A Preliminary Study on the Ability of the Trypsin-Like Peptidase Activity Assay Kit to Detect Periodontitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Data Collection
2.2.1. Questionnaire Administration
2.2.2. TLP-AA-Kit
2.2.3. Oral Health Examinations
2.2.4. Data Collection of Other Characteristics
2.3. Statistical Analyses
3. Results
3.1. Study Population Characteristics
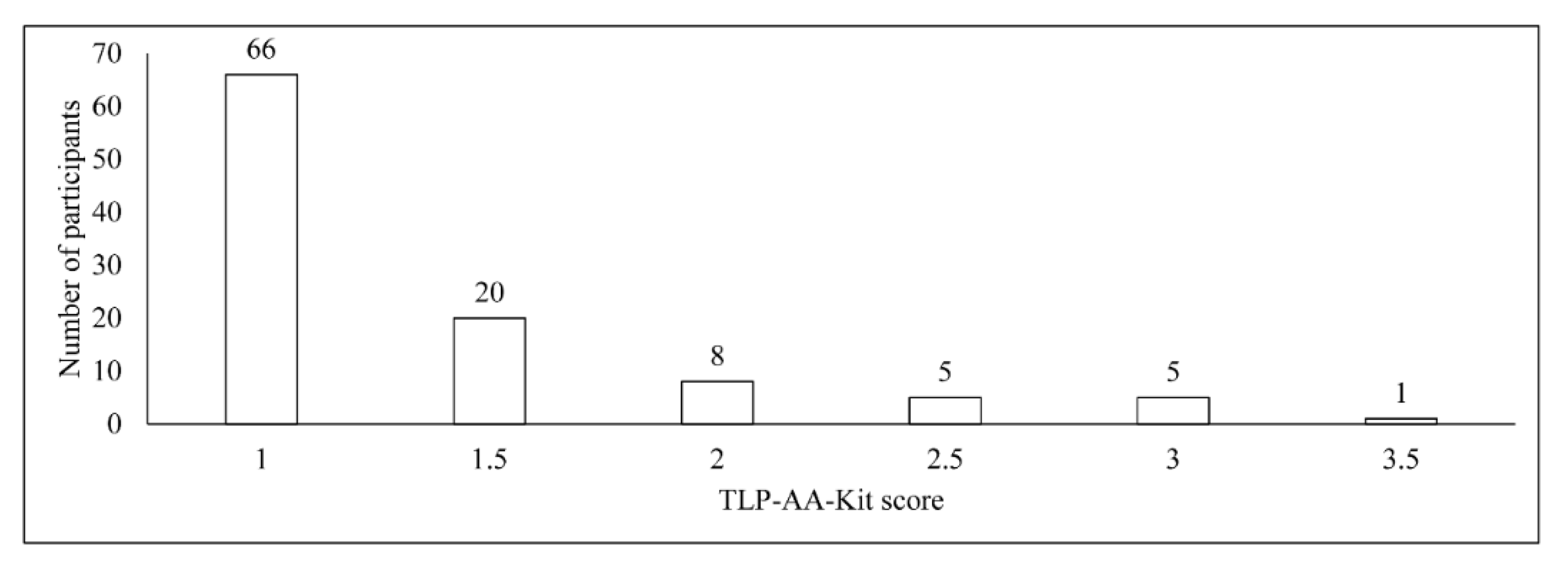
3.2. TLP-AA-Kit Score
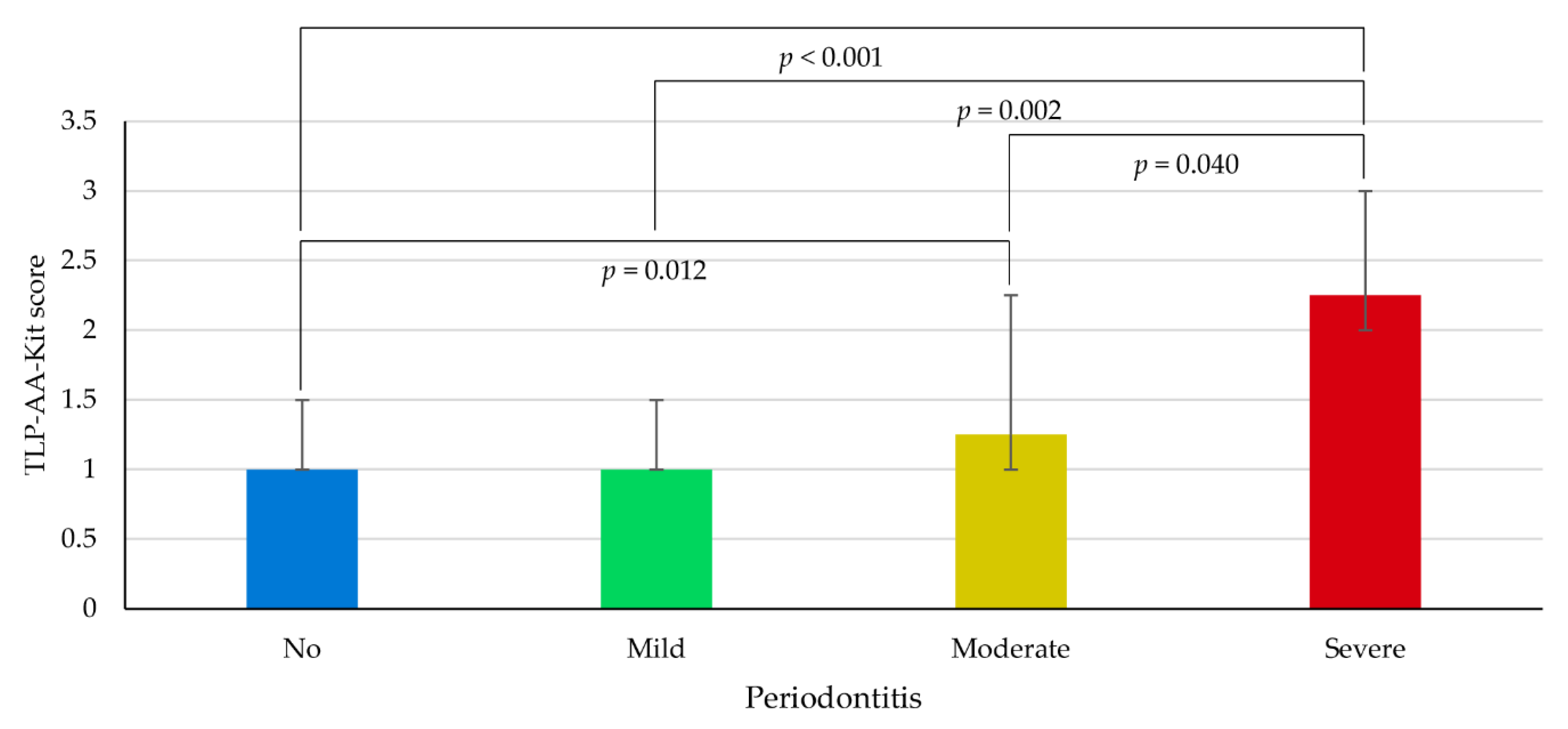
3.3. Association between TLP-AA-Kit Score and Periodontitis
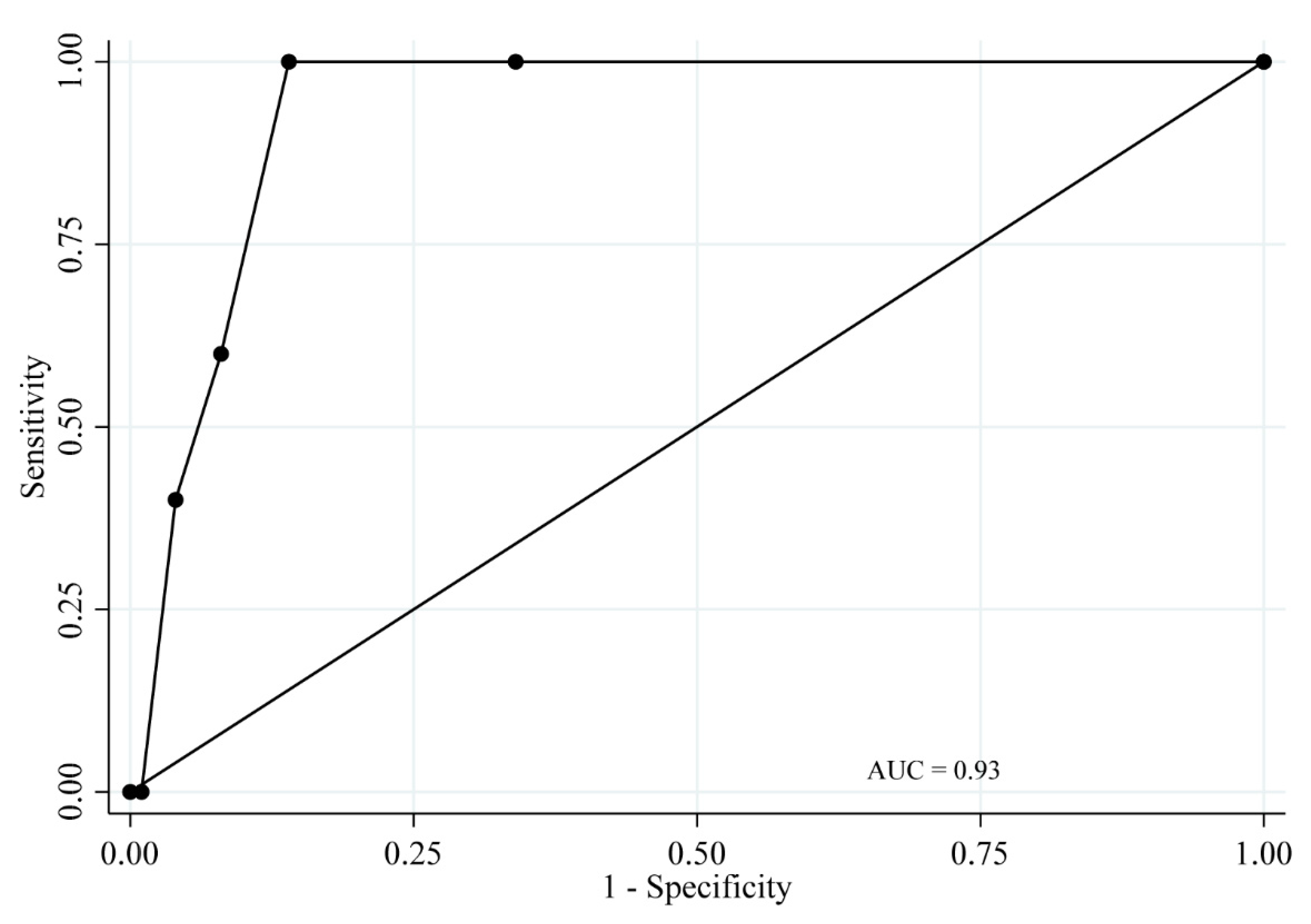
3.4. ROC Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- The Ministry of Health, Labour, and Welfare. Survey of Dental Diseases. 2016. Available online: https://www.mhlw.go.jp/toukei/list/dl/62-28-02.pdf (accessed on 2 August 2020). (In Japanese).
- Eke, P.I.; Dye, B.A.; Wei, L.; Slade, G.D.; Thornton-Evans, G.O.; Borgnakke, W.S.; Taylor, G.W.; Page, R.C.; Beck, J.D.; Genco, R.J. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J. Periodontol. 2015, 86, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Giudice, A.L.; Polizzi, A.; Alibrandi, A.; Patini, R.; Ferlito, S. Periodontitis and Tooth Loss Have Negative Systemic Impact on Circulating Progenitor Cell Levels: A Clinical Study. Genes 2019, 10, 1022. [Google Scholar] [CrossRef] [Green Version]
- Isola, G.; Alibrandi, A.; Currò, M.; Matarese, M.; Ricca, S.; Matarese, G.; Ientile, R.; Kocher, T. Evaluation of salivary and serum asymmetric dimethylarginine (ADMA) levels in patients with periodontal and cardiovascular disease as subclinical marker of cardiovascular risk. J. Periodontol. 2020, 91, 1076–1084. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Dias-Pereira, A.C.; Branco-de-Almeida, L.S.; Martins, C.C.; Paiva, S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal. Res. 2017, 52, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Linden, G.J.; Lyons, A.; Scannapieco, F.A. Periodontal systemic associations: Review of the evidence. J. Clin. Periodontol. 2013, 40 (Suppl. 14), S8–S19. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Aguilera, E.; Suvan, J.; Buti, J.; Czesnikiewicz-Guzik, M.; Barbosa Ribeiro, A.; Orlandi, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Periodontitis is associated with hypertension: A systematic review and meta-analysis. Cardiovasc. Res. 2019, 116, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International diabetes Federation and the European Federation of Periodontology. Diabetes Res. Clin. Pract. 2018, 137, 231–241. [Google Scholar] [CrossRef]
- Saremi, A.; Nelson, R.G.; Tulloch-Reid, M.; Hanson, R.L.; Sievers, M.L.; Taylor, G.W.; Shlossman, M.; Bennett, P.H.; Genco, R.; Knowler, W.C. Periodontal disease and mortality in type 2 diabetes. Diabetes Care 2005, 28, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Southerland, J.H.; Moss, K.; Taylor, G.W.; Beck, J.D.; Pankow, J.; Gangula, P.R.; Offenbacher, S. Periodontitis and diabetes associations with measures of atherosclerosis and CHD. Atherosclerosis 2012, 222, 196–201. [Google Scholar] [CrossRef]
- Sato, M.; Iwasaki, M.; Yoshihara, A.; Miyazaki, H. Association between periodontitis and medical expenditure in older adults: A 33-month follow-up study. Geriatr. Gerontol. Int. 2016, 16, 856–864. [Google Scholar] [CrossRef]
- The Japan Dental Association. Dental Medical Awareness Survey. Available online: https://www.jda.or.jp/jda/release/pdf/DentalMedicalAwarenessSurvey_h30.pdf (accessed on 2 August 2020). (In Japanese).
- Kimura, H.; Goda, K.; Takeda, N.; Hirao, T.; Fukunaga, I.; Kageyama, H.; Jitsunari, F. Oral health promotion in the workplace and relative factors. J. Dent. Health 2001, 51, 37–47. (In Japanese) [Google Scholar] [CrossRef]
- Yamamoto, T.; Koyama, R.; Tamaki, N.; Maruyama, T.; Tomofuji, T.; Ekuni, D.; Yamanaka, R.; Azuma, T.; Morita, M. Validity of a questionnaire for periodontitis screening of Japanese employees. J. Occup. Health 2009, 51, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Polizzi, A.; Iorio-Siciliano, V.; Alibrandi, A.; Ramaglia, L.; Leonardi, R. Effectiveness of a nutraceutical agent in the non-surgical periodontal therapy: A randomized, controlled clinical trial. Clin. Oral. Investig. 2020. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Thornton-Evans, G.O.; Wei, L.; Borgnakke, W.S.; Dye, B.A. Accuracy of NHANES periodontal examination protocols. J. Dent. Res. 2010, 89, 1208–1213. [Google Scholar] [CrossRef]
- Daly, C.G.; Mitchell, D.H.; Highfield, J.E.; Grossberg, D.E.; Stewart, D. Bacteremia due to periodontal probing: A clinical and microbiological investigation. J. Periodontol. 2001, 72, 210–214. [Google Scholar] [CrossRef]
- Ishihara, K. Role of bacterial protease in periodontitis. J. Jpn. Soc. Periodontol. 2008, 50, 3–10. (In Japanese) [Google Scholar] [CrossRef]
- Laughon, B.E.; Syed, S.A.; Loesche, W.J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J. Clin. Microbiol. 1982, 15, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Loesche, W.J.; Lopatin, D.E.; Giordano, J.; Alcoforado, G.; Hujoel, P. Comparison of the benzoyl-DL-arginine-naphthylamide (BANA) test, DNA probes, and immunological reagents for ability to detect anaerobic periodontal infections due to Porphyromonas gingivalis, Treponema denticola, and Bacteroides forsythus. J. Clin. Microbiol. 1992, 30, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Shimazaki, Y.; Akifusa, S.; Takeshita, T.; Shibata, Y.; Doi, Y.; Hata, J.; Ninomiya, T.; Hirakawa, Y.; Kiyohara, Y.; Yamashita, Y. Effectiveness of the Salivary Occult Blood Test as a Screening Method for Periodontal Status. J. Periodontol. 2011, 82, 581–587. [Google Scholar] [CrossRef]
- Nishinaga, E.; Maki, R.; Saito, K.; Fukasawa, T.; Suzuki, N.; Uchiyama, C.; Yamamoto, T.; Murakoshi, M.; Odera, M.; Fukuta, I.; et al. Development of comprehensive salivary test system:—Efficiency of a newly-developed salivary multi-test system (AL-55). Jpn. J. Conserv. Dent. 2015, 58, 219–228. (In Japanese) [Google Scholar] [CrossRef]
- Nishinaga, E.; Uchiyama, C.; Maki, R.; Saito, K.; Fukasawa, T.; Suzuki, N.; Yamamoto, T.; Murakoshi, M.; Odera, M.; Fukuta, I.; et al. Development of Comprehensive Salivary Test System:—Validity and Reliability of a Newly-developed Salivary Multi-test System (AL-55) Compared with Standard Methods. Jpn. J. Conserv. Dent. 2015, 58, 321–330. (In Japanese) [Google Scholar] [CrossRef]
- Ikehara, S.; Iso, H.; Toyoshima, H.; Date, C.; Yamamoto, A.; Kikuchi, S.; Kondo, T.; Watanabe, Y.; Koizumi, A.; Wada, Y.; et al. Alcohol consumption and mortality from stroke and coronary heart disease among Japanese men and women: The Japan collaborative cohort study. Stroke 2008, 39, 2936–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iso, H.; Baba, S.; Mannami, T.; Sasaki, S.; Okada, K.; Konishi, M.; Tsugane, S. Alcohol consumption and risk of stroke among middle-aged men: The JPHC Study Cohort I. Stroke 2004, 35, 1124–1129. [Google Scholar] [CrossRef]
- Glavind, L.; Löe, H. Errors in the clinical assessment of periodontal destruction. J. Periodontal. Res. 1967, 2, 180–184. [Google Scholar] [CrossRef]
- Michalowicz, B.S.; Hodges, J.S.; Pihlstrom, B.L. Is change in probing depth a reliable predictor of change in clinical attachment loss? J. Am. Dent. Assoc. 2013, 144, 171–178. [Google Scholar] [CrossRef]
- Eke, P.I.; Page, R.C.; Wei, L.; Thornton-Evans, G.; Genco, R.J. Update of the case definitions for population-based surveillance of periodontitis. J. Periodontol. 2012, 83, 1449–1454. [Google Scholar] [CrossRef] [PubMed]
- Saxlin, T.; Ylostalo, P.; Suominen-Taipale, L.; Mannisto, S.; Knuuttila, M. Association between periodontal infection and obesity: Results of the Health 2000 Survey. J. Clin. Periodontol. 2011, 38, 236–242. [Google Scholar] [CrossRef]
- Shimizu, T.; Ueda, T.; Sakurai, K. New method for evaluation of tongue-coating status. J. Oral Rehabil. 2007, 34, 442–447. [Google Scholar] [CrossRef]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Gatsonis, C.A.; Glasziou, P.P.; Irwig, L.; Lijmer, J.G.; Moher, D.; Rennie, D.; de Vet, H.C.; et al. STARD 2015: An Updated List of Essential Items for Reporting Diagnostic Accuracy Studies. Radiology 2015, 277, 826–832. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.E.; Holtzman, D.; Bolen, J.; Stanwyck, C.A.; Mack, K.A. Reliability and validity of measures from the Behavioral Risk Factor Surveillance System (BRFSS). Soz. Praventivmed. 2001, 46 (Suppl. 1), S3. [Google Scholar]
- Carra, M.C.; Gueguen, A.; Thomas, F.; Pannier, B.; Caligiuri, G.; Steg, P.G.; Zins, M.; Bouchard, P. Self-report assessment of severe periodontitis: Periodontal screening score development. J. Clin. Periodontol. 2018, 45, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Blicher, B.; Joshipura, K.; Eke, P. Validation of self-reported periodontal disease: A systematic review. J. Dent. Res. 2005, 84, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Echeverría, J.J.; Echeverría, A.; Caffesse, R.G. Adherence to supportive periodontal treatment. Periodontology 2000 2019, 79, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Abbood, H.M.; Hinz, J.; Cherukara, G.; Macfarlane, T.V. Validity of Self-Reported Periodontal Disease: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 1474–1483. [Google Scholar] [CrossRef]
- Rosenman, R.; Tennekoon, V.; Hill, L.G. Measuring bias in self-reported data. Int. J. Behav. Healthc. Res. 2011, 2, 320–332. [Google Scholar] [CrossRef]
- Nomura, Y.; Okada, A.; Kakuta, E.; Gunji, T.; Kajiura, S.; Hanada, N. A new screening method for periodontitis: An alternative to the community periodontal index. BMC Oral Health 2016, 16, 64. [Google Scholar] [CrossRef] [Green Version]
- Kc, S.; Wang, X.Z.; Gallagher, J.E. Diagnostic sensitivity and specificity of host-derived salivary biomarkers in periodontal disease amongst adults: Systematic review. J. Clin. Periodontol. 2020, 47, 289–308. [Google Scholar] [CrossRef]
- Kuboniwa, M.; Sakanaka, A.; Hashino, E.; Bamba, T.; Fukusaki, E.; Amano, A. Prediction of Periodontal Inflammation via Metabolic Profiling of Saliva. J. Dent. Res. 2016, 95, 1381–1386. [Google Scholar] [CrossRef]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Balsa-Castro, C.; Nibali, L.; Donos, N.; Tomas, I. Accuracy of single molecular biomarkers in gingival crevicular fluid for the diagnosis of periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2019, 46, 1166–1182. [Google Scholar] [CrossRef]
- Kudo, C.; Naruishi, K.; Maeda, H.; Abiko, Y.; Hino, T.; Iwata, M.; Mitsuhashi, C.; Murakami, S.; Nagasawa, T.; Nagata, T.; et al. Assessment of the plasma/serum IgG test to screen for periodontitis. J. Dent. Res. 2012, 91, 1190–1195. [Google Scholar] [CrossRef]
- Loesche, W.J.; Syed, S.A.; Stoll, J. Trypsin-like activity in subgingival plaque. A diagnostic marker for spirochetes and periodontal disease? J. Periodontol. 1987, 58, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loomer, P.M. Microbiological diagnostic testing in the treatment of periodontal diseases. Periodontology 2000 2004, 34, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Holtfreter, B.; Albandar, J.M.; Dietrich, T.; Dye, B.A.; Eaton, K.A.; Eke, P.I.; Papapanou, P.N.; Kocher, T. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: Proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J. Clin. Periodontol. 2015, 42, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45, S1–S8. [Google Scholar] [CrossRef]

| Total | Periodontitis | |||||
|---|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | |||
| n = 105 | n = 72 | n = 11 | n = 17 | n = 5 | p * | |
| n of teeth, median (IQR) | 28 (28–28) | 28 (28–28) | 28 (28–28) | 28 (27–28) | 28 (25–28) | 0.200 |
| Average PPD (mm), median (IQR) | 1.9 (1.7–2.1) | 1.8 (1.7–1.9) a | 2.1 (1.9–2.2) b | 2.1 (2.0–2.3) b,c | 2.5 (2.3–2.6) c | <0.001 |
| Average CAL (mm), median (IQR) | 1.9 (1.7–2.1) | 1.8 (1.7–1.9) a | 2.0 (1.9–2.1) b | 2.2 (2.1–2.3) b,c | 2.6 (2.4–2.7) c | <0.001 |
| BOP (%), median (IQR) | 7.1 (4.8–15.5) | 6.5 (3.3–10.7) a | 12.5 (6.5–22.0) b | 14.9 (4.8–22.0) b | 19.6 (8.3–41.7) b | 0.029 |
| Denture use, n (%) | 2 (1.9%) | 2 (2.8%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.820 |
| Presence of dental plaque, n (%) | 0.470 | |||||
| None | 43 (41.0%) | 33 (45.8%) | 5 (45.5%) | 3 (17.6%) | 2 (40.0%) | |
| Plaque on gingival margins only | 42 (40.0%) | 27 (37.5%) | 3 (27.3%) | 10 (58.8%) | 2 (40.0%) | |
| Plaque also elsewhere | 20 (19.0%) | 12 (16.7%) | 3 (27.3%) | 4 (23.5%) | 1 (20.0%) | |
| TCI (%), median (IQR) | 50.0 (38.9–50.0) | 50.0 (38.9–50.0) | 50.0 (38.9–50.0) | 50.0 (38.9–50.0) | 50.0 (50.0–55.6) | 0.710 |
| Tooth brushing ≥2 times/day, n (%) | 89 (84.8%) | 58 (80.6%) | 11 (100%) | 16 (94.1%) | 4 (80.0%) | 0.240 |
| Interdental cleaning devices use, n (%) | 58 (55.2%) | 41 (56.9%) | 7 (63.6%) | 7 (41.2%) | 3 (60.0%) | 0.610 |
| Regular dental check-ups, n (%) | 21 (20.0%) | 14 (19.4%) | 5 (45.5%) | 1 (5.9%) | 1 (20.0%) | 0.086 |
| Age, mean (s.d.) | 40.1 (12.5) | 37.3 (11.8) a | 39.3 (14.0) a,b | 47.7 (8.0) b,c | 56.6 (9.4) c | <0.001 |
| Sex, n (%) | ||||||
| Men | 71 (67.6%) | 46 (63.9%) | 7 (63.6%) | 14 (82.4%) | 4 (80.0%) | 0.460 |
| Women | 34 (32.4%) | 26 (36.1%) | 4 (36.4%) | 3 (17.6%) | 1 (20.0%) | |
| High-risk drinking, n (%) | 20 (19.0%) | 14 (19.4%) | 0 (0%) | 5 (29.4%) | 1 (20.0%) | 0.290 |
| Current smoker, n (%) | 7 (6.7%) | 6 (8.3%) | 0 (0%) | 1 (5.9%) | 0 (0%) | 0.690 |
| Habitual exercise, n (%) | 37 (35.2%) | 25 (34.7%) | 6 (54.5%) | 3 (17.6%) | 3 (60.0%) | 0.140 |
| Poor sleep, n (%) | 33 (31.4%) | 25 (34.7%) | 1 (9.1%) | 5 (29.4%) | 2 (40.0%) | 0.370 |
| Obesity, n (%) | 12 (11.4%) | 6 (8.3%) | 0 (0%) | 5 (29.4%) | 1 (20.0%) | 0.048 |
| Diabetes, n (%) | 17 (16.2%) | 8 (11.1%) | 2 (18.2%) | 7 (41.2%) | 0 (0%) | 0.017 |
| Yes | No | p * | ||||
|---|---|---|---|---|---|---|
| Antibiotic usage within a month | n (%) | 15 | (14.3) | 90 | (85.7) | |
| TLP-AA-Kit score, median (IQR) | 1.0 | (1.0–2.0) | 1.0 | (1.0–1.5) | 0.865 | |
| Tooth brushing within an hour | n (%) | 43 | (41) | 62 | (59.1) | |
| TLP-AA-Kit score, median (IQR) | 1.0 | (1.0–1.5) | 1.0 | (1.0–1.5) | 0.200 | |
| Tongue cleaning within an hour | n (%) | 9 | (8.6) | 96 | (91.4) | |
| TLP-AA-Kit score, median (IQR) | 1.0 | (1.0–1.0) | 1.0 | (1.0–1.5) | 0.360 | |
| Mouth rinse usage within an hour | n (%) | 6 | (5.7) | 99 | (94.3) | |
| TLP-AA-Kit score, median (IQR) | 1.25 | (1.0–1.5) | 1.0 | (1.0–1.5) | 0.548 | |
| Taken a meal within an hour | n (%) | 74 | (70.5) | 31 | (29.5) | |
| TLP-AA-Kit score, median (IQR) | 1.0 | (1.0–1.5) | 1.0 | (1.0–2.0) | 0.227 | |
| Smoking within an hour | n (%) | 5 | (4.8) | 100 | (95.2) | |
| TLP-AA-Kit score, median (IQR) | 1.5 | (1.0–1.5) | 1.0 | (1.0–1.5) | 0.320 | |
| Parameter | ρ | p |
|---|---|---|
| Average PPD | 0.224 | 0.022 |
| Average CAL | 0.235 | 0.016 |
| BOP | 0.164 | 0.095 |
| Moderate/Severe Periodontitis | |||||
| TLP-AA-Kit Score Value | Sensitivity | Specificity | Classification | LR+ | LR− |
| ≥1 | 100.0% | 0% | 21.0% | 1.00 | |
| ≥1.5 | 59.1% | 68.7% | 66.7% | 1.89 | 0.60 |
| ≥2 | 54.6% | 91.6% | 83.8% | 6.47 | 0.50 |
| ≥2.5 | 31.8% | 95.2% | 81.9% | 6.60 | 0.72 |
| ≥3 | 22.7% | 98.8% | 82.9% | 18.86 | 0.78 |
| ≥3.5 | 4.6% | 100% | 80.0% | 0.95 | |
| ≥4 | 0% | 100% | 79.1% | 1.00 | |
| Severe Periodontitis | |||||
| TLP-AA-Kit Score Value | Sensitivity | Specificity | Classification | LR+ | LR− |
| ≥1 | 100% | 0% | 4.8% | 1.00 | |
| ≥1.5 | 100% | 66.0% | 67.6% | 2.94 | 0 |
| ≥2 | 100% | 86.0% | 86.7% | 7.14 | 0 |
| ≥2.5 | 60.0% | 92.0% | 90.5% | 7.50 | 0.43 |
| ≥3 | 40.0% | 96.0% | 93.3% | 10.00 | 0.63 |
| ≥3.5 | 0% | 99.0% | 94.3% | 0 | 1.01 |
| ≥4 | 0% | 100% | 95.2% | 1.00 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasaki, M.; Usui, M.; Ariyoshi, W.; Nakashima, K.; Nagai-Yoshioka, Y.; Inoue, M.; Nishihara, T. A Preliminary Study on the Ability of the Trypsin-Like Peptidase Activity Assay Kit to Detect Periodontitis. Dent. J. 2020, 8, 98. https://doi.org/10.3390/dj8030098
Iwasaki M, Usui M, Ariyoshi W, Nakashima K, Nagai-Yoshioka Y, Inoue M, Nishihara T. A Preliminary Study on the Ability of the Trypsin-Like Peptidase Activity Assay Kit to Detect Periodontitis. Dentistry Journal. 2020; 8(3):98. https://doi.org/10.3390/dj8030098
Chicago/Turabian StyleIwasaki, Masanori, Michihiko Usui, Wataru Ariyoshi, Keisuke Nakashima, Yoshie Nagai-Yoshioka, Maki Inoue, and Tatsuji Nishihara. 2020. "A Preliminary Study on the Ability of the Trypsin-Like Peptidase Activity Assay Kit to Detect Periodontitis" Dentistry Journal 8, no. 3: 98. https://doi.org/10.3390/dj8030098
APA StyleIwasaki, M., Usui, M., Ariyoshi, W., Nakashima, K., Nagai-Yoshioka, Y., Inoue, M., & Nishihara, T. (2020). A Preliminary Study on the Ability of the Trypsin-Like Peptidase Activity Assay Kit to Detect Periodontitis. Dentistry Journal, 8(3), 98. https://doi.org/10.3390/dj8030098






