Do Lasers Have an Adjunctive Role in Initial Non-Surgical Periodontal Therapy? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
- Randomized controlled clinical trials;
- At least 10 patients per group;
- Chronic periodontitis;
- Laser used in test group;
- Interventions: the test groups received laser therapy additional to conventional treatment and one of the control groups received conventional treatment only;
- Follow up: at least 6 months.
- Case series/case reports/pilot studies;
- Studies without control group;
- Laser used as monotherapy in the test group;
- Surgical approach;
- Follow-up less than 6 months;
- Less than 10 patients per group;
- aPDT or other adjuncts applied.
2.2. Data Extraction
- Citation (first author and publication year);
- Type of study/number of samples/pocket depth;
- Test/control groups;
- Examined parameters;
- Laser protocol/number of sessions involved;
- Follow-up;
- Outcome.
2.3. Quality Assessment
- Randomization?
- Sample size calculation and required sample numbers included?
- Baseline situation similar to that of the test group?
- Blinding?
- Parameters of laser use described appropriately, and associated calculations correct?
- Power meter used?
- Numerical results available (statistics)?
- No missing outcome data?
- All samples/patients completed the follow-up evaluation?
- Correct interpretation of data acquired?
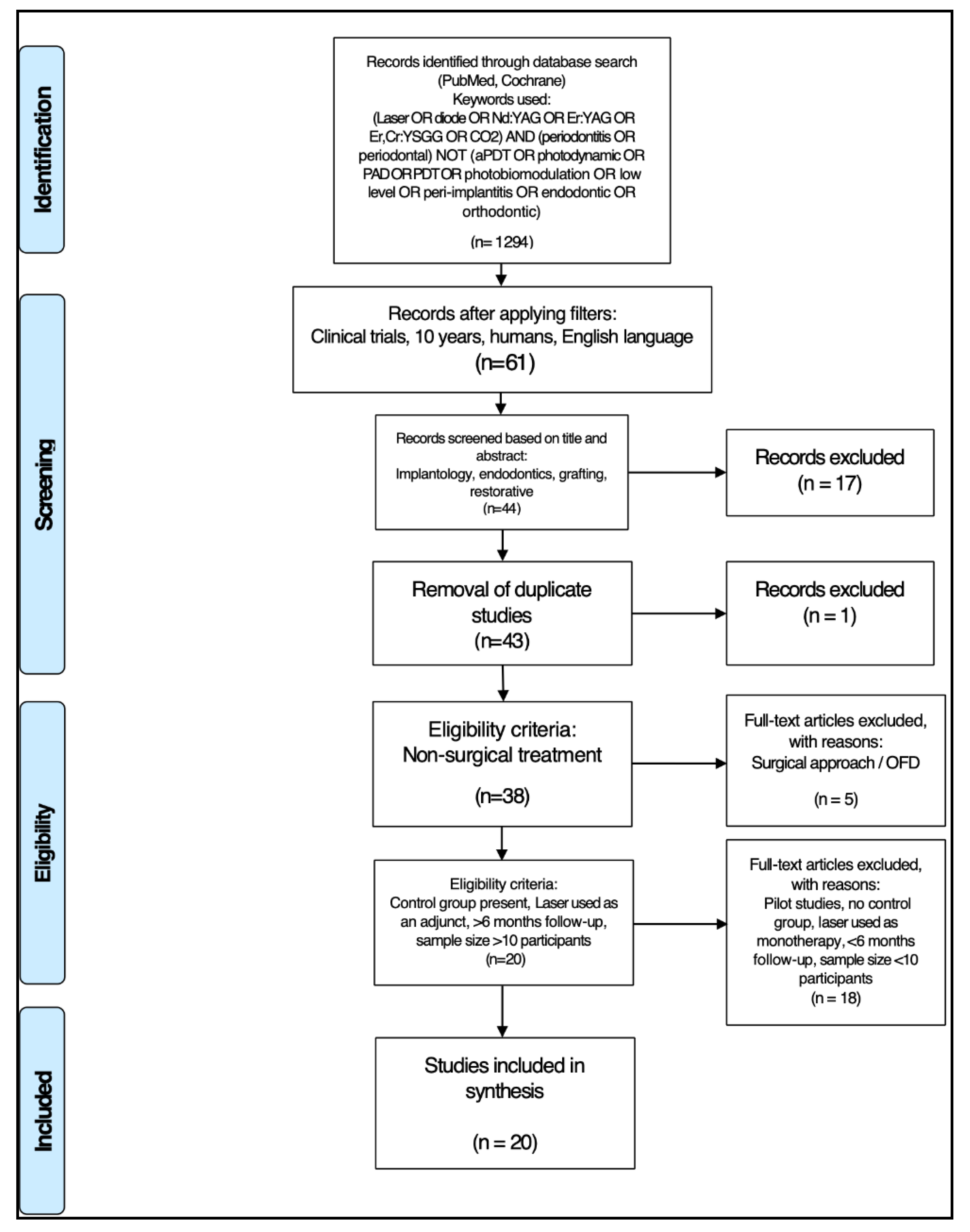
3. Results
3.1. Primary Outcome
3.2. Data Presentation
3.3. Quality Assessment Presentation
3.4. Analysis of Data
- Tip or spot size: 5/14
- Frequency: 2/14
- Fluence/irradiance (either missing or wrongly calculated): 5/14
- Pulse duration: 9/14
- Irradiation time: 8/14
4. Discussion
4.1. Background
4.2. Group Selection at Baseline
4.3. Laser Parameters
4.3.1. Power
4.3.2. Pulse Duration
4.3.3. Power Meter Measurement
4.3.4. Fiber/Tip Size
4.3.5. Irradiation Time
4.4. Treatment Protocols
4.5. Other Clinical Measurements and Influencing Factors
4.5.1. Gingival Crevicular Fluid Level (GCF) Sampling
4.5.2. Smoking
4.5.3. Halitosis
4.5.4. Follow-Up
4.6. General Comments
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eke, P.I.; Zhang, X.; Lu, H.; Wei, L.; Thornton-Evans, G.; Greenlund, K.; Holt, J.; Croft, J. Predicting Periodontitis at State and Local Levels in the United States. J. Dent. Res. 2016, 95, 515–522. [Google Scholar] [CrossRef]
- Garcia, R.I.; Compton, R.; Dietrich, T. Risk assessment and periodontal prevention in primary care. Periodontology 2000 2016, 71, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontology 2000 2006, 40, 11–28. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.; Takei, H.; Klokkevold, P.; Carranza, F. Carranza’s Clinical Periodontology, 11th ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; p. 448. ISBN 978-1-4377-0416-7. [Google Scholar]
- Drisko, C.L. Periodontal Debridement: Still the Treatment of Choice. J. Évid. Based Dent. Pract. 2014, 14, 33–41.e1. [Google Scholar] [CrossRef]
- Cobb, C.M. Non-Surgical Pocket Therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, G.M.; Ash, M.M.; Caffesse, R.G. The Effectiveness of Subgingival Scaling and Root Planing in Calculus Removal. J. Periodontol. 1981, 52, 119–123. [Google Scholar] [CrossRef]
- Schenk, G.; Flemmig, T.F.; Lob, S.; Ruckdeschel, G.; Hickel, R. Lack of antimicrobial effect on periodonto-pathic bacteria by ultrasonic and sonic scalers in vitro. J. Clin. Periodontol. 2000, 27, 116–119. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S.; And, K.J. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Myers, T.D.; Myers, W.D.; Stone, R.M. First soft tissue study utilizing a pulsed Nd: YAG dental laser. Northwest Dent. 1989, 68, 14–17. [Google Scholar]
- Mizutani, K.; Aoki, A.; Coluzzi, D.; Yukna, R.; Wang, C.-Y.; Pavlic, V.; Izumi, Y. Lasers in minimally invasive periodontal and peri-implant thera-py. Periodontology 2000 2016, 71, 185–212. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Ciurescu, C.E.; Cosgarea, R.; Ciurescu, D.; Gheorghiu, A.; Popa, D.; Franzen, R.; Arweiler, N.B.; Sculean, A.; Gutknecht, N. Adjunctive use of InGaAsP and Er,Cr:YSGG lasers in nonsurgical periodontal therapy: A randomized controlled clinical study. Quintessence Int. 2019, 50, 436–447. [Google Scholar] [PubMed]
- Zhou, X.; Lin, M.; Zhang, D.; Song, Y.; Wang, Z. Efficacy of Er: YAG laser on periodontitis as an adjunctive non-surgical treatment: A split-mouth randomized controlled study. J. Clin. Periodontol. 2019, 46, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Celik, T.Z.; Saglam, E.; Ercan, C.; Akbas, F.; Nazaroglu, K.; Tunali, M. Clinical and Microbiological Effects of the Use of Erbium: Yttrium–Aluminum–Garnet Laser on Chronic Periodontitis in Addition to Nonsurgical Periodontal Treatment: A Randomized Clinical Trial—6 Months Follow-Up. Photobiomodulation Photomed. Laser Surg. 2019, 37, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Abduljabbar, T.; Vohra, F.; Kellesarian, S.V.; Javed, F. Efficacy of scaling and root planning with and without adjunct Nd: YAG laser therapy on clinical periodontal parameters and gingival crevicular fluid interleukin 1-beta and tumor necrosis factor-alpha levels among patients with periodontal disease: A prospective randomized split-mouth clinical study. J. Photochem. Photobiol. B Boil. 2017, 169, 70–74. [Google Scholar] [CrossRef]
- Magaz, V.R.; Alemany, A.S.; Alfaro, F.H.; Molina, J.N. Efficacy of Adjunctive Er, Cr:YSGG Laser Application Following Scaling and Root Planing in Periodontally Diseased Patients. Int. J. Periodontics Restor. Dent. 2016, 36, 715–721. [Google Scholar] [CrossRef]
- Dereci, Ö.; Hatipoğlu, M.; Sindel, A.; Tozoglu, S.; Ustun, K. The efficacy of Er,Cr: YSGG laser supported periodontal therapy on the reduction of peridodontal disease related oral malodor: A randomized clinical study. Head Face Med. 2016, 12, 20. [Google Scholar] [CrossRef]
- Sanz-Sánchez, I.; Ortiz-Vigón, A.; Matos, R.; Herrera, D.; Sanz, M. Clinical Efficacy of Subgingival Debridement With Adjunctive Erbium: Yttrium-Aluminum-Garnet Laser Treatment in Patients With Chronic Periodontitis: A Randomized Clinical Trial. J. Periodontol. 2015, 86, 527–535. [Google Scholar] [CrossRef]
- Ustun, K.; Erciyas, K.; Sezer, U.; Şenyurt, S.Z.; Gundogar, H.; Üstün, Ö.; Oztuzcu, S. Clinical and Biochemical Effects of 810 nm Diode Laser as an Adjunct to Periodontal Therapy: A Randomized Split-Mouth Clinical Trial. Photomed. Laser Surg. 2014, 32, 61–66. [Google Scholar] [CrossRef]
- Saglam, M.; Kantarci, A.; Dundar, N.; Hakki, S.S.; Saglam, M. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: A randomized, controlled clinical trial. Lasers Med. Sci. 2012, 29, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Dilsiz, A.; Canakci, V.; Aydin, T. Clinical Effects of Potassium–Titanyl–Phosphate Laser and Photodynamic Therapy on Outcomes of Treatment of Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2013, 84, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Alves, V.T.E.; De Andrade, A.K.P.; Toaliar, J.M.; Conde, M.C.; Zezell, D.M.; Cai, S.; Pannuti, C.M.; De Micheli, G. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: A 6-month clinical trial. Clin. Oral Investig. 2012, 17, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Zingale, J.; Harpenau, L.; Chambers, D.; Lundergan, W. Effectiveness of root planing with diode laser curettage for the treatment of periodontitis. J. Calif. Dent. Assoc. 2012, 40, 786–793. [Google Scholar] [PubMed]
- Slot, D.E.; Timmerman, M.F.; Versteeg, P.A.; Van Der Velden, U.; Van Der Weijden, F.A. Adjunctive clinical effect of a water-cooled Nd: YAG laser in a periodontal maintenance care programme: A randomized controlled trial. J. Clin. Periodontol. 2012, 39, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Giannopoulou, C.; Cappuyns, I.; Cancela, J.; Cionca, N.; Mombelli, A. Effect of Photodynamic Therapy, Diode Laser, and Deep Scaling on Cytokine and Acute-Phase Protein Levels in Gingival Crevicular Fluid of Residual Periodontal Pockets. J. Periodontol. 2012, 83, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Eltas, A.; Orbak, R. Clinical Effects of Nd: YAG Laser Applications During Nonsurgical Periodontal Treatment in Smoking and Nonsmoking Patients with Chronic Periodontitis. Photomed. Laser Surg. 2012, 30, 360–366. [Google Scholar] [CrossRef]
- Eltas, A.; Orbak, R. Effect of 1,064-nm Nd: YAG laser therapy on GCF IL-1β and MMP-8 levels in patients with chronic periodontitis. Lasers Med Sci. 2011, 27, 543–550. [Google Scholar] [CrossRef]
- Qadri, T.; Javed, F.; Poddani, P.; Tunér, J.; Gustafsson, A. Long-term effects of a single application of a water-cooled pulsed Nd: YAG laser in supplement to scaling and root planing in patients with periodontal inflammation. Lasers Med. Sci. 2010, 26, 763–766. [Google Scholar] [CrossRef]
- Kelbauskiene, S.; Baseviciene, N.; Goharkhay, K.; Moritz, A.; Machiulskiene, V. One-year clinical results of Er,Cr:YSGG laser application in addition to scaling and root planing in patients with early to moderate periodontitis. Lasers Med. Sci. 2010, 26, 445–452. [Google Scholar] [CrossRef]
- Rotundo, R.; Nieri, M.; Cairo, F.; Franceschi, D.; Mervelt, J.; Bonaccini, D.; Esposito, M.; Pini-Prato, G. Lack of adjunctive benefit of Er: YAG laser in non-surgical periodontal treatment: A randomized split-mouth clinical trial. J. Clin. Periodontol. 2010, 37, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Lopes, B.M.V.; Theodoro, L.H.; Melo, R.F.; Thompson, G.M.D.A.; Marcantonio, R.A.C. Clinical and Microbiologic Follow-Up Evaluations After Non-Surgical Periodontal Treatment With Erbium:YAG Laser and Scaling and Root Planing. J. Periodontol. 2010, 81, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Donos, N.; Calciolari, E.; Brusselaers, N.; Goldoni, M.; Bostanci, N.; Belibasakis, G.N. The adjunctive use of host modulators in non-surgical periodontal therapy. A systemat-ic review of randomized, placebo-controlled clinical studies. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 199–238. [Google Scholar] [CrossRef] [PubMed]
- Peron, D.; Bergamo, A.; Prates, R.; Vieira, S.S.; Carvalho, P.D.T.C.D.; Serra, A.J. Photodynamic antimicrobial chemotherapy has an overt killing effect on periodontal pathogens? A systematic review of experimental studies. Lasers Med. Sci. 2019, 34, 1527–1534. [Google Scholar] [CrossRef]
- Coluzzi, D.J.; Aoki, A.; Chininforush, N. Laser Treatment of Periodontal and Peri-implant Disease in Lasers in Dentistry—Current Concepts; Coluzzi, D.J., Parker, S.P.A., Eds.; Springer Nature: Cham, Switzerland, 2017; pp. 295–309. ISBN 978-3-319-51943-2. [Google Scholar]
- Heitz-Mayfield, L.J.A.; Lang, N.P. Surgical and nonsurgical periodontal therapy. Learned and unlearned concepts. Periodontology 2000 2013, 62, 218–231. [Google Scholar] [CrossRef]
- Newman, M.; Takei, H.; Klokkevold, P.; Carranza, F. Carranza’s Clinical Periodontology, 11th ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 525–543. ISBN 978-1-4377-0416-7. [Google Scholar]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontology 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Parker, S. Lasers and soft tissue: Periodontal therapy. Br. Dent. J. 2007, 202, 309–315. [Google Scholar] [CrossRef]
- Dilsiz, A.; Sevinc, S. KTP laser therapy as an adjunctive to scaling and root planing in treatment of chronic periodontitis. Acta Odontol. Scand. 2014, 72, 681–686. [Google Scholar] [CrossRef]
- Amaroli, A.; Barbieri, R.; Signore, A.; Marchese, A.; Parker, S.; De Angelis, N.; Benedicenti, S. Simultaneous photoablative and photodynamic 810-nm diode laser therapy as an adjunct to non-surgical periodontal treatment: An in-vitro study. Minerva Stomatol. 2020, 69, 1–7. [Google Scholar] [CrossRef]
- Gómez, C.; Bisheimer, M.; Costela, A.; Garcia-Moreno, I.; García, A.; García, J.A. Evaluation of the Effects of Er:YAG and Nd: YAG Lasers and Ultrasonic Instrumentation on Root Surfaces. Photomed. Laser Surg. 2009, 27, 43–48. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, Y.; Shu, R. Er,Cr: YSGG Laser Application for the Treatment of Periodontal Furcation Involvements. Photomed. Laser Surg. 2017, 35, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Sumra, N.; Kulshrestha, R.; Umale, V.; Chandurkar, K. Lasers in non-surgical periodontal treatment—A review. J. Cosmet. Laser Ther. 2018, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Belal, M.H.; Watanabe, H. Comparative Study on Morphologic Changes and Cell Attachment of Periodontitis-Affected Root Surfaces Following Conditioning with CO2and Er: YAG Laser Irradiations. Photomed. Laser Surg. 2014, 32, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Nevins, M.; Nevins, M.L.; Parma-Benfenati, S.; Benfenati, M.R.; Schupbach, P.; Chen, C.-Y.; Sava, C.; Sava, C.; Trifan, M.; Kim, D.M. Human Clinical and Histologic Evaluations of Laser-Assisted Periodontal Therapy with a 9.3-μm CO2 Laser System. Int. J. Periodontics Restor. Dent. 2020, 40, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M.; Low, S.B.; Coluzzi, D.J. Lasers and the Treatment of Chronic Periodontitis. Dent. Clin. N. Am. 2010, 54, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, M.; Bani, D.; Viti, C.; Tani, A.; Lorenzini, L.; Zecchi-Orlandini, S.; Formigli, L. Comparative Evaluation of the Effects of Different Photoablative Laser Irradiation Protocols on the Gingiva of Periodontopathic Patients. Photomed. Laser Surg. 2012, 30, 222–230. [Google Scholar] [CrossRef]
- Van Swol, C.F.; Slaa, E.T.; Verdaasdonk, R.M.; De La Rosette, J.J.; Boon, T.A. Variation in output power of laser prostatectomy fibers: A need for power measurements. Urology 1996, 47, 672–678. [Google Scholar] [CrossRef]
- Gris Sanchez, I. Fabrication and Applications of Low OH Photonic Crystal Fibres. Ph.D Thesis, University of Bath, Claverton Down, UK, 2012. [Google Scholar]
- Harrington, J.A. Theoretical Foundations of Infrared Fiber Optic Transmission: Solid-Core Fibers. In Infrared Fibers and Their Applications; SPIE-Intl Soc Optical Eng: Bellingham, WA, USA, 2010; pp. 11–38. [Google Scholar]
- Yoshida, K.; Furui, Y.; Sentsui, S.; Kuroha, T. Loss factors in optical fibres. Opt. Quantum Electron. 1981, 13, 85–89. [Google Scholar] [CrossRef]
- Lasers in Dentistry—Current Concepts. Lasers Dent. Current Concept. 2017, 29–56. [CrossRef]
- Zhu, H.; Zhang, S.; Ahn, C. Sample size considerations for split-mouth design. Stat. Methods Med. Res. 2015, 26, 2543–2551. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2017, 76, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Bergström, J. Cigarette smoking as risk factor in chronic periodontal disease. Community Dent. Oral Epidemiol. 1989, 17, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Greenman, J. Halitosis (breath odor). Periodontology 2000 2008, 48, 66–75. [Google Scholar] [CrossRef]
- American Academy of Periodontology. Position Paper, Periodontal Maintenance. J. Periodontol. 2003, 74, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Carranza, F.; Camargo, P. The Periodontal Pocket. In Carranza’s Clinical Periodontology, 11th ed.; Elsevier Saunders: St. Louis, MO, USA, 2012; pp. 127–139. [Google Scholar]
- Parker, S.; Cronshaw, M.; Anagnostaki, E.; Bordin-Aykroyd, S.R.; Lynch, E. Systematic Review of Delivery Parameters Used in Dental Photobiomodulation Therapy. Photobiomodulation Photomed. Laser Surg. 2019, 37, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Al-Falaki, R.; Cronshaw, M.; Hughes, F.J. Treatment outcome following use of the erbium, chromium:yttrium, scandium, gallium, garnet laser in the non-surgical management of peri-implantitis: A case series. Br. Dent. J. 2014, 217, 453–457. [Google Scholar] [CrossRef]
- Gutknecht, N.; Van Betteray, C.; Ozturan, S.; Vanweersch, L.; Franzen, R. Laser Supported Reduction of Specific Microorganisms in the Periodontal Pocket with the Aid of an Er,Cr: YSGG Laser: A Pilot Study. Sci. World J. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
| Citation [Ref] | Type of Study/Number of Samples/Pocket Depth | Test/Control Groups | Aim/Approach | Laser Protocol/Number of Sessions | Follow-Up | Outcomes Including Stated PD Reduction and CAL Gain with Statistical Significance |
|---|---|---|---|---|---|---|
| Ciurescu et al., 2019 [14] | Parallel group RCT 38 Pts. Chronic periodontitis 1 pocket per quadrant ≥5 mm | Test (19) 940 Diode, Er,Cr:YSGG + US Control (19) US + hand instruments | PPD, BOP, CAL PCR microbiological. analysis | (i) diode, 940 nm: 1.5 W (day 0)–2 W (day 7), non-initiated 300 μm tip, sinusoidal retracting movements, 30 s for mono-rooted–60 s for multirooted/3 sessions: day 0, 7, 60 (ii) Er,Cr:YSGG 2780 nm: AvP 1.5 W, 30 Hz, 50 μs pulse, 45 mj/pulse, 500 μm radial tip, 10 s/mm pocket for mono-rooted–15 s/mm pocket for multi-rooted/2 sessions: day 7, 60 | 2 m tx and 6 months | Test group significantly better in PD, CAL, BOP Pg, Td, Tf, Pi, Pm, Fn, En compared to control. At 6 months, compared to control, PD reduction 1.19 mm; CAL 0.98 mm p < 0.001 for both. |
| Zhou et al., 2019 [15] | Randomized, single-blinded, controlled trial/25 patients/Split mouth, chronic periodontitis one pocket per quad ≥4 mm and BOP | one quadrant SRP + Er:YAG/one quadrant SRP | PPD, CAL, BI, PI at baseline, 3 months, and 6 months | Er:YAG (2940 nm) Hard tissue: 100 mJ 15 Hz Chisel tip coronal to apical in slow parallel paths. Soft tissue: 50 mJ 30 Hz Conical tip 800 μm | 3 months, and 6 months | Er:YAG + SRP: PD and CAL sig. difference between groups at 3 + 6 mo. Differences clinically small (0.11 mm PD 0.2 mm CAL at 6 mo) p < 0.03 for both |
| Celik et al., 2019 [16] | Parallel group RCT 38 pts. 4 teeth in each quadrant, had a at least 4 pockets with PD ≥5 mm | SRP + Er:YAG 19 patients/SRP 19 patients | PPD, CAL, PI, BOP Microbiological evaluation using PCR | Er:YAG 2940 nm 150 mJ 10 Hz water irrigation 600 µm tip Coronal to apical 15–20° | 3 months, 6 months | Test group significantly better than control in CAL, PD. At 6 months, compared to control, PD reduction 0.3–0.8 mm; CAL 0.5–0.8 mm p < 0.05 No significant difference in Pg Tf Td (Porphyromonas gingivalis, Tannerella forsythia Treponema denticola) |
| Abduljabbar et al., 2017 [17] | Split-mouth RCT/28 male patients with PD ≥4 mm | SRP + Nd:YAG/SRP | PI, BOP and PPD and GCF IL-1β (interleukin 1-beta)and TNF-α tumor necrosis factor-alpha) levels | Nd:YAG 1064 nm. Av P 4 W/80 mJ pp/50 Hz. Pulse width 350 μs peak power 240 W; Irrad: 1430 W/cm2 60–120 s/tooth. Total energy/tooth 240–480 J. | 3 months, and 6 months | Test group significantly better in PI, BOP, PD and GCF IL-1β and TNF-α levels compared with SRP alone. At 6 months compared to control, PD reduction listed as 1.0 mm p < 0.01. |
| Magaz et al., 2016 [18] | Split mouth RCT/30 pts. PD ≥4 mm + BOP | SRP + Er,Cr:YSGG/ SRP | PI, BOP, PPD, GR, CAL | Er,Cr:YSGG 2780 nm Av P 1.0 W, 50 mJ, 20 Hz. Air 10%/Water 15%, 60 s/tooth, 5–15°, 600 µm tip | 6 weeks and 6 months | No significant difference between test and control groups. |
| Dereci et al., 2016 [19] | Parallel group RCT 60 pts/ 2 teeth with PD ≥5 mm + Halitosis | SRP + Er,Cr:YSGG/ SRP | PI, PPD, CAL, BOP, Halitosis VSC | Er,Cr:YSGG 2780 nm Av P: 1.5 W 30 Hz/Air 11%/Water 20%. 140 μs pulse. 600 µm radial firing tip. 10° apical to coronal 3 sessions/day 0, 2, 7 | 1, 3, 6 months | Test group significantly better in BOP and halitosis reduction (VSC) compared to control |
| Sanz-Sánchez et al., 2015 [20] | Parallel-group RCT /37 patients/ ≥4 teeth per quadrant, one with PD ≥4.5 mm, BOP Chronic periodontitis | SRP ultrasonic + Er:YAG (17) patients)/SRP ultrasonic (20 patients) | PD, REC, CAL, BOP | Er:YAG 2940 nm 160 mJ 10 Hz Sapphire tip | 3, 6, 12 Months | Test group achieved a significantly lower percentage of PD ≥4.5 mm (p = 0.004) No significant difference between the groups for mean PD reduction (p = 0.08) or other clinical parameters. At 12 months, compared to control, PD reduction 0.16 and CAL 0.13 mm. |
| Üstün et al., 2014 [21] | Split-mouth RCT 19 pts PD 4–7 mm incisors or canines in two quadrants | SRP + diode laser 810 nm/ SRP | PI, GI, CAL, PPD GCF IL-1β flow cytometry | Diode 810 nm P 2.5 W Duty cycle 50% Av.P. 1.5 W 320 μm fiber, sweeping motion, slightly initiated tip, apical to coronal sweeping motion, 20 s per site/4 sites 1 session day 0 | 1, 3, and 6 months | Test group: At 1 month PPD, GI and GCF IL-1β significantly better p < 0.05 At 3 months PPD, CAL, GI and GCF IL-1β significantly better p < 0.05 At 6 months PPD, CAL, and GCF IL-1β significantly better p < 0.05. At 6 months compared to control, PD reduction 0.24 mm and CAL 0.45 mm. Both p < 0.05 |
| Saglam et al., 2014 [22] | Parallel-group, RCT 30 pts. 2 teeth/quadrant PD ≥5 mm | SRP + diode 940 nm/ SRP | PI, GI, BOP, PPD, CAL GCF assay IL-1β, IL-6 (interleukin-6), IL-8 (interleukin-8), MMP-1 (matrix metalloproteinase-1), MMP-8 (matrix metalloproteinaise-8, TIMP-1(tissue inhibitor matrix metalloproteinase-1) | Diode 940 nm Av.P.1.5 W. Pulse length 20 ms on /20 ms off 10 s/buccal 10 s/lingual 15 J/cm2 fluence 300 μm tip Sweeping motion apical to coronal 1 session day 0 | 1, 3, and 6 months | Test group significant better compared to control: At 1 mo PPD, GI BOP, MMP-8 At 3 months BOP, TIMP-1 At 6 months PI, GI, TIMP-1. At 6 months compared to control, PD reduction 1.0 mm and CAL 0.2 mm Both p < 0.05. |
| Dilsiz et al., 2013 [23] | Split-mouth RCT/24 patients ≥4 non-adjacent teeth with PD ≥5 mm, BOP and bone loss Chronic periodontitis | SRP + KTP (potassium titanyl phosphate) (1)/ SRP + aPDT (2), SRP (3) (aPDT: MB + 808 nm) note: the aPDT group (2) was not included in our review. | PI, GI, BOP, PD, CAL | KTP 532 nm 2 applications: 0.8 W/50 ms on/50 off. 30 s. 200 μm/11.7 J/cm2 sweeping motion horizontally and coronally. 1 session: day 0 | 6 months | SRP + KTP group: Significant difference in PD and CAL compared to both other control groups. At 6 months in group 1 compared to control, PD reduction 2.08 and CAL 2,42 mm. Both p <0.001 |
| Euzebio Alves et al., 2013 [24] | Split-mouth RCT/36 patients, one pair of contra-lateral single rooted teeth with PD >5 mm Chronic periodontitis | SRP + diode 808 nm/ SRP Full mouth debridement Only 36/36 teeth evaluated | CAL, PD, PI, BOP Microbiological Analysis CFU count Pg, Pi, Aa (Porphyromonas gingivalis, Prevotella intermedia and Aggregatibacter actinomycetemcomitans) | Diode 808 nm, 400 m fiber, 1.5 W CW, Irradiance 1193.7 W/cm2 Sweeping motion coronally parallel to tooth, 20 s/pocket. 2 sessions: day 0, 7 | 6 weeks, 6 months | No significant differences between groups |
| Zingale et al., 2012 [25] | Split-mouth RCT/25 pts. At least 5 pockets with PD ≥5–9 mm | 2 test groups: Laser + SRP(1) Laser + SRP + laser sealing(2) 3 control groups: SRP only(3) papillae reflection + SRP + flap closure(4) No treatment(5) | PPD, BOP, CAL | Diode 810 nm 0.8 W CW 400 µm fibre initiated, 30–45 s per tooth (same parameters for curettage and sealing) | 3, 6 months | No significant differences between treatment groups |
| Slot et al., 2012 [26] | Split-mouth RCT, 30 pts, At least two sites per quadrant with PD ≥5 mm, attachment loss ≥2 mm, BOP and bone loss. Moderate to severe generalised periodontitis | SRP + Nd:YAG/ SRP | Post-op pain, bleeding, swelling evaluation PD, REC, BOP | From reference Slot 2011 | 1 day post-op pain, bleeding, swelling evaluation 6 months PD, REC, BOP | Pain, bleeding, swelling reported significantly worse in test group No significant difference between groups in PD, REC, BOP |
| Giannopoulou et al., 2012 [27] | Split-mouth three-arm parallel-design RCT 32 pts. Per quadrant PD ≥5 mm +/CAL loss ≥ 2 mm + BOP | SRP + Diode 810 nm 1 Quadrant (1) SRP + aPDT 1 Quadrant (2) SRP 1 Quadrant (3) Note SRP + aPDT group (2) not included in our review. | PPD, BOP, REC, GCF levels of 22 different biomarkers, cytokines, acute-phase proteins evaluated | Diode laser 810 nm 1 W 60 s per tooth. | 2 weeks, 2, 6 months | No significant differences between groups at any time Remaining pockets >4 mm 25% in test group vs 9% in both other groups (p = 0.034) |
| Eltas et al., 2012 (smokers) [28] | Split-mouth –4 armed RCT/52 patients 2 teeth per quadrant with PD 4–6 and bone loss, Chronic periodontitis | SRP + Laser (52 teeth from 26 patients- smokers) SRP + Laser (52 teeth from 26 patients non-smokers) SRP (52 teeth from 26 patients smokers) SRP (52 teeth from 26 patients non-smokers) | PI, CAL, PD, GI, GCF (volume) | Nd:YAG 1064 nm. Av P 1 W 100 mJ, 10 Hz Apical-coronal sweeping motion 120 s/ tooth. | 1, 6 months | Statistically significant PD, GI, CGF improvement between non-smoker test group and all other groups at 6 months. (p < 0.05) At 6 months, the non-smoker group had PD reduction of 0.5 compared to all other groups. Statistically significant GI, CGF improvement between non-smoker test group and both smoker groups at 6 months. (p < 0.05) No significant differences in any parameters between test and control in the smoker group. |
| Eltas et al., 2012 [29] | Split-mouth, RCT 20 pts/40 teeth PD ≥ 4–6 mm/CAL loss ≥2 mm+ | Nd:YAG + SRP(1tooth/patient) SRP. (1tooth/patient) Full mouth debridement. Only 2 teeth evaluated | PI, GI, PPD, CAL. GCF IL-1β and MMP-8 levels | Nd:YAG 1064 nm. Av P 1 W 100 mJ 10 Hz Apical-coronal sweeping motion 120 s/tooth 200 µm fibre | 3, 9 months | 9 months test group significantly better results in PPD, CAL, GI, and GCF values. At 9 months compared to control, PD reduction 0.91 and CAL 1.17 mm. Both p < 0.001. IL-1β and MMP-8 no sig. diff. |
| Qadri et al., 2011 [30] | Split-mouth RCT 22 pts at least six pockets of 4–8 mm on each side of the mandible | SRP + Nd:YAG/SRP | PI, GI, PPD, and marginal bone loss (measured on dig. BW radiographs. GCF vol. | Nd:YAG 1064 nm Av P. 4 W, 80 mJ/pulse, 50 Hz 350 μs pulse w/a 9 20–30° angulation Tx 60 and 120 s, depending on accessibility. | Median follow-up time 20 months (range 12–39 months) | PI, GI, PPD, marginal bone loss and GCF-volume significantly improved compared to control group Pocket depth reduction compared to control 1.61 mm at 20 months p < 0.001 |
| Kelbauskiene et al., 2011 [31] | Split-mouth RCT/ 30 patients PD 4–6 mm on at least one site of a single rooted tooth. At least two quadrants are included. Chronic periodontitis | SRP + Er,Cr:YSGG 8–9 teeth per patient 509 sites test 579 control and | PI, BOP, PD, REC, CAL | Er,Cr:YSGG 2780 nm 1 W Av P. 20 Hz/600 μm tip 10%w/a 5–15° from coronal to apical (until root surface “acid-etched” appearance) Inner epithelial lining to the depth of pocket removed, 5 mm of outer epithelium removed, root surface conditioning. 3 sessions/day 0,7,14. 2nd and 3rd visits the amount of inner epithelial lining removed 1 mm less than the previous session. | 2,3,6,12 months | Test group: Statistically significant differences in BOP, PD, and CAL compared to control. (p < 0.001) No differences for PI, REC |
| Rotundo et al., 2010 [32] | Split-mouth 4-armed RCT 27 pts. At least 2 teeth per quad PD ≥4–9 mm + BOP | SRP + Er:YAG (1) SRP (2) Er:YAG (3) Only supragingival debridement (4) Each one quadrant | PPD, BOP, PI, REC, CAL. Full mouth plaque & bleeding scores in addition. VAS pain. | Er:YAG 2940 nm 150 mJ 10 Hz 500 µm tip Water Coronal to apical 20° angulation | 6 months | No significant difference in CAL between groups No p-values for all parameters except VAS and CAL |
| Lopes et al., 2010 [33] | Split-mouth 4 armed RCT/ 19 patients 4 non-adjacent sites in different quadrants with BOP and PD 5–9 mm. | SRP + laser(1)/ laser only(2)/ SRP(3)/ no treatment(4) 76 sites, 42 single or double rooted teeth, 34 multirooted teeth. Note: Group 2 was not included in our review because of monotherapy. | PD, GR, CAL, PI, GI, BOP GCF Microbiol analysis PCR Aa, Pg, Pi, Tf, Pn | Er:YAG, 2940 nm 100 mJ/10 Hz/AP 1.0 W, 12.9 J/cm2 1.1 × 0.5 mm tip 30 s per site Apico-coronal movement 30° angulation Total irradiation time 180–240 s for each patient. | 1, 3, 6, 12 months | SRP + laser statistically sig bacterial reduction at 6 and 12 months (p < 0.05) No statistically sig diff for PB, CAL, GI, BOP and PD. |
| Citation [Ref] | Randomization | Sample Size Calculation and Required Number Included | Baseline Situation Similar | Blinding | Parameters of laser Use Described and Calculations Correct | Power-Meter Used | Numerical Results Available (Stats) | No Missing Out-Come Data | All Samples/Patients Completed the Follow-Up | Correct Interpretation of Data | Total Score/10 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PERIO | |||||||||||
| Ciurescu et al., 2019 [14] | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| Zhou, X et al., 2019 [15] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
| Celik et al., 2019 [16] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
| Abduljabbar et al., 2017 [17] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 9 |
| Magaz et al., 2016 [18] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
| Dereci et al., 2016 [19] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 7 |
| Sanz-Sánchez et al., 2015 [20] | Yes | Yes | No | Yes | No | No | Yes | Yes | Yes | No | 6 |
| Üstün et al., 2014 [21] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | 8 |
| Saglam et al., 2014 [22] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 9 |
| Dilsiz et al., 2013 [23] | Yes | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 8 |
| Euzebio Alves et al., 2013 [24] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 10 |
| Zingale et al., 2012 [25] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 7 |
| Slot et al., 2012 [26] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 7 |
| Giannopoulou et al., 2012 [27] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | No | Yes | 7 |
| Eltas et al., 2012 (Smokers) [28] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | 7 |
| Eltas S et al., 2012 [29] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 7 |
| Qadri et al., 2011 [30] | Yes | No | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 7 |
| Kelbauskiene et al., 2011 [31] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
| Rotundo et al., 2010 [32] | Yes | Yes | Yes | Yes | No | No | No | Yes | Yes | No | 6 |
| Lopes et al., 2010 [33] | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coluzzi, D.; Anagnostaki, E.; Mylona, V.; Parker, S.; Lynch, E. Do Lasers Have an Adjunctive Role in Initial Non-Surgical Periodontal Therapy? A Systematic Review. Dent. J. 2020, 8, 93. https://doi.org/10.3390/dj8030093
Coluzzi D, Anagnostaki E, Mylona V, Parker S, Lynch E. Do Lasers Have an Adjunctive Role in Initial Non-Surgical Periodontal Therapy? A Systematic Review. Dentistry Journal. 2020; 8(3):93. https://doi.org/10.3390/dj8030093
Chicago/Turabian StyleColuzzi, Donald, Eugenia Anagnostaki, Valina Mylona, Steven Parker, and Edward Lynch. 2020. "Do Lasers Have an Adjunctive Role in Initial Non-Surgical Periodontal Therapy? A Systematic Review" Dentistry Journal 8, no. 3: 93. https://doi.org/10.3390/dj8030093
APA StyleColuzzi, D., Anagnostaki, E., Mylona, V., Parker, S., & Lynch, E. (2020). Do Lasers Have an Adjunctive Role in Initial Non-Surgical Periodontal Therapy? A Systematic Review. Dentistry Journal, 8(3), 93. https://doi.org/10.3390/dj8030093






