Association between Vitamin D and Candida-Associated Denture Stomatitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Questionnaire
2.3. Clinical Examination
2.4. Cultivation and Identification of Candida spp.
2.5. Serum Vitamin D Measurement
2.6. Ethical Considerations
2.7. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Candidal Infection and the Severity of Denture Stomatitis
3.3. Serum Vitamin D Level
3.4. Vitamin D Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 1. Factors influencing distribution of Candida species in the oral cavity. Aust. Dent. J. 1998, 43, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Samaranayake, L. Oral mucosal and salivary gland infections. In Essential Microbiology for Dentistry, 4th ed.; Samaranayake, L., Ed.; Churcill Livnigstone Elsivier: Edinburg, UK, 2012; pp. 307–321. [Google Scholar]
- Jontell, M.; Holmstrup, P. Red and white lesion of oral mucosa. In Burkets Oral Medicine, 11th ed.; Greenberg, M.S., Glick, M., Ship, J.A., Eds.; BC Decker, Inc.: Hamilton, ON, Canada, 2008; pp. 77–106. [Google Scholar]
- Salerno, C.; Pascale, M.; Contaldo, M.; Esposito, V.; Busciolano, M.; Milillo, L.; Guida, A.; Petruzzi, M.; Serpico, R. Candida-associated denture stomatitis. Med. Oral Patol. Oral Cir. Bucal. 2011, 16, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Coco, B.J.; Bagg, J.; Cross, L.J.; Jose, A.; Cross, J.; Ramage, G. Mixed Candida albicans and Candida glabrata populations associated with the pathogenesis of denture stomatitis. Oral Microbiol. Immunol. 2008, 23, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Sanitá, P.V.; Pavarina, A.C.; Giampaolo, E.T.; Silva, M.M.; Mima, E.G.; Ribeiro, D.G.; Vergani, C.E. Candida spp. prevalence in well controlled type 2 diabetic patients with denture stomatitis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 726–733. [Google Scholar] [CrossRef]
- Webb, B.C.; Thomas, C.J.; Willcox, M.D.; Harty, D.W.; Knox, K.W. Candida-associated denture stomatitis. Aetiology and management: A review. Part 2. Oral diseases caused by Candida species. Aust. Dent. J. 1998, 43, 160–166. [Google Scholar] [CrossRef]
- Newton, A.V. Denture sore mouth. Br. Dent. J. 1962, 112, 357–359. [Google Scholar]
- Sardi, J.C.; Scorzoni, L.; Bernardi, T.; Fusco-Almeida, A.M.; Mendes Giannini, M.J. Candida species: Current epidemiology, pathogenicity, biofilm formation, natural antifungal products and new therapeutic options. J. Med. Microbiol. 2013, 62, 10–24. [Google Scholar] [CrossRef]
- Bilhan, H.; Sulun, T.; Erkose, G.; Kurt, H.; Erturan, Z.; Kutay, O.; Bilgin, T. The role of Candida albicans hyphae and Lactobacillus in denture-related stomatitis. Clin. Oral Investig. 2009, 13, 363–368. [Google Scholar] [CrossRef]
- Compagnoni, M.A.; Souza, R.F.; Marra, J.; Pero, A.C.; Barbosa, D.B. Relationship between Candida and nocturnal denture wear: Quantitative study. J. Oral Rehabil. 2007, 34, 600–605. [Google Scholar] [CrossRef]
- Santos, A.L.S.; Carvalho, I.S.; Prata, J.M.; Martins, M.B.; Souza, L.O.P.; Galdono, A.C.M.; Braga-Silva, L.A.; Branquinha, M.H.; Rodrigues, S.M.; Sousa, L.V.N.F. Candida Albicans Involvement in Denture-Related Stomatitis: A Serious and Real Clinical Concern. J. Infect. Dis. Diagn. 2016, 1, 104. [Google Scholar] [CrossRef]
- Darwazeh, A.M.G.; Darwazeh, T.A. What makes oral candidiasis recurrent infection? A clinical view. J. Mycol. 2014. [Google Scholar] [CrossRef]
- Stein, S.H.; Tipton, D.A. Vitamin D and its impact on oral health--an update. J. Tenn. Dent. Assoc. 2011, 91, 30–33. [Google Scholar]
- Prietl, B.; Treiber, G.; Pieber, T.R.; Amrein, K. Vitamin D and immune function. Nutrients 2013, 5, 2502–2521. [Google Scholar] [CrossRef] [PubMed]
- Basit, S. Vitamin D in health and disease: A literature review. Br. J. Biomed. Sci. 2013, 70, 161–172. [Google Scholar] [CrossRef]
- Youssef, D.A.; Miller, C.W.; El-Abbassi, A.M.; Cutchins, D.C.; Cutchins, C.; Grant, W.B.; Peiris, A.N. Antimicrobial implications of vitamin D. Derm. Endocrinol. 2011, 3, 220–229. [Google Scholar] [CrossRef]
- Lim, J.H.; Ravikumar, S.; Wang, Y.M.; Thamboo, T.P.; Ong, L.; Chen, J.; Goh, J.G.; Tay, S.H.; Chengchen, L.; Win, M.S.; et al. Bimodal influence of vitamin D in host response to systemic Candida infection-vitamin D dose matters. J. Infect. Dis. 2015, 212, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Antonoglou, G.N.; Knuuttila, M.; Niemelä, O.; Raunio, T.; Karttunen, R.; Vainio, O.; Hedberg, P.; Ylöstalo, P.; Tervonen, T. Low serum level of 1,25(OH)2 D is associated with chronic periodontitis. J. Periodontal Res. 2015, 50, 274–280. [Google Scholar] [CrossRef]
- Kramer, I.R.; Pindborg, J.J.; Bezroukov, V.; Infirri, J.S. Guide to epidemiology and diagnosis of oral mucosal diseases and conditions. World Health Organization. Community Dent. Oral Epidemiol. 1980, 8, 1–26. [Google Scholar] [PubMed]
- McKenzie, D.W. Serum germ tube identification of Candida albicans. J. Clin. Pathol. 1962, 15, 563–565. [Google Scholar] [CrossRef]
- Joshi, K.R.; Solanki, A.; Prakash, P. Morphological identification of Candida species on glucose agar, rice extract agar and corn meal agar with and without Tween-80. Indian J. Pathol. Microbiol. 1993, 36, 48–52. [Google Scholar]
- Durán-Valle, M.T.; Sanz-Rodríguez, N.; Muñoz-Paraíso, C.; Almagro-Moltó, M.; Gómez-Garcés, J.L. Identification of clinical yeasts by Vitek MS system compared with API ID 32 C. Med. Mycol. 2014, 52, 342–349. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, V.V.; Khadilkar, A.V. Use of vitamin D in various disorders. Indian J. Pediatr. 2013, 80, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Chen, T.C. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 2008, 87, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.G.; Anderson, P.H.; Morris, H.A. Vitamin D and bone health. Scand. J. Clin. Lab. Invest. Suppl. 2012, 243, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Pencina, M.J.; Booth, S.L.; Jacques, P.F.; Ingelsson, E.; Lanier, K.; Benjamin, E.J.; D’Agostino, R.B.; Wolf, M.; Vasan, R.S. Vitamin D deficiency and risk of cardiovascular disease. Circulation 2008, 117, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Nadir, M.A.; Szwejkowski, B.R.; Witham, M.D. Vitamin D and cardiovascular prevention. Cardiovasc. Ther. 2010, 28, 5–12. [Google Scholar] [CrossRef]
- Griz, L.H.; Bandeira, F.; Gabbay, M.A.; Dib, S.A.; Carvalho, E.F. Vitamin D and diabetes mellitus: An update 2013. Arq. Bras. Endocrinol. Metabol. 2014, 58, 1–8. [Google Scholar] [CrossRef]
- Pappa, H.M.; Grand, R.J.; Gordon, C.M. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm. Bowel Dis. 2006, 12, 1162–1174. [Google Scholar] [CrossRef]
- Mesliniene, S.; Ramrattan, L.; Giddings, S.; Sheikh-Ali, M. Role of vitamin D in the onset, progression, and severity of multiple sclerosis. Endocr. Pract. 2013, 19, 129–136. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez Mena, J.M.; Brenner, H. Vitamin D and cancer: An overview on epidemiological studies. Adv. Exp. Med. Biol. 2014, 810, 17–32. [Google Scholar] [PubMed]
- Miragliotta, G.; Miragliotta, L. Vitamin D and infectious diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 267–271. [Google Scholar] [CrossRef]
- Dini, C.; Bianchi, A. The potential role of vitamin D for prevention and treatment of tuberculosis and infectious diseases. Ann. Ist. Super. Sanita 2012, 48, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. 2020, 14, 561–565. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of Vitamin D Deficiency and Treatment with COVID-19 Incidence. medRxiv 2020, 13, 2020.05.08.20095893. [Google Scholar] [CrossRef]
- Amano, Y.; Komiyama, K.; Makishima, M. Vitamin D and periodontal disease. J. Oral Sci. 2009, 51, 11–20. [Google Scholar] [CrossRef]
- Zhan, Y.; Samietz, S.; Holtfreter, B.; Hannemann, A.; Meisel, P.; Nauck, M.; Völzke, H.; Wallaschofski, H.; Dietrich, T.; Kocher, T. Prospective Study of Serum 25-hydroxy Vitamin D and Tooth Loss. J. Dent. Res. 2014, 93, 639–644. [Google Scholar] [CrossRef]
- Jimenez, M.; Giovannucci, E.; Krall Kaye, E.; Joshipura, K.J.; Dietrich, T. Predicted vitamin D status and incidence of tooth loss and periodontitis. Public Health Nutr. 2014, 17, 844–852. [Google Scholar] [CrossRef]
- Fathi, N.; Ahmadian, E.; Shahi, S.; Roshangar, L.; Khan, H.; Kouhsoltani, M.; Maleki Dizaj, S.; Sharifi, S. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed. Pharmacother. 2019, 109, 391–401. [Google Scholar] [CrossRef]
- Antonoglou, G.; Knuuttila, M.; Niemelä, O.; Hiltunen, L.; Raunio, T.; Karttunen, R.; Vainio, O.; Ylöstalo, P.; Tervonen, T. Serum 1,25(OH)D level increases after elimination of periodontal inflammation in T1DM subjects. J. Clin. Endocrinol. Metab. 2013, 98, 3999–4005. [Google Scholar] [CrossRef]
- Adams, J.S.; Liu, P.T.; Chun, R.; Modlin, R.L.; Hewison, M. Vitamin D in defense of the human immune response. Ann. N. Y. Acad. Sci. 2007, 1117, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Scocchi, M.; Pacor, S.; Tossi, A.; Nobili, D.; Basaglia, G.; Busetti, M.; Gennaro, R. Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J. Antimicrob. Chemother. 2006, 58, 950–959. [Google Scholar] [CrossRef]
- Lee, W.J.; Cha, H.W.; Sohn, M.Y.; Lee, S.J.; Kim, D.W. Vitamin D increases expression of cathelicidin in cultured sebocytes. Arch. Dermatol. Res. 2012, 304, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Tomalka, J.; Azodi, E.; Narra, H.P.; Patel, K.; O’Neill, S.; Cardwell, C.; Hall, B.A.; Wilson, J.M.; Hise, A.G. β-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J. Immunol. 2015, 194, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Sroussi, H.Y.; Burke-Miller, J.; French, A.L.; Adeyemi, O.M.; Weber, K.M.; Lu, Y.; Cohen, M. Association among vitamin D, oral candidiasis, and calprotectinemia in HIV. J. Dent. Res. 2012, 91, 666–670. [Google Scholar] [CrossRef]
- Van der Wielen, R.P.; Löwik, M.R.; Van den Berg, H.; De Groot, L.C.; Haller, J.; Moreiras, O.; Van Staveren, W.A. Serum vitamin D concentrations among elderly people in Europe. Lancet 1995, 346, 207–210. [Google Scholar] [CrossRef]
- Jacques, P.F.; Felson, D.T.; Tucker, K.L.; Mahnken, B.; Wilson, P.W.; Rosenberg, I.H.; Rush, D. Plasma 25-hydroxyvitamin D and its determinants in an elderly population sample. Am. J. Clin. Nutr. 1997, 66, 929–936. [Google Scholar] [CrossRef]
- Spiro, A.; Buttriss, J.L. Vitamin D: An overview of vitamin D status and intake in Europe. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef]
- Bonilla, E. Treatment of chromoblastomycosis with calciferol; report of three cases. AMA Arch. Derm. Syphilol. 1954, 70, 666–667. [Google Scholar] [CrossRef]
- Cantorna, M.T.; Yu, S.; Bruce, D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol. Asp. Med. 2008, 29, 369–375. [Google Scholar] [CrossRef] [PubMed]
| Demographic Data | Groups | p Value | |
|---|---|---|---|
| CADS | Control | ||
| Gender | |||
| Female/n (%) | 25 (78.13) | 25 (78.13) | p = 1 * |
| Male/n (%) | 7 (21.87) | 7 (21.87) | |
| Age/years | |||
| Average | 68.9 | 68.9 | p = 1 ** |
| Standard deviation | 8.4 | 8.4 | |
| Min. | 53 | 53 | |
| Max. | 83 | 83 | |
| Groups | |||||
|---|---|---|---|---|---|
| CADS | Control | p Value | |||
| (n = 32) | (n = 32) | ||||
| Vitamin D/nmol/L | |||||
| mean ± SD | 54.68 ± 17.07 | 56.82 ± 17.75 | p = 0.622 * | ||
| 95% CI | 48.53–60.83 | 50.49–63.14 | |||
| median | female | male | female | male | |
| (5th–95th percentile) | (n = 25) | (n = 7) | (n = 25) | (n = 7) | |
| 48.2 | 69.7 | 51.2 | 66.57 | p = 0.16 ** | |
| (30.4–78.8) | (42.1–83.7) | (30.4–78.5) | (41–98.2) | ||
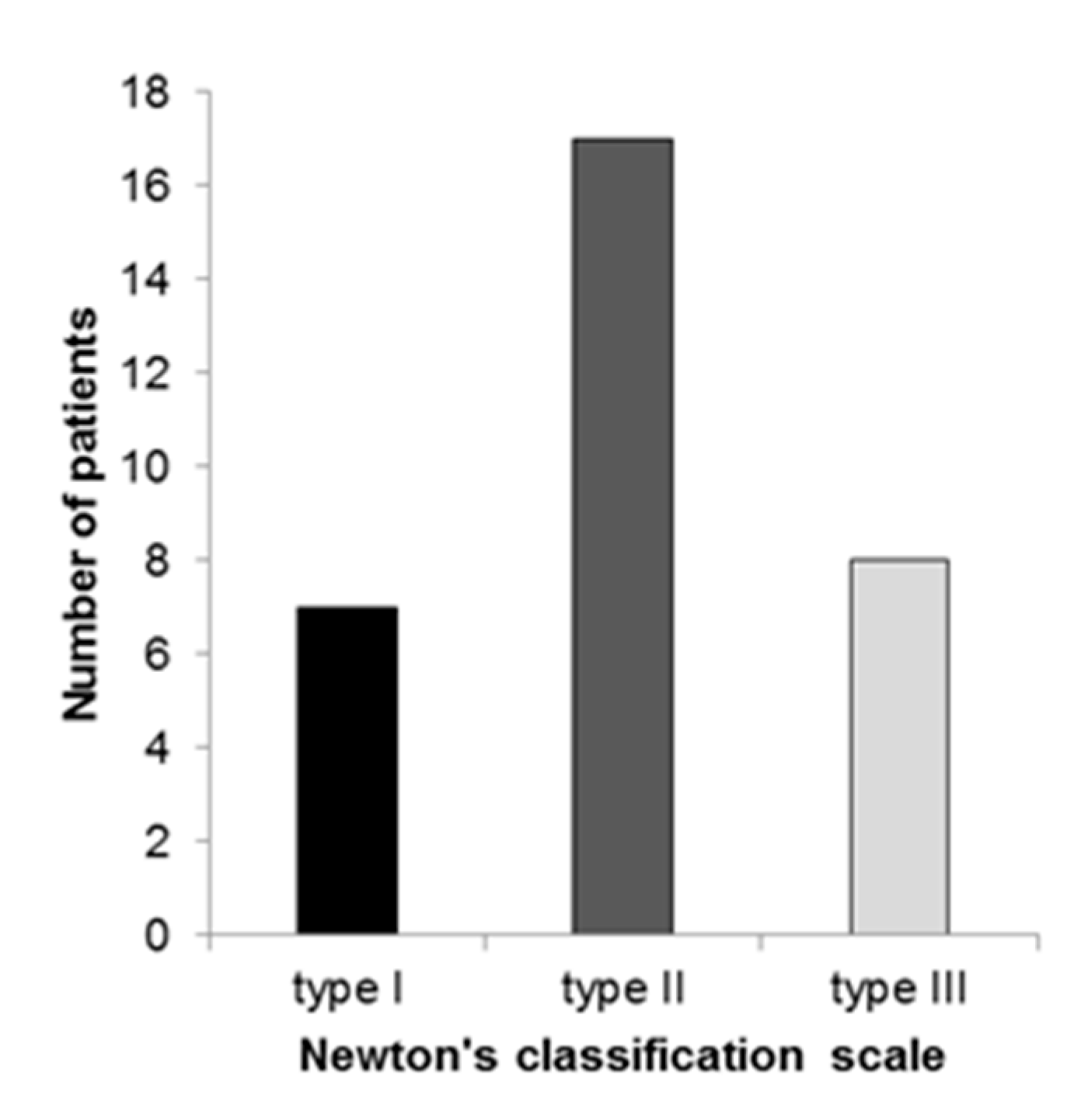
| Groups | p Value | ||||
|---|---|---|---|---|---|
| Type I DS | Type II DS | Type III DS | Control | ||
| (n = 7) | (n = 17) | (n = 8) | (n = 32) | ||
| Vitamin D/nmol/L | |||||
| median | 57.4 | 50.3 | 43.4 | 54.35 | p = 0.601 * |
| (5th–95th percentile) | (33.50–78.80) | (36.50–83.70) | (26.80–74.80) | (30.40–80.60) | |
| Vitamin D Status | Groups | ||
|---|---|---|---|
| CADS | Control | p Value | |
| n (%) | n (%) | ||
| sufficiency (>75 nmol/L) | 5 (15.63) | 6 (18.75) | |
| insufficiency (>50–≤75 nmol/L) | 11 (34.77) | 13 (40.625) | p = 0.75 * |
| moderate deficiency (>25–≤50 nmol/L) | 16 (50) | 13 (40.625) | |
| severe deficiency (≤25 nmol/L) | 0 | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muhvić-Urek, M.; Saltović, E.; Braut, A.; Kovačević Pavičić, D. Association between Vitamin D and Candida-Associated Denture Stomatitis. Dent. J. 2020, 8, 121. https://doi.org/10.3390/dj8040121
Muhvić-Urek M, Saltović E, Braut A, Kovačević Pavičić D. Association between Vitamin D and Candida-Associated Denture Stomatitis. Dentistry Journal. 2020; 8(4):121. https://doi.org/10.3390/dj8040121
Chicago/Turabian StyleMuhvić-Urek, Miranda, Ema Saltović, Alen Braut, and Daniela Kovačević Pavičić. 2020. "Association between Vitamin D and Candida-Associated Denture Stomatitis" Dentistry Journal 8, no. 4: 121. https://doi.org/10.3390/dj8040121
APA StyleMuhvić-Urek, M., Saltović, E., Braut, A., & Kovačević Pavičić, D. (2020). Association between Vitamin D and Candida-Associated Denture Stomatitis. Dentistry Journal, 8(4), 121. https://doi.org/10.3390/dj8040121




