, C.; Kim, Y.H.; Seo, K.S.; Chae, J.I.; et al. The influence of feed energy density and a formulated additive on rumen and rectal temperature in hanwoo steers. Asian Australas. J. Anim. Sci. 2014, 27, 1652–1662. [Google Scholar]
- Quesnel, H.; Renaudin, A.; Le Floc’h, N.; Jondreville, C.; Père, M.C.; Taylor-Pickard, J.A.; Le Dividich, J. Effect of organic and inorganic selenium sources in sow diets on colostrum production and piglet response to a poor sanitary environment after weaning. ANM 2008, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tufarelli, V.; Ceci, E.; Laudadio, V. 2-Hydroxy-4-Methylselenobutanoic Acid as New Organic Selenium Dietary Supplement to Produce Selenium-Enriched Eggs. Biol. Trace Elem. Res. 2016, 171, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F.; Fisinin, V.I. Selenium in poultry breeder nutrition: An update. Anim. Feed Sci. Technol. 2014, 191, 1–15. [Google Scholar] [CrossRef]
- Khalili, M.; Chamani, M.; Amanlou, H.; Nikkhah, A.; Sadeghi, A. The effect of feeding inorganic and organic selenium sources on the performance and content of selenium in milk of transition dairy cows. Acta Sci. Anim. Sci. 2019, 41, 44691. [Google Scholar] [CrossRef]
- Gromadzińska, J.; Reszka, E.; Bruzelius, K.; Wąsowicz, W.; Åkesson, B. Selenium and cancer: Biomarkers of selenium status and molecular action of selenium supplements. Eur. J. Nutr. 2008, 47, 29–50. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Gaweł-Bęben, K.; Bujak, T.; Nizioł-Łukaszewska, Z.; Antosiewicz, B.; Jakubczyk, A.; Karaś, M.; Rybczyńska, K. Stevia Rebaudiana Bert. Leaf Extracts as a Multifunctional Source of Natural Antioxidants. Molecules 2015, 20, 5468–5486. [Google Scholar] [CrossRef] [Green Version]
- Callow, E.H. Comparative studies of meat. II. The changes in the carcass during growth and fattening, and their relation to the chemical composition of the fatty and muscular tissues. J. Agric. Sci. 1948, 38, 174–199. [Google Scholar] [CrossRef]
- Mehdi, Y.; Clinquart, A.; Hornick, J.-L.; Cabaraux, J.-F.; Istasse, L.; Dufrasne, I. Meat composition and quality of young growing Belgian Blue bulls offered a fattening diet with selenium enriched cereals. Can. J. Anim. Sci. 2015, 95, 465–473. [Google Scholar] [CrossRef]
- Waldenstedt, L. Nutritional factors of importance for optimal leg health in broilers: A review. Anim. Feed Sci. Technol. 2006, 126, 291–307. [Google Scholar] [CrossRef]
- Zhang, S.; Xie, Y.; Li, M.; Yang, H.; Li, S.; Li, J.; Xu, Q.; Yang, W.; Jiang, S. Effects of Different Selenium Sources on Meat Quality and Shelf Life of Fattening Pigs. Animals 2020, 10, 615. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.S.; Lee, J.H.; Lee, H.J.; Jang, S.S.; Lee, J.J.; Choi, Y.I. Effect of stevia and charcoal as an alternative to antibiotics on carcass characteristics and meat quality in finishing pigs. Food Sci. Anim. Resour. 2012, 32, 835–841. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.C.; Belk, K.E.; Sofos, J.N.; Tatum, J.D.; Williams, S.N. Economic implications of improved color stability in beef. In Antioxidants in Muscle Foods: Nutritional Strategies to Improve Quality; Decker, E.A., Faustman, C., Lopez-Bote, C.J., Eds.; Wiley Interscience: New York, NY, USA, 2000; pp. 397–426. [Google Scholar]
- Macit, M.; Aksakal, V.; Emsen, E.; Aksu, M.I.; Karaoglu, M.; Esenbuga, N. Effects of vitamin E supplementation on performance and meat quality traits of Morkaraman male lambs. Meat Sci. 2003, 63, 51–55. [Google Scholar] [CrossRef]
- Boiago, M.M.; Borba, H.; Leonel, F.R.; Giampietro-Ganeco, A.; Ferrari, F.B.; Stefani, L.M.; Souza, P.A. de Sources and levels of selenium on breast meat quality of broilers. Cienc. Rural 2014, 44, 1692–1698. [Google Scholar] [CrossRef] [Green Version]
- Boonkaewwan, C.; Burodom, A. Anti-inflammatory and immunomodulatory activities of stevioside and steviol on colonic epithelial cells: Anti-inflammatory and immunomodulatory activities of stevia compounds. J. Sci. Food Agric. 2013, 93, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Chatsudthipong, V.; Muanprasat, C. Stevioside and related compounds: Therapeutic benefits beyond sweetness. Pharmacol. Ther. 2009, 121, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.C.R.; Ordoñez, Y.B.M.; Basto, Á.M.; Campos, M.R.S. Antioxidant capacity of leaf extracts from two Stevia rebaudiana Bertoni varieties adapted to cultivation in Mexico. Nutr. Hosp. 2015, 31, 1163–1170. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Xu, L.; Wu, P.; Xie, H.; Jiang, Y.; Chen, F.; Wei, X. Polyphenols from longan seeds and their radical-scavenging activity. Food Chem. 2009, 116, 433–436. [Google Scholar] [CrossRef]
- Mukai, K.; Nagai, S.; Ohara, K. Kinetic study of the quenching reaction of singlet oxygen by tea catechins in ethanol solution. Free Radic. Biol. Med. 2005, 39, 752–761. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Berchielli, T.T.; Vaz Pires, A.; Oliveira, S.G. Nutrição de Ruminantes. Jaboticabal Funep 2006, 1, 200–583. [Google Scholar]
- Richard, D.; Bausero, P.; Schneider, C.; Visioli, F. Polyunsaturated fatty acids and cardiovascular disease. Cell. Mol. Life Sci. 2009, 66, 3277–3288. [Google Scholar] [CrossRef] [PubMed]
- Netto, A.S.; Zanetti, M.A.; Claro, G.R.D.; de Melo, M.P.; Vilela, F.G.; Correa, L.B. Effects of Copper and Selenium Supplementation on Performance and Lipid Metabolism in Confined Brangus Bulls. Asian Australas. J. Anim. Sci. 2014, 27, 488–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, M.; Cardozo, P.W.; Calsamiglia, S.; Fievez, V. Effects of saponins, quercetin, eugenol, and cinnamaldehyde on fatty acid biohydrogenation of forage polyunsaturated fatty acids in dual-flow continuous culture fermenters1. J. Anim. Sci. 2008, 86, 3045–3053. [Google Scholar] [CrossRef]
- Li, Z.T.; Lin, T.; Shen, J.X.; Chen, W.Y.; Sun, C. Effect of rosemary on antibacterial and freshness of cold meat. Food Res. Dev. 2017, 38, 181–186. [Google Scholar]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation technologies for fresh meat—A review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Kostadinović, L.; Lević, J.; Popović, S.T.; Čabarkapa, I.; Puvača, N.; Djuragic, O.; Kormanjoš, S. Dietary inclusion of Artemisia absinthium for management of growth performance, antioxidative status and quality of chicken meat. Eur. Poult. Sci. 2015, 79. [Google Scholar] [CrossRef]
- Hęś, M. Protein-lipid interactions in different meat systems in the presence of natural antioxidants—A review. Polish J. Food Nutr. Sci. 2017, 67, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Mikulski, D.; Jankowski, J.; Zdun Czyk, Z.; Wróblewska, M.; Sartowska, K.; Majewska, T. The effect of selenium source on performance, carcass traits, oxidative status of the organism, and meat quality of turkeys. J. Anim. Feed Sci. 2009, 18, 518–530. [Google Scholar] [CrossRef] [Green Version]

| Composition | Amount |
|---|---|
| Ingredient (%, as-fed basis) | |
| Corn grain | 43.28 |
| Corn gluten feed | 11.81 |
| Wheat | 10.47 |
| Palm kernel expeller | 9.03 |
| Coconut meal | 8.29 |
| Lupin | 5.91 |
| Tapioca | 3.41 |
| Molasses | 3.05 |
| Rapeseed meal | 2.06 |
| Wheat flour | 1.97 |
| Soybean meal | 0.50 |
| Vitamin mineral premix A | 0.22 |
| Chemical composition (%DM) | |
| Total digestible nutrients (TDN) | 74.12 |
| Crude protein | 12.50 |
| Crude fat | 3.61 |
| Crude fiber | 6.70 |
| Crude ash | 5.48 |
| Calcium | 0.84 |
| Phosphorus | 0.36 |
| Neutral detergent fiber (NDF) | 21.70 |
| Acid detergent fiber (ADF) | 10.70 |
| Item | CON (1) | TRT (2) | SEM | p-Value |
|---|---|---|---|---|
| Initial BW | 647.00 | 652.00 | 22.01 | 0.32 |
| Final BW | 717.79 b | 751.93 a | 31.47 | 0.04 |
| Weight gain (kg) | 0.30 b | 0.42 a | 0.14 | 0.03 |
| Feed intake (kg/Day) | 11.32 | 11.43 | 0.22 | 0.51 |
| Item | CON (1) | TRT (2) | SEM | p-Value |
|---|---|---|---|---|
| Moisture (%) | 56.83 b | 63.89 a | 1.23 | 0.01 |
| Crude protein (%) | 22.48 b | 25.26 a | 0.80 | 0.03 |
| Crude fat (%) | 16.62 a | 12.45 b | 0.95 | 0.01 |
| Crude ash (%) | 1.18 | 1.29 | 0.05 | 0.14 |
| Cholesterol (mg/100 g) | 50.27 | 49.74 | 0.04 | 0.92 |
| Items | CON (1) | TRT (2) | SEM | p-Value |
|---|---|---|---|---|
| Yield traits | ||||
| Carcass weight (kg) | 446.68 | 449.57 | 20.46 | 0.87 |
| Loin area (cm2) | 100.50 | 103.33 | 4.42 | 0.67 |
| Back fat thickness (mm) | 16.67 | 18.33 | 2.81 | 0.68 |
| Quality traits | ||||
| Marbling score (3) | 4.83 | 6.33 | 0.78 | 0.20 |
| Meat color (4) | 4.83 | 4.83 | 0.17 | 1.00 |
| Fat color (5) | 3.17 | 2.83 | 0.19 | 0.18 |
| Texture (6) | 1.17 | 1.00 | 0.08 | 0.34 |
| Maturity (7) | 2.00 | 1.89 | 0.16 | 0.21 |
| Quality grade | 0:3:1:2:0 | 2:1:3:0:0 | – | – |
| Meat point (8) | 3.17 | 3.83 | 0.40 | 0.26 |
| Item | CON (1) | TRT (2) | SEM | p-Value |
|---|---|---|---|---|
| Meat color | ||||
| CIE L* | 29.04 | 29.21 | 0.73 | 0.87 |
| CIE a* | 14.94 b | 18.45 a | 0.81 | 0.03 |
| CIE b* | 3.58 | 5.87 | 0.70 | 0.07 |
| Drip loss (%) | 21.14 a | 16.48 b | 0.75 | 0.00 |
| Cooking loss (%) | 15.81 | 16.30 | 0.71 | 0.70 |
| WHC (%) | 12.81 | 13.30 | 1.26 | 0.78 |
| Shear force (kg) | 5.38 a | 3.93 b | 0.35 | 0.01 |
| Items | CON (1) | TRT (2) | SEM | p-Value |
|---|---|---|---|---|
| C10:0 | 0.03 | 0.04 | 0.00 | 0.08 |
| C12:0 | 0.08 | 0.08 | 0.00 | 0.69 |
| C14:0 | 3.35 | 3.39 | 0.11 | 0.80 |
| C15:0 | 0.20 b | 0.25 a | 0.01 | 0.008 |
| C16:0 | 26.65 a | 24.05 b | 0.60 | 0.04 |
| C17:0 | 0.92 a | 0.15 b | 0.03 | <0.0001 |
| C18:0 | 8.33 | 8.67 | 0.15 | 0.13 |
| C20:0 | 0.08 a | 0.07 b | 0.00 | 0.004 |
| C14:1 | 0.30 a | 0.25 b | 0.02 | 0.04 |
| C16:1 | 5.92 a | 5.62 b | 0.06 | 0.007 |
| C18:1 | 47.19 | 46.09 | 0.58 | 0.24 |
| C24:1 | 0.06 b | 0.08 a | 0.00 | 0.01 |
| C18:2 | 0.06 b | 0.10 a | 0.01 | 0.004 |
| C18:3 | 0.22 b | 0.36 a | 0.04 | 0.008 |
| C20:3 | 0.13 b | 0.16 a | 0.01 | 0.04 |
| C20:4 | 0.20 b | 0.26 a | 0.01 | 0.03 |
| ΣSFA (3) | 39.69 | 36.75 | 0.72 | 0.16 |
| ΣMUFA (4) | 53.46 | 52.04 | 0.58 | 0.13 |
| ΣPUFA (5) | 0.63 b | 0.88 a | 0.05 | 0.04 |
| ΣPUFA/SFA | 0.015 | 0.024 | 0.04 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.G.; Rathnayake, D.; Mun, H.S.; Dilawar, M.A.; Pov, S.; Yang, C.J. Sensory Attributes, Microbial Activity, Fatty Acid Composition and Meat Quality Traits of Hanwoo Cattle Fed a Diet Supplemented with Stevioside and Organic Selenium. Foods 2021, 10, 129. https://doi.org/10.3390/foods10010129
Shin YG, Rathnayake D, Mun HS, Dilawar MA, Pov S, Yang CJ. Sensory Attributes, Microbial Activity, Fatty Acid Composition and Meat Quality Traits of Hanwoo Cattle Fed a Diet Supplemented with Stevioside and Organic Selenium. Foods. 2021; 10(1):129. https://doi.org/10.3390/foods10010129
Chicago/Turabian StyleShin, Yong Geum, Dhanushka Rathnayake, Hong Seok Mun, Muhammad Ammar Dilawar, Sreynak Pov, and Chul Ju Yang. 2021. "Sensory Attributes, Microbial Activity, Fatty Acid Composition and Meat Quality Traits of Hanwoo Cattle Fed a Diet Supplemented with Stevioside and Organic Selenium" Foods 10, no. 1: 129. https://doi.org/10.3390/foods10010129
APA StyleShin, Y. G., Rathnayake, D., Mun, H. S., Dilawar, M. A., Pov, S., & Yang, C. J. (2021). Sensory Attributes, Microbial Activity, Fatty Acid Composition and Meat Quality Traits of Hanwoo Cattle Fed a Diet Supplemented with Stevioside and Organic Selenium. Foods, 10(1), 129. https://doi.org/10.3390/foods10010129