Association between Periodontitis and Genetic Polymorphisms in Interleukins among Patients with Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
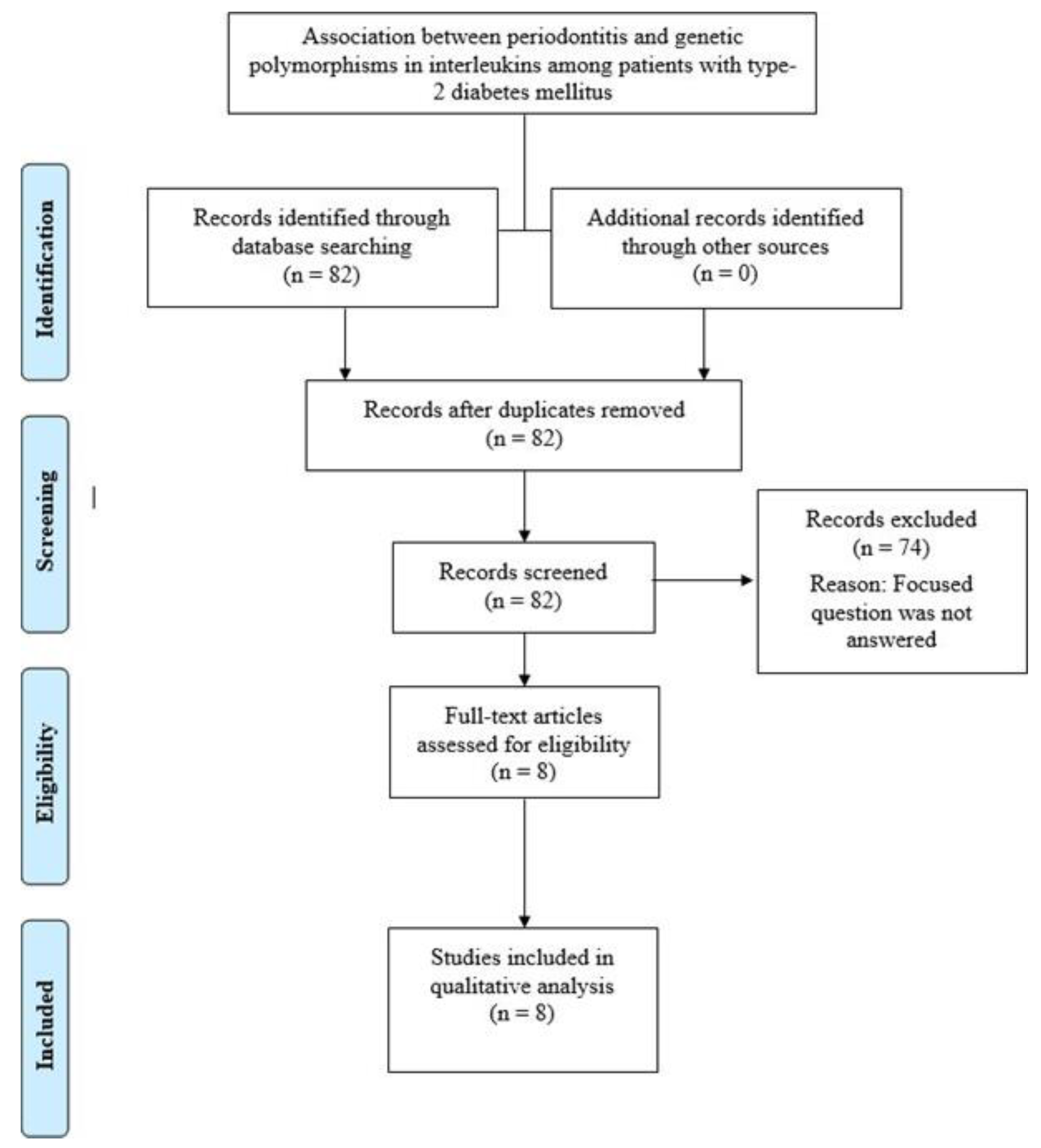
2.2. Eligibility Criteria and Literature Search
3. Results
3.1. General Characteristics
3.2. Outcomes
3.3. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bascones-Martínez, A.; Muñoz-Corcuera, M.; Bascones-Ilundain, J. Diabetes and periodontitis: A bidirectional relationship. Med. Clin. 2015, 145, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Papapanou, P.N. Diabetes mellitus and periodontitis: A tale of two common interrelated diseases. Nat. Rev. Endocrinol. 2011, 7, 738–748. [Google Scholar] [CrossRef] [PubMed]
- Alasqah, M.; Mokeem, S.; Alrahlah, A.; Al-Hamoudi, N.; Abduljabbar, T.; Akram, Z.; Vohra, F.; Javed, F. Periodontal parameters in prediabetes, type 2 diabetes mellitus, and non-diabetic patients. Braz. Oral Res. 2018, 32, e81. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Askar, M.; Al-Hezaimi, K. Cytokine profile in the gingival crevicular fluid of periodontitis patients with and without type 2 diabetes: A literature review. J. Periodontol. 2012, 83, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Al-Kheraif, A.A.; Salazar-Lazo, K.; Yanez-Fontenla, V.; Aldosary, K.M.; Alshehri, M.; Malmstrom, H.; Romanos, G.E. Periodontal inflammatory conditions among smokers and never-smokers with and without type 2 diabetes mellitus. J. Periodontol. 2015, 86, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Näsström, K.; Benchimol, D.; Altamash, M.; Klinge, B.; Engström, P.E. Comparison of periodontal and socioeconomic status between subjects with type 2 diabetes mellitus and non-diabetic controls. J. Periodontol. 2007, 78, 2112–2119. [Google Scholar] [CrossRef]
- Mealey, B.L.; Oates, T.W. Diabetes mellitus and periodontal diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef]
- Graves, D.T.; Ding, Z.; Yang, Y. The impact of diabetes on periodontal diseases. Periodontol. 2000 2020, 82, 214–224. [Google Scholar] [CrossRef]
- Gurav, A.N. Advanced glycation end products: A link between periodontitis and diabetes mellitus? Curr. Diabetes Rev. 2013, 9, 355–361. [Google Scholar] [CrossRef]
- Akram, Z.; Alqahtani, F.; Alqahtani, M.; Al-Kheraif, A.A.; Javed, F. Levels of advanced glycation end products in gingival crevicular fluid of chronic periodontitis patients with and without type-2 diabetes mellitus. J. Periodontol. 2020, 91, 396–402. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, S.; Albiero, M.L.; Vieira, G.H.A.; Wang, J.; Feng, J.Q.; Graves, D.T. Diabetes Activates Periodontal Ligament Fibroblasts via NF-κB In Vivo. J. Dent. Res. 2018, 97, 580–588. [Google Scholar] [CrossRef]
- Zhao, B.; Li, R. The association between periodontitis and interleukin-6 genetic polymorphism-174 G/C: A meta-analysis. Arch. Oral Biol. 2018, 96, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.W.; Li, J.C.; Zhang, M.; Wang, J.Z.; Li, B.H.; Liu, Y.; Liao, S.Q.; Zhang, M.J.; Gao, C.Y.; Zhang, L.L. Influence of interleukin-6 gene -174G>C polymorphism on development of atherosclerosis: A meta-analysis of 50 studies involving 33,514 subjects. Gene 2013, 529, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Borilova Linhartova, P.; Poskerova, H.; Tomandlova, M.; Bartova, J.; Kankova, K.; Fassmann, A.; Izakovicova Holla, L. Interleukin-1 gene variability and plasma levels in czech patients with chronic periodontitis and diabetes mellitus. Int. J. Dent. 2019, 2019, 6802349, PMC6348895. [Google Scholar] [CrossRef] [PubMed]
- López, N.J.; Valenzuela, C.Y.; Jara, L. Interleukin-1 gene cluster polymorphisms associated with periodontal disease in type 2 diabetes. J. Periodontol. 2009, 80, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, L.; Vijayshree Priyadharshini, J.; Sivapathasundharam, B. Association among interleukin-6 gene polymorphisms, type 2 diabetes mellitus, and chronic periodontitis: A pilot study. J. Investig. Clin. Dent. 2017, 8, e12230. [Google Scholar] [CrossRef] [PubMed]
- Deppe, H.; Mücke, T.; Wagenpfeil, S.; Kesting, M.; Karl, J.; Noe, S.; Sculean, A. Are selected IL-1 polymorphisms and selected subgingival microorganisms significantly associated to periodontitis in type 2 diabetes patients? A clinical study. BMC Oral Health 2015, 15, 143. [Google Scholar] [CrossRef]
- Cirelli, T.; Nepomuceno, R.; Rios, A.C.S.; Orrico, S.R.P.; Cirelli, J.A.; Theodoro, L.H.; Barros, S.P.; Scarel-Caminaga, R.M. Genetic polymorphisms in the Interleukins IL1B, IL4, and IL6 are associated with concomitant periodontitis and type 2 diabetes mellitus in Brazilian patients. J. Periodontal. Res. 2020, 55, 918–930. [Google Scholar] [CrossRef]
- Petrovic, S.M.; Nikolic, N.; Toljic, B.; Arambasic-Jovanovic, J.; Milicic, B.; Milicic, T.; Jotic, A.; Vidakovic, M.; Milasin, J.; Pucar, A. The association of tumor necrosis factor alpha, lymphotoxin alpha, tumor necrosis factor receptor 1 and tumor necrosis factor receptor 2 gene polymorphisms and serum levels with periodontitis and type 2 diabetes in Serbian population. Arch. Oral Biol. 2020, 120, 104929. [Google Scholar] [CrossRef]
- Xiao, L.M.; Yan, Y.X.; Xie, C.J.; Fan, W.H.; Xuan, D.Y.; Wang, C.X.; Chen, L.; Sun, S.Y.; Xie, B.Y.; Zhang, J.C. Association among interleukin-6 gene polymorphism, diabetes and periodontitis in a Chinese population. Oral Dis. 2009, 15, 547–553. [Google Scholar] [CrossRef]
- Raunio, T.; Knuuttila, M.; Hiltunen, L.; Karttunen, R.; Vainio, O.; Tervonen, T. IL-6(-174) genotype associated with the extent of periodontal disease in type 1 diabetic subjects. J. Clin. Periodontol. 2009, 36, 11–17. [Google Scholar] [CrossRef]
- Struch, F.; Dau, M.; Schwahn, C.; Biffar, R.; Kocher, T.; Meisel, P. Interleukin-1 gene polymorphism, diabetes, and periodontitis: Results from the Study of Health in Pomerania (SHIP). J. Periodontol. 2008, 79, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Jin, Y.; Miao, Y.; Wang, Y.; Zhou, Y.; Lin, X. IL-10 secreting B cells regulate periodontal immune response during periodontitis. Odontology 2020, 108, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.R.P.; Vasconcelos, A.; de Carvalho França, L.F.; Di Lenardo, D.; Nascimento, H.M.S.; Vasconcelos, D.F.P. Association between the rs1143634 polymorphism in interleukin-1B and chronic periodontitis: Results from a meta-analysis composed by 54 case/control studies. Gene 2018, 668, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhao, L.; Sun, Y.; Li, L.; Xu, Y. Interleukin-1 receptor antagonist polymorphism (rs2234663) and periodontitis susceptibility: A meta-analysis. Arch. Oral Biol. 2012, 57, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Toker, H.; Görgün, E.P.; Korkmaz, E.M. Analysis of IL-6, IL-10 and NF-κB Gene Polymorphisms in Aggressive and Chronic Periodontitis. Cent. Eur. J. Public Health 2017, 25, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Downs, S.H.; Black, N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Linares-Espinós, E.; Hernández, V.; Domínguez-Escrig, J.L.; Fernández-Pello, S.; Hevia, V.; Mayor, J.; Padilla-Fernández, B.; Ribal, M.J. Methodology of a systematic review. Actas Urol. Esp. 2018, 42, 499–506. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Mehmood, A.; Saeed, A.; Al-Hezaimi, K.; Samaranayake, L.P. Association between glycemic status and oral Candida carriage in patients with prediabetes. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 53–58. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Saeed, A.; Mehmood, A.; Bain, C. Whole salivary interleukin-6 and matrix metalloproteinase-8 levels in patients with chronic periodontitis with and without prediabetes. J. Periodontol. 2014, 85, e130–e135. [Google Scholar] [CrossRef]
- Javed, F.; Al-Kheraif, A.A.; Al Amri, M.D.; Alshehri, M.; Vohra, F.; Al-Askar, M.; Malmstrom, H.; Romanos, G.E. Periodontal Status and Whole Salivary Cytokine Profile among Smokers and Never-Smokers With and Without Prediabetes. J. Periodontol. 2015, 86, 890–898. [Google Scholar] [CrossRef]
- Javed, F.; Sundin, U.; Altamash, M.; Klinge, B.; Engström, P.E. Self-perceived oral health and salivary proteins in children with type 1 diabetes. J. Oral Rehabil. 2009, 36, 39–44. [Google Scholar] [CrossRef]
- Aldakheel, F.M.; Alduraywish, S.A.; Jhugroo, P.; Jhugroo, C.; Divakar, D.D. Quantification of pathogenic bacteria in the subgingival oral biofilm samples collected from cigarette-smokers, individuals using electronic nicotine delivery systems and non-smokers with and without periodontitis. Arch. Oral Biol. 2020, 117, 104793. [Google Scholar] [CrossRef] [PubMed]
- Al-Sowygh, Z.H.; Al-Kheraif, A.A.; Akram, Z.; Vohra, F.; Javed, F. Peri-implant soft tissue inflammatory parameters and crestal bone loss among waterpipe (narghile) smokers and never-smokers with and without type 2 diabetes mellitus. J. Periodontol. 2018, 89, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Li, X. Impact of diabetes and its treatments on skeletal diseases. Front. Med. 2013, 7, 81–90. [Google Scholar] [CrossRef]
- Fiorellini, J.P.; Nevins, M.L.; Norkin, A.; Weber, H.P.; Karimbux, N.Y. The effect of insulin therapy on osseointegration in a diabetic rat model. Clin. Oral Implants Res. 1999, 10, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Kraut, D.; Gerstenfeld, L.C.; Graves, D.T. Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 2003, 144, 346–352. [Google Scholar] [CrossRef]
- Nevins, M.L.; Karimbux, N.Y.; Weber, H.P.; Giannobile, W.V.; Fiorellini, J.P. Wound healing around endosseous implants in experimental diabetes. Int. J. Oral Maxillofac. Implants 1998, 13, 620–629. [Google Scholar] [CrossRef]
- Westfelt, E.; Rylander, H.; Blohme, G.; Jonasson, P.; Lindhe, J. The effect of periodontal therapy in diabetics. Results after 5 years. J. Clin. Periodontol. 1996, 23, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Klingspor, L.; Sundin, U.; Altamash, M.; Klinge, B.; Engström, P.E. Periodontal conditions, oral Candida albicans and salivary proteins in type 2 diabetic subjects with emphasis on gender. BMC Oral Health 2009, 9, 12. [Google Scholar] [CrossRef] [PubMed]
| Author et al. | Study Design | Patients (n) | Age Range | Groups | Assessed Interleukin Polymorphisms | Outcome |
|---|---|---|---|---|---|---|
| Borilova Linhartova et al. [14] | CS/CC | 1106 patients | 30–77 years | Gp-1: Patients with periodontitis Gp-2: Patients with type-1 DM Gp-3: Patients with periodontitis and type-1 DM Gp-4: Patients with type-2 DM Gp-5: Patients with periodontitis and type-2 DM | IL-1 gene |
|
| López et al. [15] | CS/CC | 544 patients | 42–68 years | Gp-1: Patients with periodontitis Gp-2: Patients with type-2 DM Gp-3: Patients without type-2 DM and periodontitis | IL-1 gene |
|
| Kavitha et al. [16] | CC | 120 patients | NR | Gp-1: Patients with periodontitis Gp-2: Patients with type-2 DM Gp-3: Patients with periodontitis and type-2 DM Gp-4: Patients without type-2 DM and periodontitis | IL-6 gene |
|
| Deppe et al. [17] | CC | 104 patients | 48–72 years | Gp-1: Patients with type-2 DM Gp-2: Patients without type-2 DM | IL-1 gene |
|
| Cirelli et al. [18] | CS/CC | 894 patients | Gp-1: Patients with periodontitis Gp-2: Patients with periodontitis Gp-3: Patients with type-2 DM | IL-1A, IL-1B, IL-4, IL-6 and IL-10 |
| |
| Petrovic et al. [19] | CC | Gp-1: Patients with periodontitis Gp-2: Patients with periodontitis and type-2 DM Gp-3: Patients without type-2 DM and periodontitis |
| |||
| Xiao et al. [20] | CS/CC | 492 patients | 40–87 years | Gp-1: Patients with periodontitis Gp-2: Patients with type-2 DM Gp-3: Patients with periodontitis and type-2 DM Gp-4: Patients without type-2 DM and periodontitis | IL-6 gene |
|
| Struch et al. [22] | CS/CC | 1515 patients | 40–60 years | Gp-1: Patients with type-2 DM Gp-2: Patients without type-2 DM | IL-1A/1B haplotype |
|
| Author et al. | Reporting (Range: 2–12) | External Validity (Range: 0–2) | Bias (Range: 0–6) | Confounding (Range: 1–6) | Power (Range: 0–1) | Total | Final Assessment |
|---|---|---|---|---|---|---|---|
| Borilova Linhartova et al. [14] | 4 | 1 | 0 | 2 | 0 | 7 | High |
| López et al. [15] | 6 | 1 | 2 | 3 | 0 | 12 | High |
| Kavitha et al. [16] | 4 | 1 | 2 | 2 | 0 | 9 | High |
| Deppe et al. [17] | 8 | 4 | 4 | 5 | 1 | 22 | Low |
| Cirelli et al. [18] | 5 | 1 | 1 | 0 | 0 | 7 | High |
| Petrovic et al. [19] | 4 | 1 | 2 | 2 | 0 | 9 | High |
| Xiao et al. [20] | 5 | 1 | 1 | 2 | 0 | 9 | High |
| Struch et al. [22] | 3 | 1 | 1 | 2 | 0 | 7 | High |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdellatif, H.M.; Binshabaib, M.S.; Shawky, H.A.; ALHarthi, S.S. Association between Periodontitis and Genetic Polymorphisms in Interleukins among Patients with Diabetes Mellitus. Dent. J. 2021, 9, 45. https://doi.org/10.3390/dj9040045
Abdellatif HM, Binshabaib MS, Shawky HA, ALHarthi SS. Association between Periodontitis and Genetic Polymorphisms in Interleukins among Patients with Diabetes Mellitus. Dentistry Journal. 2021; 9(4):45. https://doi.org/10.3390/dj9040045
Chicago/Turabian StyleAbdellatif, Hoda M., Munerah Saleh Binshabaib, Heba A. Shawky, and Shatha Subhi ALHarthi. 2021. "Association between Periodontitis and Genetic Polymorphisms in Interleukins among Patients with Diabetes Mellitus" Dentistry Journal 9, no. 4: 45. https://doi.org/10.3390/dj9040045
APA StyleAbdellatif, H. M., Binshabaib, M. S., Shawky, H. A., & ALHarthi, S. S. (2021). Association between Periodontitis and Genetic Polymorphisms in Interleukins among Patients with Diabetes Mellitus. Dentistry Journal, 9(4), 45. https://doi.org/10.3390/dj9040045






