High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extrusion Trials
2.3. Cryo-Imaging
2.4. Micro-CT
2.5. Protein Solubility
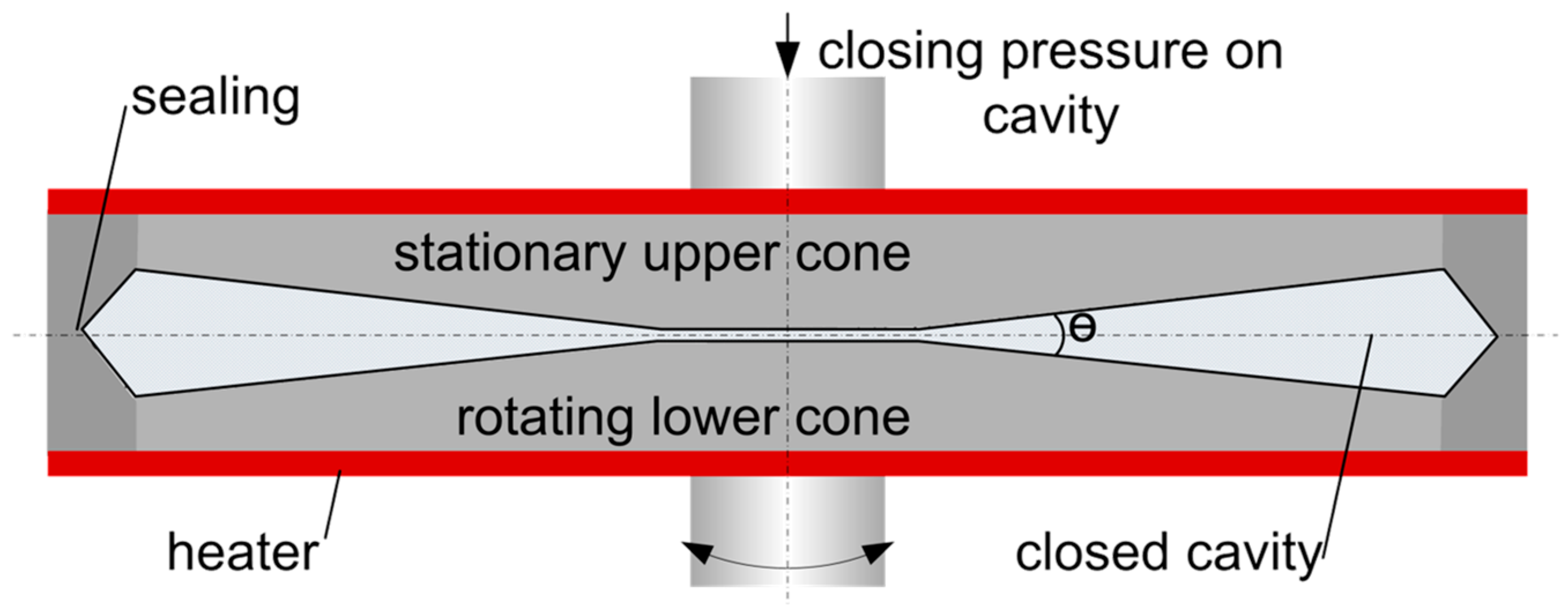
2.6. Rheological Measurements
2.7. Statistical Analysis
3. Results
3.1. Product Structure of Extruded Soy Protein
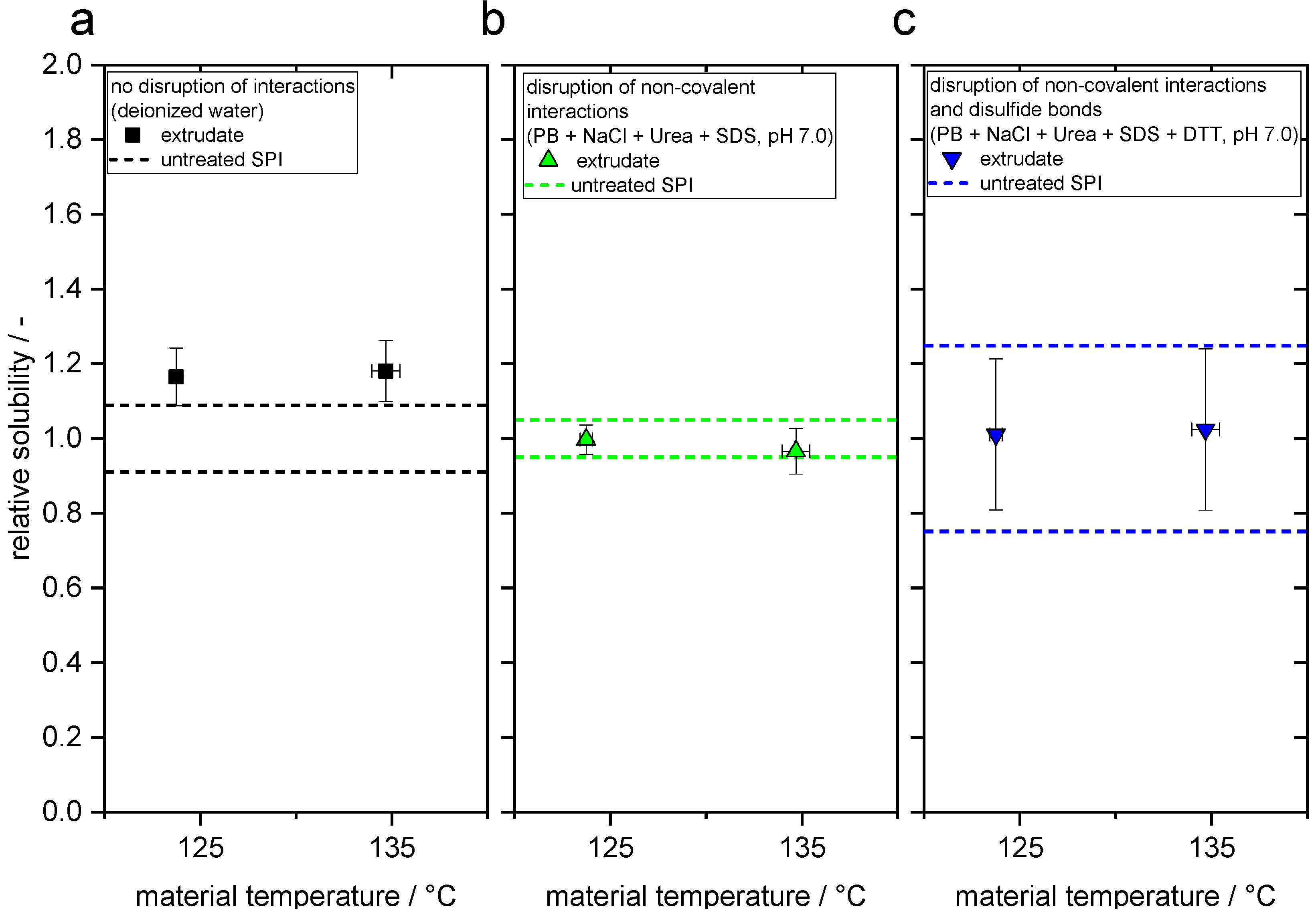
3.2. Changes in Protein–Protein Interactions through Extrusion Processing
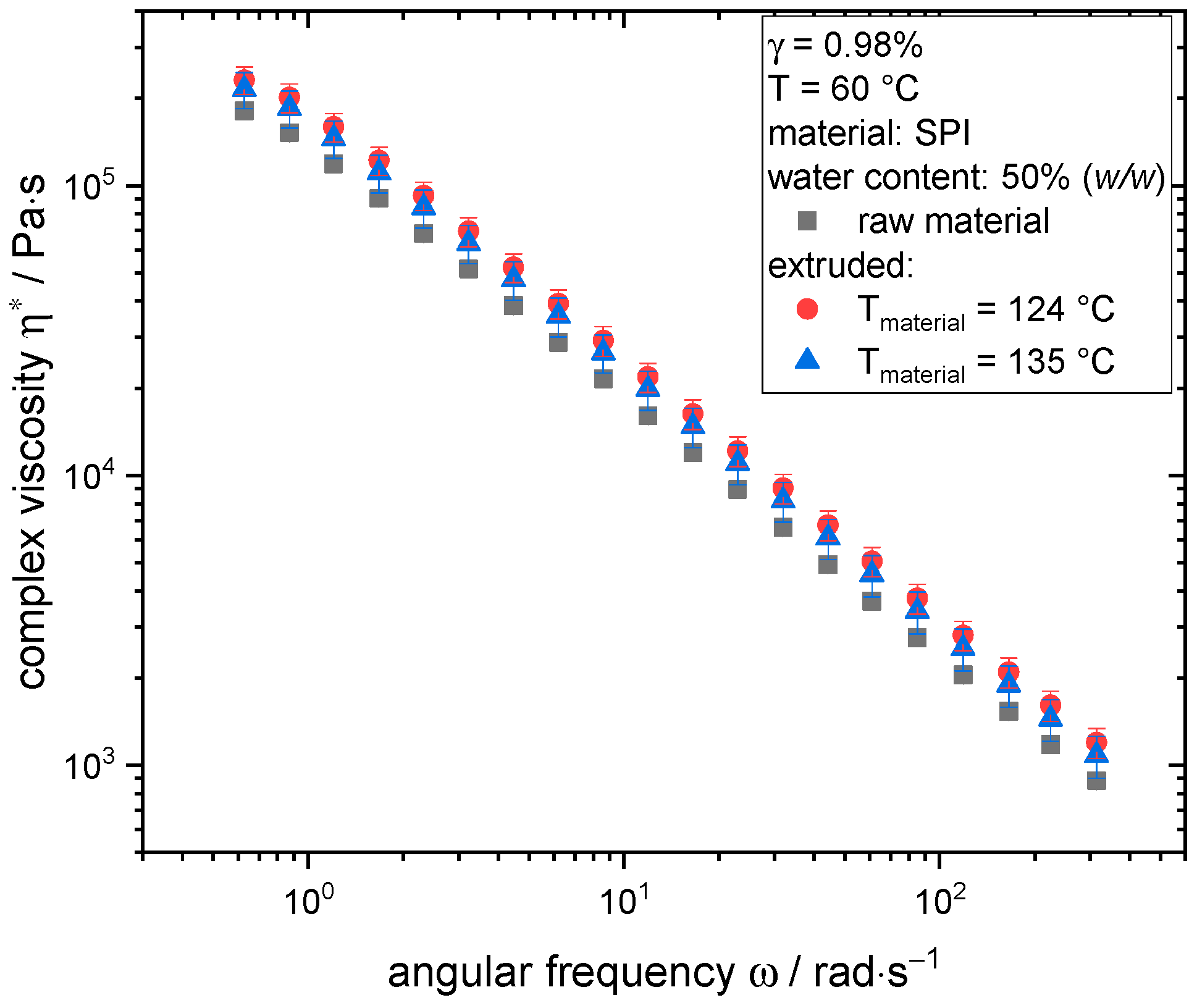
3.3. Changes in Rheological Properties through Extrusion Processing
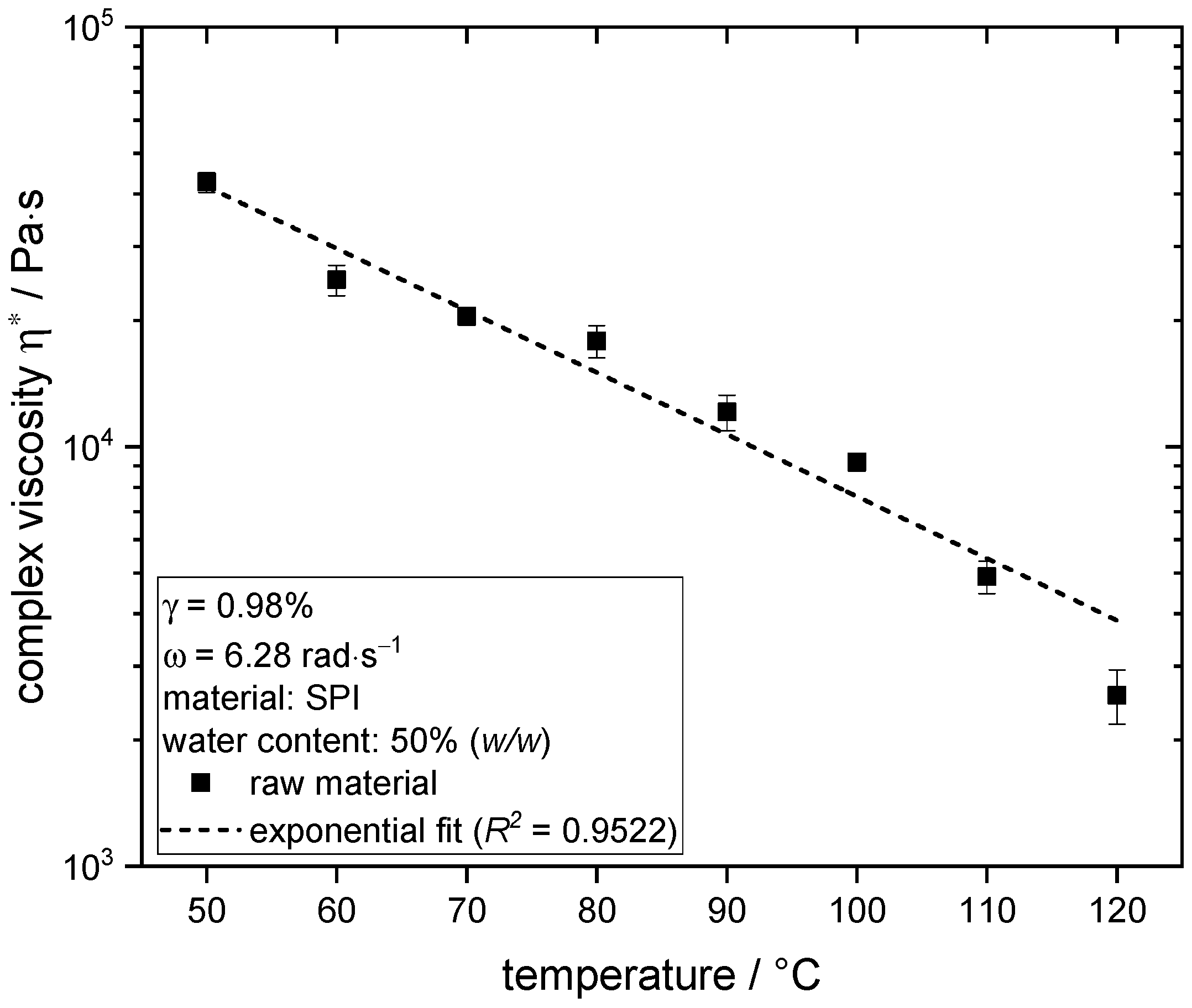
3.4. Influence of Temperature on Rheological Properties of Soy Protein
3.5. Influence of Processing Conditions on the Extrudate Microstructure
3.6. Water Distribution in Soy Protein Extrudates
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dwyer, J.T. Nutritional Consequences of Vegetarianism. Annu. Rev. Nutr. 1991, 11, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Demeyer, D.; Honikel, K.; De Smet, S. The World Cancer Research Fund report 2007: A challenge for the meat processing industry. Meat Sci. 2008, 80, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- McEachern, M.G.; Schröder, M.J.A. The Role of Livestock Production Ethics in Consumer Values Towards Meat. J. Agric. Environ. Ethics 2002, 15, 221–237. [Google Scholar] [CrossRef]
- Berndsen, M.; Van Der Pligt, J. Risks of meat: The relative impact of cognitive, affective and moral concerns. Appetite 2005, 44, 195–205. [Google Scholar] [CrossRef]
- Troy, D.; Kerry, J.P. Consumer perception and the role of science in the meat industry. Meat Sci. 2010, 86, 214–226. [Google Scholar] [CrossRef]
- Pimentel, D.; Houser, J.; Preiss, E.; White, O.; Fang, H.; Mesnick, L.; Barsky, T.; Tariche, S.; Schreck, J.; Alpert, S. Water Resources: Agriculture, the Environment, and Society. Bioscience 1997, 47, 97–106. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [CrossRef]
- Steinfeld, H. Livestock’s long Shadow. Environmental Issues and Options; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISBN 9789251055717. [Google Scholar]
- Aaslyng, M.D.; Oksama, M.; Olsen, E.V.; Bejerholm, C.; Baltzer, M.; Andersen, G.; Bredie, W.L.P.; Byrne, D.V.; Gabrielsen, G. The impact of sensory quality of pork on consumer preference. Meat Sci. 2007, 76, 61–73. [Google Scholar] [CrossRef]
- Huffman, K.L.; Miller, M.F.; Hoover, L.C.; Wu, C.K.; Brittin, H.C.; Ramsey, C.B. Effect of beef tenderness on consumer satisfaction with steaks consumed in the home and restaurant. J. Anim. Sci. 1996, 74, 91–97. [Google Scholar] [CrossRef]
- Hoek, A.C.; Luning, P.A.; Weijzen, P.; Engels, W.; Kok, F.J.; De Graaf, C. Replacement of meat by meat substitutes. A survey on person- and product-related factors in consumer acceptance. Appetite 2011, 56, 662–673. [Google Scholar] [CrossRef]
- Elzerman, J.E.; Hoek, A.C.; Van Boekel, M.A.; Luning, P.A. Consumer acceptance and appropriateness of meat substitutes in a meal context. Food Qual. Prefer. 2011, 22, 233–240. [Google Scholar] [CrossRef]
- Furnols, M.F.I.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.L. Method for Preparing a Protein Product. U.S. Patent 3496858A, 9 December 1966. [Google Scholar]
- Atkinson, W.T. Meat-Like Protein Food Product. U.S. Patent 3488770A, 7 March 1969. [Google Scholar]
- Rakosky, J. Soy products for the meat industry. J. Agric. Food Chem. 1970, 18, 1005–1009. [Google Scholar] [CrossRef]
- Cumming, D.; Stanley, D.; Deman, J. Texture—Strueture Relationships in Texturized Soy Protein II. Textural Properties and Ultrastructure of an Extruded Soybean Product. Can. Inst. Food Sci. Technol. J. 1972, 5, 124–128. [Google Scholar] [CrossRef]
- Aguilera, J.M.; Kosikowski, F.V. Soybean extruded product: A response surface analysis. J. Food Sci. 1976, 41, 647–651. [Google Scholar] [CrossRef]
- Buckle, T.S.; Zapata, L.E.; Sanoval, A.M.; Ben-Gera, I.; Shomer, I. Relationship between structure and texture of extruded soy and cottonseed flour products. J. Food Qual. 1977, 1, 253–266. [Google Scholar] [CrossRef]
- Taranto, M.V.; Cegla, G.F.; Bell, K.R.; Rhee, K.C. Textured cottonseed and soy flours: A microscopic analysis. J. Food Sci. 1978, 43, 767–771. [Google Scholar] [CrossRef]
- Puski, G.; Konwinski, A.H. Process of Making a Soy-Based Meat Substitute. U.S. Patent 3950564A, 2 August 1974. [Google Scholar]
- Kitabatake, N.; Mégard, D.; Cheftel, J.C. Continuous Gel Formation by HTST Extrusion-Cooking: Soy Proteins. J. Food Sci. 1985, 50, 1260–1265. [Google Scholar] [CrossRef]
- Gwiazda, S.; Noguchi, A.; Saio, K. Microstructural studies of texturized vegetable protein products: Effects of oil addition and transformation of raw materials in various sections of a twin screw extruder. Food Struct. 1987, 6, 57–61. [Google Scholar]
- Isobe, S.; Noguchi, A. High moisture extrusion with twin screw extruder—Fate of soy protein during the repetition of extrusion cooking. Nippon Shokuhin Kogyo Gakkaishi 1987, 34, 456–461. [Google Scholar] [CrossRef]
- Sheard, P.R.; Mitchell, J.R.; Ledward, D.A. Extrusion behaviour of different soya isolates and the effect of particle size. Int. J. Food Sci. Technol. 1986, 21, 627–641. [Google Scholar] [CrossRef]
- Liu, K.S.; Hsieh, F.-H. Protein–Protein Interactions in High Moisture-Extruded Meat Analogs and Heat-Induced Soy Protein Gels. J. Am. Oil Chem. Soc. 2007, 84, 741–748. [Google Scholar] [CrossRef]
- Osen, R.; Toelstede, S.; Wild, F.; Eisner, P.; Schweiggert-Weisz, U. High moisture extrusion cooking of pea protein isolates: Raw material characteristics, extruder responses, and texture properties. J. Food Eng. 2014, 127, 67–74. [Google Scholar] [CrossRef]
- Burgess, L.; Stanley, D. A Possible Mechanism for Thermal Texturization of Soybean Protein. Can. Inst. Food Sci. Technol. J. 1976, 9, 228–231. [Google Scholar] [CrossRef]
- Jeunink, J.; Cheftel, J.C. Chemical and physicochemical changes in field bean and soybean proteins texturized by extrusion. J. Food Sci. 1979, 44, 1322–1325. [Google Scholar] [CrossRef]
- Hager, D.F. Effects of extrusion upon soy concentrate solubility. J. Agric. Food Chem. 1984, 32, 293–296. [Google Scholar] [CrossRef]
- Baird, D.G. The effect of heat and shear on the viscoelastic properties of soy flour dough. J. Food Process. Eng. 1982, 5, 231–245. [Google Scholar] [CrossRef]
- Sheard, P.R.; Ledward, D.A.; Mitchell, J.R. Role of carbohydrates in soya extrusion. Int. J. Food Sci. Technol. 2007, 19, 475–483. [Google Scholar] [CrossRef]
- Sheard, P.R.; Fellows, A.; Ledward, D.A.; Mitchell, J.R. Macromolecular changes associated with the heat treatment of soya isolate. Int. J. Food Sci. Technol. 2007, 21, 55–60. [Google Scholar] [CrossRef]
- Prudencio, S.H.; Arêas, J.G. Protein-Protein Interactions in the Extrusion of Soya at Various Temperatures and Moisture Contents. J. Food Sci. 1993, 58, 378–381. [Google Scholar] [CrossRef]
- Simonsky, R.; Stanley, D. Texture-Structure Relationships in Textured Soy Protein. V. Influence of pH and Protein Acylation on Extrusion Texturization. Can. Inst. Food Sci. Technol. J. 1982, 15, 294–301. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.; Hsieh, F. Texture and Chemical Characteristics of Soy Protein Meat Analog Extruded at High Moisture. J. Food Sci. 2000, 65, 264–269. [Google Scholar] [CrossRef]
- Liu, K.; Hsieh, F.-H. Protein–Protein Interactions during High-Moisture Extrusion for Fibrous Meat Analogues and Comparison of Protein Solubility Methods Using Different Solvent Systems. J. Agric. Food Chem. 2008, 56, 2681–2687. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Chemical cross-linking and molecular aggregation of soybean protein during extrusion cooking at low and high moisture content. LWT 2011, 44, 957–962. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y. Effects of the specific mechanical energy on the physicochemical properties of texturized soy protein during high-moisture extrusion cooking. J. Food Eng. 2014, 121, 32–38. [Google Scholar] [CrossRef]
- Samard, S.; Gu, B.; Ryu, G.-H. Effects of extrusion types, screw speed and addition of wheat gluten on physicochemical characteristics and cooking stability of meat analogues. J. Sci. Food Agric. 2019, 99, 4922–4931. [Google Scholar] [CrossRef]
- Zahari, I.; Ferawati, F.; Helstad, A.; Ahlström, C.; Östbring, K.; Rayner, M.; Purhagen, J. Development of High-Moisture Meat Analogues with Hemp and Soy Protein Using Extrusion Cooking. Foods 2020, 9, 772. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Functional properties of food proteins and role of protein-polysaccharide interaction. Food Hydrocoll. 1991, 4, 429–468. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Thermoplastic extrusion-the mechanism of the formation of extrudate structure and properties. J. Am. Oil Chem. Soc. 1993, 70, 417–424. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B.; Grinberg, V.Y.; Gurov, A.N. Some physicochemical approaches to the problem of protein texturization. J. Agric. Food Chem. 1985, 33, 151–159. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Creation of Fibrous Structures by Spinneretless Spinning. In Food Structure: Its Creation and Evaluation; Symposium; 44th Easter School in Agricultural Science; Papers; Mitchell, J.R., Blanshard, J.M.V., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 1987; pp. 181–196. ISBN 9781855733961. [Google Scholar]
- Tolstoguzov, V. Some physico-chemical aspects of protein processing into foodstuffs. Food Hydrocoll. 1988, 2, 339–370. [Google Scholar] [CrossRef]
- Krause, S. Polymer Compatibility. J. Macromol. Sci. Part C 1972, 7, 251–314. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Hamoen, R.; Boom, R.M.; Van Der Goot, A.J. Understanding fiber formation in a concentrated soy protein isolate—Pectin blend. J. Food Eng. 2018, 222, 84–92. [Google Scholar] [CrossRef]
- Schreuders, F.K.; Dekkers, B.L.; Bodnár, I.; Erni, P.; Boom, R.M.; Van Der Goot, A.J. Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J. Food Eng. 2019, 261, 32–39. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. (Eds.) History of Modern Soy Protein Ingredients. Isolates, Concentrates, and Textured Soy Protein Products (1911–2016): Extensively Annotated Bibliography and Sourcebook; Soyinfo Center: Lafayette, CA, USA, 2016; ISBN 1928914837. [Google Scholar]
- Wolf, W.J. Soybean proteins. Their functional, chemical, and physical properties. J. Agric. Food Chem. 1970, 18, 969–976. [Google Scholar] [CrossRef]
- Preece, K.; Hooshyar, N.; Zuidam, N. Whole soybean protein extraction processes: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef]
- Belter, P.A.; Smith, A.K. Protein denaturation in soybean meal during processing. J. Am. Oil Chem. Soc. 1952, 29, 170–174. [Google Scholar] [CrossRef]
- Cogan, U.; Yaron, A.; Berk, Z.; Mizrahi, S. Isolation of soybean protein: Effect of processing conditions on yields and purity. J. Am. Oil Chem. Soc. 1967, 44, 321–324. [Google Scholar] [CrossRef]
- Moure, A.; Sineiro, J.; Domínguez, H.; Parajó, J.C. Functionality of oilseed protein products: A review. Food Res. Int. 2006, 39, 945–963. [Google Scholar] [CrossRef]
- Añón, M.C.; Sorgentini, D.A.; Wagner, J.R. Relationships between Different Hydration Properties of Commercial and Laboratory Soybean Isolates. J. Agric. Food Chem. 2001, 49, 4852–4858. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Wei, Y.M.; Zhang, B. Characterization of water state and distribution in textured soybean protein using DSC and NMR. J. Food Eng. 2010, 100, 522–526. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System parameters and product properties response of soybean protein extruded at wide moisture range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Fang, Y.; Zhang, B.; Wei, Y.; Li, S. Effects of specific mechanical energy on soy protein aggregation during extrusion process studied by size exclusion chromatography coupled with multi-angle laser light scattering. J. Food Eng. 2013, 115, 220–225. [Google Scholar] [CrossRef]
- Lin, S.; Huff, H.E.; Hsieh, F. Extrusion Process Parameters, Sensory Characteristics, and Structural Properties of a High Moisture Soy Protein Meat Analog. J. Food Sci. 2002, 67, 1066–1072. [Google Scholar] [CrossRef]
- Park, J.-H.; Chatpaisarn, A.; Ryu, G.-H. Effects of Gluten and Moisture Contents on Texturization of Extruded Soy Protein Isolate. J. Korean Soc. Food Sci. Nutr. 2017, 46, 473–480. [Google Scholar] [CrossRef]
- Mégard, D.; Kitabatake, N.; Cheftel, J.C. Continuous Restructuring of Mechanically Deboned Chicken Meat by HTST Extrusion-Cooking. J. Food Sci. 1985, 50, 1364–1369. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, H.S.; Hu, Y.H. Extruder Responses, Textural Properties and Color of Meat Analogs Made by High Moisture Soybean Residue and Soy Protein Isolate. Adv. Mater. Res. 2013, 1670–1676. [Google Scholar] [CrossRef]
- Dekkers, B.L.; De Kort, D.W.; Grabowska, K.J.; Tian, B.; Van As, H.; Van Der Goot, A.J. A combined rheology and time domain NMR approach for determining water distributions in protein blends. Food Hydrocoll. 2016, 60, 525–532. [Google Scholar] [CrossRef]
- Schreuders, F.; Bodnár, I.; Erni, P.; Boom, R.M.; Van Der Goot, A.J. Water redistribution determined by time domain NMR explains rheological properties of dense fibrous protein blends at high temperature. Food Hydrocoll. 2020, 101, 105562. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Emin, M.A.; Schuchmann, H.P. Process conditions influencing wheat gluten polymerization during high moisture extrusion of meat analog products. J. Food Eng. 2017, 198, 28–35. [Google Scholar] [CrossRef]
- Pietsch, V.L.; Bühler, J.M.; Karbstein, H.P.; Emin, M. High moisture extrusion of soy protein concentrate: Influence of thermomechanical treatment on protein-protein interactions and rheological properties. J. Food Eng. 2019, 251, 11–18. [Google Scholar] [CrossRef]
- Zuber, M.; Laaß, M.; Hamann, E.; Kretschmer, S.; Hauschke, N.; Van De Kamp, T.; Baumbach, T.; Koenig, T. Augmented laminography, a correlative 3D imaging method for revealing the inner structure of compressed fossils. Sci. Rep. 2017, 7, 41413. [Google Scholar] [CrossRef] [PubMed]
- Siebert, T.; Zuber, M.; Hamann, E.; Baumbach, T.; Karbstein, H.P.; Gaukel, V. Micro-CT visualization of structure development during freeze-drying processes. Dry. Technol. 2019, 38, 376–384. [Google Scholar] [CrossRef]
- Emin, M.; Quevedo, M.; Wilhelm, M.; Karbstein, H. Analysis of the reaction behavior of highly concentrated plant proteins in extrusion-like conditions. Innov. Food Sci. Emerg. Technol. 2017, 44, 15–20. [Google Scholar] [CrossRef]
- Wittek, P.; Zeiler, N.; Karbstein, H.P.; Emin, M.A. Analysis of the complex rheological properties of highly concentrated proteins with a closed cavity rheometer. Appl. Rheol. 2020, 30, 64–76. [Google Scholar] [CrossRef]
- Akdogan, H. High moisture food extrusion. Int. J. Food Sci. Technol. 1999, 34, 195–207. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Böcker, L.; Müssner, C.; Stirnemann, E.; Haberkorn, I.; Adelmann, H.; Handschin, S.; Windhab, E.J.; Mathys, A. Extruded meat analogues based on yellow, heterotrophically cultivated Auxenochlorella protothecoides microalgae. Innov. Food Sci. Emerg. Technol. 2020, 59, 102275. [Google Scholar] [CrossRef]
- Cheftel, J.C.; Kitagawa, M.; Quéguiner, C. New protein texturization processes by extrusion cooking at high moisture levels. Food Rev. Int. 1992, 8, 235–275. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liu, H.; Yoon, A.; Rizvi, S.S.H.; Wang, Q. Changes in conformation and quality of vegetable protein during texturization process by extrusion. Crit. Rev. Food Sci. Nutr. 2019, 59, 3267–3280. [Google Scholar] [CrossRef]
- Arrese, E.L.; Sorgentini, D.A.; Wagner, J.R.; Anon, M.C. Electrophoretic, solubility and functional properties of commercial soy protein isolates. J. Agric. Food Chem. 1991, 39, 1029–1032. [Google Scholar] [CrossRef]
- Wagner, J.R.; Sorgentini, D.A.; Añón, M.C. Relation between Solubility and Surface Hydrophobicity as an Indicator of Modifications during Preparation Processes of Commercial and Laboratory-Prepared Soy Protein Isolates. J. Agric. Food Chem. 2000, 48, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Berry, G.C.; Fox, T. The viscosity of polymers and their concentrated solutions. In Fortschritte der Hochpolymeren-Forschung; Springer: Berlin/Heidelberg, Germany, 1968; pp. 261–357. ISBN 3-540-04032-3. [Google Scholar]
- Baird, D.G. Dynamic viscoelastic properties of soy isolate doughs. J. Texture Stud. 1981, 12, 1–16. [Google Scholar] [CrossRef]
- Quevedo, M.; Jandt, U.; Kulozik, U.; Karbstein, H.P.; Emin, M. Investigation on the influence of high protein concentrations on the thermal reaction behaviour of β-lactoglobulin by experimental and numerical analyses. Int. Dairy J. 2019, 97, 99–110. [Google Scholar] [CrossRef]
- Hayashi, N.; Noma, K.; Hayakawa, I.; Fujio, Y. Influence of time-temperature history and strain history on the melt rheology of soy protein isolate at an elevated temperature. Int. J. Food Sci. Technol. 2007, 27, 297–304. [Google Scholar] [CrossRef]
- Dekkers, B.L.; Boom, R.M.; Van Der Goot, A.J. Structuring processes for meat analogues. Trends Food Sci. Technol. 2018, 81, 25–36. [Google Scholar] [CrossRef]
- Chen, A.H.; Jao, Y.C.; Larkin, J.W.; Goldstein, W.E. Rheological model of soy dough in extrusion. J. Food Process. Eng. 1978, 2, 337–342. [Google Scholar] [CrossRef]
- Wang, J.-S.; Porter, R.S. On the viscosity-temperature behavior of polymer melts. Rheol. Acta 1995, 34, 496–503. [Google Scholar] [CrossRef]
- Murillo, J.S.; Osen, R.; Hiermaier, S.; Ganzenmüller, G.C. Towards understanding the mechanism of fibrous texture formation during high-moisture extrusion of meat substitutes. J. Food Eng. 2019, 242, 8–20. [Google Scholar] [CrossRef]
- Siebert, T.; Becker, A.; Bunzel, M.; Zuber, M.; Hamann, E.; Baumbach, T.; Karbstein, H.P.; Gaukel, V. Evaluation of the usefulness of serial combination processes for drying of apples. Dry. Technol. 2019, 38, 1274–1290. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Jiang, Y.; Faisal, S.; Wei, L.; Cao, C.; Yan, W.; Wang, Q. Converting Peanut Protein Biomass Waste into “Double Green” Meat Substitutes Using a High-Moisture Extrusion Process: A Multiscale Method to Explore a Process for Forming a Meat-Like Fibrous Structure. J. Agric. Food Chem. 2019, 67, 10713–10725. [Google Scholar] [CrossRef] [PubMed]
- Osen, R.; Schweiggert-Weisz, U. High-Moisture Extrusion: Meat Analogues. In Reference Module in Food Science; Smithers, G.W., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Palanisamy, M.; Töpfl, S.; Aganovic, K.; Berger, R.G. Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT 2018, 87, 546–552. [Google Scholar] [CrossRef]
- Yuryev, V.P.; Zasypkin, D.V.; Alexeyev, V.V.; Genin, Y.V.; Ezernitskaya, M.G.; Tolstoguzov, V.B. Structure of protein texturates obtained by thermoplastic extrusion. Food/Nahrung 1990, 34, 607–613. [Google Scholar] [CrossRef]


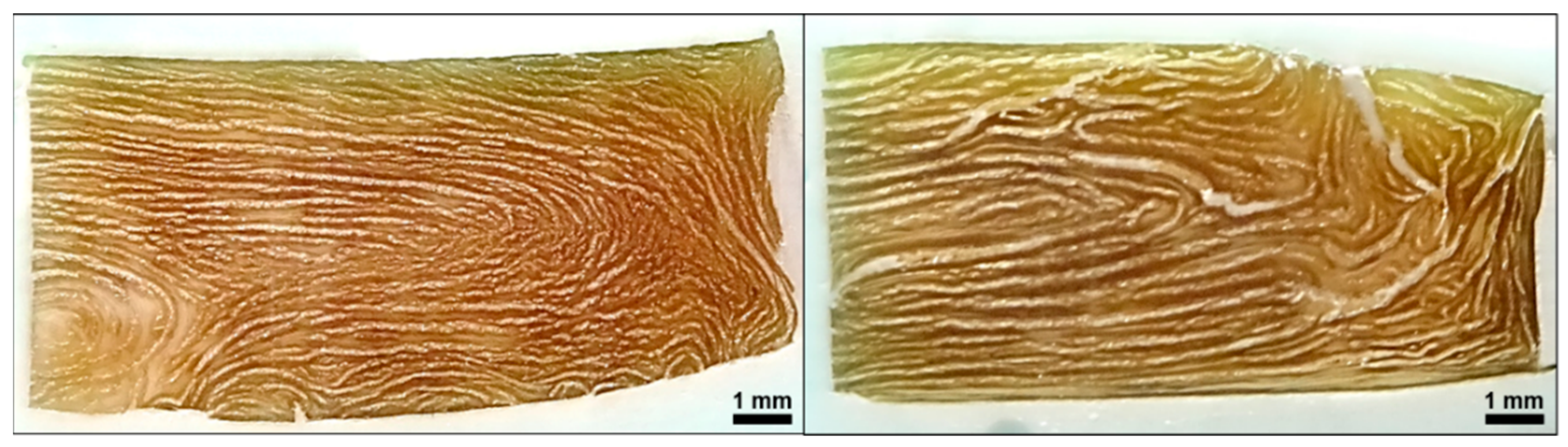

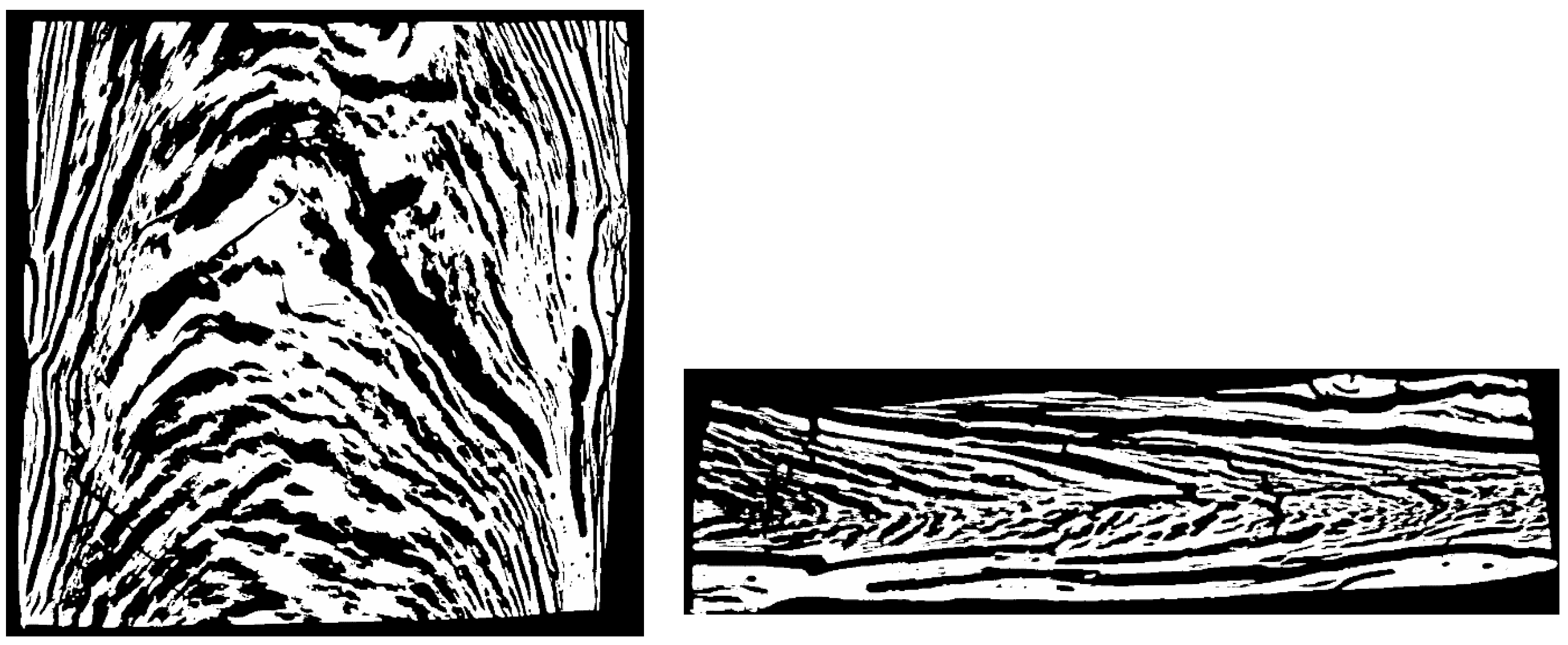
| Setting No. | 1 | 2 |
|---|---|---|
| Material temperature/°C | 124 | 135 |
| Temperature barrel 2/°C | 40 | 40 |
| Temperature barrel 3/°C | 60 | 60 |
| Temperature barrel 4/°C | 80 | 90 |
| Temperature barrel 5/°C | 110 | 130 |
| Temperature barrel 6/°C | 123 | 135 |
| Temperature barrel 7/°C | 124 | 140 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wittek, P.; Zeiler, N.; Karbstein, H.P.; Emin, M.A. High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure. Foods 2021, 10, 102. https://doi.org/10.3390/foods10010102
Wittek P, Zeiler N, Karbstein HP, Emin MA. High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure. Foods. 2021; 10(1):102. https://doi.org/10.3390/foods10010102
Chicago/Turabian StyleWittek, Patrick, Nicole Zeiler, Heike P. Karbstein, and M. Azad Emin. 2021. "High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure" Foods 10, no. 1: 102. https://doi.org/10.3390/foods10010102
APA StyleWittek, P., Zeiler, N., Karbstein, H. P., & Emin, M. A. (2021). High Moisture Extrusion of Soy Protein: Investigations on the Formation of Anisotropic Product Structure. Foods, 10(1), 102. https://doi.org/10.3390/foods10010102





