Management of Peri-Implantitis Lesions without the Use of Systemic Antibiotics: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources, Search Strategy and Study Selection
2.4. Risk of Bias (RoB) in Individual Studies
2.5. Data Synthesis: Evaluating the Effect of the Intervention
2.6. Additional Analysis
3. Results
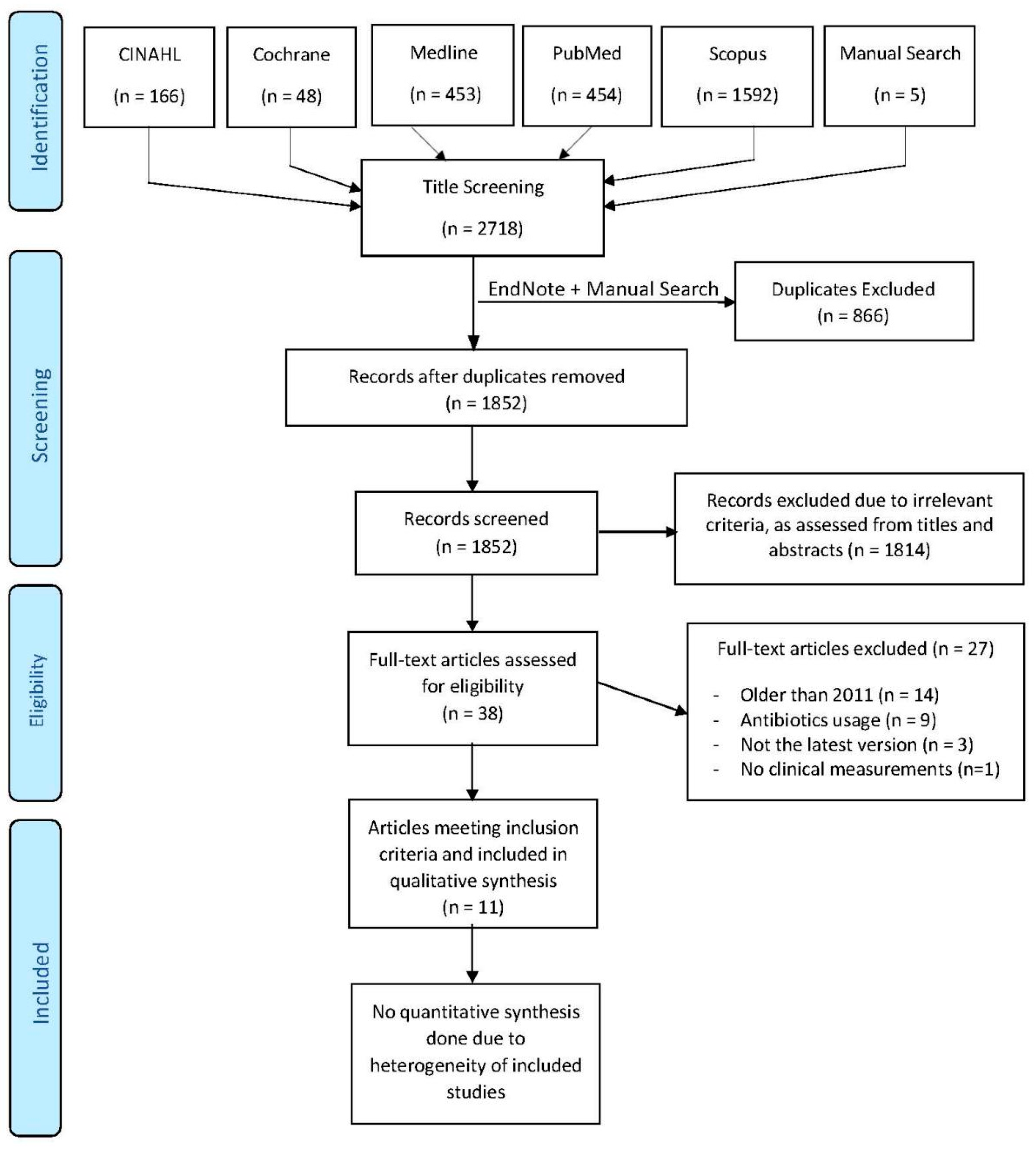
3.1. Literature Search and Screening Process
3.2. Study Characteristics
3.2.1. Non-Surgical Techniques
3.2.2. Surgical Techniques
3.3. Data Analysis
3.4. Risk of Bias
3.5. Quality of the Evidence
4. Discussion
4.1. Overall Completeness and Applicability of Evidence
4.1.1. Inclusion Criteria
4.1.2. Exclusion Criteria
4.1.3. Outcome Measures
4.1.4. Interventions
4.1.5. Sample Sizes
4.1.6. Randomisation and Blinding
4.1.7. Follow-Up
4.1.8. Statistical Analysis
4.1.9. Drop Outs, Withdrawals and Failures
4.2. Quality of the Evidence
- Non-surgical debridement + CHX chips;
- Non-surgical debridement + matrix chips;
- Surgical resective treatment + 0.12% CHX and 0.05% CPC;
- Surgical resective treatment + 2% CHX.
4.3. Practical Implications
4.3.1. Non-Surgical Therapies
4.3.2. Surgical Therapies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacing missing teeth: Treatment of peri-implantitis. Cochrane Database Syst. Rev. 2012, 1, CD004970. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Berglundh, T. Periimplant diseases: Where are we now? Consensus of the seventh european workshop on periodontology. J. Clin. Periodontol. 2011, 38, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Chapple, I.L. On behalf of working group 4 of the viii european workshop on periodontology* clinical research on peri-implant diseases: Consensus report of working group 4. J. Clin. Periodontol. 2012, 39, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Berglundh, T.; Genco, R.; Aass, A.M.; Demirel, K.; Derks, J.; Figuero, E.; Giovannoli, J.L.; Goldstein, M.; Lambert, F.; et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J. Clin. Periodontol. 2015, 42, S152–S157. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.-L. Peri-implantitis. J. Clin. Periodontol. 2018, 45, S246–S266. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S278–S285. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.-M. Long time follow up of implant therapy and treatment of peri-implantitis. Swed. Dent. J. Suppl. 2007, 188, 7–66. [Google Scholar]
- Atieh, M.; Alsabeeha, N.H.; Faggion, C.M.; Duncan, W. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2012, 84, 1–15. [Google Scholar] [CrossRef]
- Chambrone, L.; Gondim, V.; Schmitter, M.; Tu, Y.-K. Comparison of the effects of treatment of peri-implant infection in animal and human studies: Systematic review and meta-analysis. Clin. Oral Implant. Res. 2010, 21, 137–147. [Google Scholar] [CrossRef]
- Machtei, E.E. Treatment alternatives to negotiate peri-implantitis. Adv. Med. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Muthukuru, M.; Zainvi, A.; Esplugues, E.O.; Flemmig, T.F. Non-surgical therapy for the management of peri-implantitis: A systematic review. Clin. Oral Implant. Res. 2012, 23, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.; Lin, G.-H.; Suarez, F.; Mac Eachern, M.; Wang, H. Surgical management of peri-implantitis: A systematic review and meta-analysis of treatment outcomes. J. Periodontol. 2014, 85, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Steven-Howe, M.; Richards, D. Surgical regenerative treatment of peri-implantitis. Evid. Based Dent. 2017, 18, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Daugela, P.; Almeida, R.F.; Saulacic, N. Surgical non-regenerative treatments for peri-implantitis: A systematic review. J. Oral Maxillofac. Res. 2016, 7, e14. [Google Scholar] [CrossRef][Green Version]
- Heitz-Mayfield, L.J.; Mombelli, A. The therapy of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 325–345. [Google Scholar] [CrossRef]
- Ramanauskaite, A.; Daugela, P.; Juodzbalys, G. Treatment of peri-implantitis: Meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int. 2016, 47, 379–393. [Google Scholar]
- Mahato, N.; Wu, X.; Wang, L. Management of peri-implantitis: A systematic review, 2010–2015. Springer Plus 2016, 5, 105. [Google Scholar] [CrossRef]
- Natto, Z.S.; Aladmawy, M.; Levi, P.A.; Wang, H.-L. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2015, 30, 338–345. [Google Scholar] [CrossRef]
- Schwarz, F.; Becker, K.; Renvert, S. Efficacy of air polishing for the non-surgical treatment of peri-implant diseases: A systematic review. J. Clin. Periodontol. 2015, 42, 951–959. [Google Scholar] [CrossRef]
- Klinge, B.; Gustafsson, A.; Berglundh, T. A systematic review of the effect of anti-infective therapy in the treatment of peri-implantitis. J. Clin. Periodontol. 2002, 29, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Moher, D.; Shamseer, L.; Clarke, M.J.; Ghersi, D.; Liberati, A.; Petticrew, M.P.; Shekelle, P.G.; Stewart, L. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.; Clarke, M.J.; Ghersi, D.; Moher, D.; Petticrew, M.P.; Stewart, L. An international registry of systematic-review protocols. Lancet 2011, 377, 108–109. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.J.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.P.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, M. Introducing GRADE: A systematic approach to rating evidence in systematic reviews and to guideline development. KT Update 2013, 1, 1–9. [Google Scholar]
- Arısan, V.; Karabuda, Z.C.; Arıcı, S.V.; Topcuoglu, N.; Kulekci, G. A randomized clinical trial of an adjunct diode laser application for the nonsurgical treatment of peri-implantitis. Photomed. Laser Surg. 2015, 33, 547–554. [Google Scholar] [CrossRef]
- John, G.; Sahm, N.; Becker, J.; Schwarz, F. Nonsurgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine. Twelve-month follow-up of a prospective, randomized, controlled clinical study. Clin. Oral Investig. 2015, 19, 1807–1814. [Google Scholar] [CrossRef]
- Machtei, E.E.; Frankenthal, S.; Levi, G.; Elimelech, R.; Shoshani, E.; Rosenfeld, O.; Tagger-Green, N.; Shlomi, B. Treatment of peri-implantitis using multiple applications of chlorhexidine chips: A double-blind, randomized multi-centre clinical trial. J. Clin. Periodontol. 2012, 39, 1198–1205. [Google Scholar] [CrossRef]
- Roos-Jansåker, A.-M.; Almhöjd, U.S.; Jansson, H. Treatment of peri-implantitis: Clinical outcome of chloramine as an adjunctive to non-surgical therapy, a randomized clinical trial. Clin. Oral Implant. Res. 2015, 28, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sahm, N.; Becker, J.; Santel, T.; Schwarz, F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: A prospective, randomized, controlled clinical study. J. Clin. Periodontol. 2011, 38, 872–878. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lindahl, C.; Jansåker, A.-M.R.; Persson, G.R. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: A randomized clinical trial. J. Clin. Periodontol. 2010, 38, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hentenaar, D.F.; De Waal, Y.C.; Strooker, H.; Meijer, H.J.A.; Van Winkelhoff, A.J.; Raghoebar, G.M. Implant decontamination with phosphoric acid during surgical peri-implantitis treatment: A RCT. Int. J. Implant. Dent. 2017, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Isehed, C.; Svenson, B.; Lundberg, P.; Holmlund, A. Surgical treatment of peri-implantitis using enamel matrix derivative, an RCT: 3- and 5-year follow-up. J. Clin. Periodontol. 2018, 45, 744–753. [Google Scholar] [CrossRef]
- Papadopoulos, C.A.; Vouros, I.; Menexes, G.C.; Konstantinidis, A. The utilization of a diode laser in the surgical treatment of peri-implantitis. A randomized clinical trial. Clin. Oral Investig. 2015, 19, 1851–1860. [Google Scholar] [CrossRef]
- De Waal, Y.C.; Raghoebar, G.M.; Slater, J.J.R.H.A.; Meijer, H.J.; Winkel, E.G.; Van Winkelhoff, A.J. Implant decontamination during surgical peri-implantitis treatment: A randomized, double-blind, placebo-controlled trial. J. Clin. Periodontol. 2012, 40, 186–195. [Google Scholar] [CrossRef]
- De Waal, Y.C.; Raghoebar, G.M.; Meijer, H.J.A.; Winkel, E.G.; Van Winkelhoff, A.J. Implant decontamination with 2% chlorhexidine during surgical peri-implantitis treatment: A randomized, double-blind, controlled trial. Clin. Oral Implant. Res. 2014, 26, 1015–1023. [Google Scholar] [CrossRef]
- Vu-Ngoc, H.; Elawady, S.S.; Mehyar, G.M.; Abdelhamid, A.H.; Mattar, O.M.; Halhouli, O.; Vuong, N.L.; Ali, C.D.M.; Hassan, U.H.; Kien, N.D.; et al. Quality of flow diagram in systematic review and/or meta-analysis. PLoS ONE 2018, 13, e0195955. [Google Scholar] [CrossRef]
- Ting, M.; Craig, J.; Balkin, B.E.; Suzuki, J.B. Peri-implantitis: A comprehensive overview of systematic reviews. J. Oral Implant. 2018, 44, 225–247. [Google Scholar] [CrossRef]
- Verdugo, F.; Laksmana, T.; Uribarri, A.; Information, P.E.K.F.C. Systemic antibiotics and the risk of superinfection in peri-implantitis. Arch. Oral Boil. 2016, 64, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Alghamdi, A.S.T.; Ahmed, A.; Mikami, T.; Ahmed, H.B.; Tenenbaum, H.C. Clinical efficacy of antibiotics in the treatment of peri-implantitis. Int. Dent. J. 2013, 63, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Carcuac, O.; Derks, J.; Charalampakis, G.; Abrahamsson, I.; Wennström, J.; Berglundh, T. Adjunctive systemic and local antimicrobial therapy in the surgical treatment of peri-implantitis: A randomized controlled clinical trial. J. Dent. Res. 2016, 95, 50–57. [Google Scholar] [CrossRef]
- Leonhardt, A.; Dahlen, G.; Renvert, S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J. Periodontol. 2003, 74, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Van Winkelhoff, A.J. Antibiotics in the treatment of peri-implantitis. Eur. J. Oral Implant. 2012, 5, S43–S50. [Google Scholar]
- Faggion, C.M.; Chambrone, L.; Listl, S.; Tu, Y.-K. Network meta-analysis for evaluating interventions in implant dentistry: The case of peri-implantitis treatment. Clin. Implant. Dent. Relat. Res. 2011, 15, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Castro, G.L.; Gallas, M.; Nunez, I.R.; Borrajo, J.L.; Alvarez, J.C.; Varela, L.G. Scanning electron microscopic analysis of diode laser-treated titanium implant surfaces. Photomed. Laser Surg. 2007, 25, 124–128. [Google Scholar] [CrossRef]
- Fontana, C.R.; Kurachi, C.; Mendonca, C.R.; Bagnato, V.S. Temperature variation at soft periodontal and rat bone tissues during a medium-power diode laser exposure. Photomed. Laser Surg. 2004, 22, 519–522. [Google Scholar] [CrossRef]
- Gonçalves, F.; Zanetti, A.L.; Zanetti, R.V.; Martelli, F.S.; Ávila-Campos, M.; Tomazinho, L.F.; Granjeiro, J.M. Effectiveness of 980-mm diode and 1064-nm extra-long-pulse neodymium-doped yttrium aluminum garnet lasers in implant disinfection. Photomed. Laser Surg. 2010, 28, 273–280. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Konstantinidis, I.; Karoussis, I.K.; Ma, X.; Chu, H. Systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J. Periodontol. 2014, 85, 1203–1213. [Google Scholar] [CrossRef]
- Stübinger, S.; Etter, C.; Miskiewicz, M.; Homann, F.; Saldamli, B.; Wieland, M.; Sader, R. Surface alterations of polished and sandblasted and acid-etched titanium implants after Er: YAG, carbon dioxide, and diode laser irradiation. Int. J. Oral Maxillofac. Implant. 2010, 25, 104–111. [Google Scholar]
- Moëne, R.; Décaillet, F.; Andersen, E.; Mombelli, A. Subgingival plaque removal using a new air-polishing device. J. Periodontol. 2010, 81, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Sculean, A.; Romanos, G.; Herten, M.; Horn, N.; Scherbaum, W.; Becker, J. Influence of different treatment approaches on the removal of early plaque biofilms and the viability of SAOS2 osteoblasts grown on titanium implants. Clin. Oral Investig. 2005, 9, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Papanicolau, P.; Rothamel, D.; Beck, B.; Herten, M.; Becker, J. Influence of plaque biofilm removal on reestablishment of the biocompatibility of contaminated titanium surfaces. J. Biomed. Mater. Res. Part A 2006, 77, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Ferrari, D.; Popovski, K.; Hartig, B.; Becker, J. Influence of different air-abrasive powders on cell viability at biologically contaminated titanium dental implants surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 83–91. [Google Scholar] [CrossRef]
- Chen, C.-J.; Ding, S.-J. Effectiveness of hypochlorous acid to reduce the biofilms on titanium alloy surfaces in vitro. Int. J. Mol. Sci. 2016, 17, 1161. [Google Scholar] [CrossRef]
- Héritier, M. Effects of phosphoric acid on root dentin surface. A scanning and transmission electron microscopic study. J. Periodontal Res. 1984, 19, 168–176. [Google Scholar] [CrossRef]
- Tastepe, C.S.; Liu, Y.; Visscher, C.M.; Wismeijer, D. Cleaning and modification of intraorally contaminated titanium discs with calcium phosphate powder abrasive treatment. Clin. Oral Implant. Res. 2012, 24. [Google Scholar] [CrossRef]
- Wheelis, S.E.; Gindri, I.M.; Valderrama, P.; Wilson, T.G.; Huang, J.; Rodrigues, D.C. Effects of decontamination solutions on the surface of titanium: Investigation of surface morphology, composition, and roughness. Clin. Oral Implant. Res. 2015, 27, 329–340. [Google Scholar] [CrossRef]
- Schwarz, F.; John, G.; Schmucker, A.; Sahm, N.; Becker, J. Combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination: A 7-year follow-up observation. J. Clin. Periodontol. 2017, 44, 337–342. [Google Scholar] [CrossRef]
- Schwarz, F.; Sahm, N.; Iglhaut, G.; Becker, J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: A randomized controlled clinical study. J. Clin. Periodontol. 2011, 38, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Avontroodt, P.; Peeters, W.; Pauwels, M.; Coucke, W.; Van Steenberghe, D. Effect of different chlorhexidine formulations in mouthrinses on de novo plaque formation. J. Clin. Periodontol. 2001, 28, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Isehed, C.; Holmlund, A.; Renvert, S.; Svenson, B.; Johansson, I.; Lundberg, P. Effectiveness of enamel matrix derivative on the clinical and microbiological outcomes following surgical regenerative treatment of peri-implantitis. A randomized controlled trial. J. Clin. Periodontol. 2016, 43, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, M.; Goharfar, Z.; Pourabbas, R.; Kashefimehr, A.; Shirmohmmadi, A. Microbiological and clinical effects of enamel matrix derivative and sustained-release micro-spherical minocycline application as an adjunct to non-surgical therapy in peri-implant mucosal inflammation. J. Korean Assoc. Oral Maxillofac. Surg. 2015, 41, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Auschill, T.M.; Donos, N.; Brecx, M.; Arweiler, N.B. Effect of an enamel matrix protein derivative (Emdogain®) on ex vivo dental plaque vitality. J. Clin. Periodontol. 2008, 28, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
| Treatment for Peri-Implantitis |
|---|
|
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Population | Healthy human patients receiving treatment for peri-implantitis lesions | Studies of human patients with chronic diseases, co-morbidities and non-human studies; Animal studies |
| Intervention | Surgical or non-surgical treatment of peri-implantitis lesions, including adjunctive treatment | Studies which allow/use pre-operative (up to 3 months prior to initiation), peri-operative and post-operative anti-microbial therapy |
| Comparator | Individuals or teeth within the same individual (including split mouth technique) not subjected to the same therapeutic variable | |
| Outcome | Resolution of peri-implantitis, including implant survival and absence of peri-implant probing pocket depths of >5 mm, suppuration, bleeding on probing (BoP) and further bone loss | Studies including patients who have previously received peri-implantitis treatment |
| Study Design | Randomised controlled trials (RCT), published or unpublished | Non-RCT, cohort studies, case reports, case series, reviews, abstracts, systematic reviews, opinions, studies with questionnaires or studies where the diagnosis/measurement of peri-implantitis was performed only on radiographs rather than clinically. |
| # | Searches | Results |
|---|---|---|
| 1 | exp Peri-implantitis/ | 1017 |
| 2 | exp Therapeutics/ | 4,367,916 |
| 3 | exp Operative Surgical Procedures/ | 2,990,228 |
| 4 | 2 OR 3 | 6,056,211 |
| 5 | 1 AND 4 | 453 |
|
|
| References | Diagnosis of Peri-Implantitis | No. of Implants | Treatment Strategies | Follow-Up | Study Parameters | Results | |
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | ||||||
| Arisan et al. (2015) | 4–6 mm of PPD <3 mm of MBL BoP, Plaque, Pain and/or SoP | 48 | Diode Laser | Mechanical debridement | 6 months | PPD PI BoP MBL Bacterial Count | Adjunctive use of a diode laser did not yield any additional positive influence on the peri-implant health compared with conventional scaling alone |
| John et al. (2015) | PPD ≥ 4 mm BoP SoP MBL ≤ 30% | 25 | Amino Acid Glycine Powder (AAD) | Mechanical Debridement with carbon curettes + Antiseptic therapy chlorhexidine (MDA) | 12 months | PI BoP PPD Mucosal Recession CAL | Both treatment procedures resulted in comparable but limited CAL gains; AAD was assoc. significantly higher BoP than MDA |
| Machtei et al. (2012) | PPD of 6–10 mm BoP MBL | 73 | Matrix Chips (MatrixC) | Chlorhexidine Chips (PerioC) | 6 months | PPD change CAL BoP | Both groups resulted in substantial improvement; CAL changes in PerioC group were significantly greater than MatrixC |
| Renvert et al. (2011) | PPD ≥ 5 mm Bone Loss ≥ 2 mm BoP | 100 | Er:YAG Laser | Air-Abrasive device | 6 months | PPD BoP Bacterial Counts | Both methods showed limited clinical improvement, but failed to reduce bacterial count |
| Roos-Jansaker et al. (2017) | MBL ≥ 2 mm PPD > 4 mm BoP and/or SoP | 32 | Local applications of chloramine gel Supra- and submucosal debridement by ultrasonic and hand instruments | Supra- and submucosal debridement by ultrasonic and hand instruments | 3 months | PI PPD CAL BoP | Adjunctive use of chloramine is equally effective in the reduction in mucosal inflammation as conventional non-surgical mechanical debridement up to 3 months |
| Sahm et al. (2011) | PPD ≥ 4 mm Bone Loss ≤ 30% BoP SoP No occlusal overload 2 mm keratinized mucosa Good PI | 43 | Amino Acid Glycine Powder (AAD) | Mechanical Debridement with carbon curettes + Antiseptic therapy chlorhexidine (MDA) | 6 months | BoP PPD CAL | Both groups revealed comparable PD reduction and CAL gains Higher changes in BoP in the AAD group |
| References | Diagnosis of Peri-Implantitis | No. of Implants | Treatment Strategies | Follow-Up | Study Parameters | Results | |
|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | ||||||
| Hentenaar et al. (2017) | MBL ≥ 2 mm PPD ≥ 5 mm BoP and/or SoP | 50 | Resective surgery with apically positioned flap Bone recontouring 35% phosphoric acid etching gel | Resective surgery with apically positioned flap Bone recontouring Sterile Saline | 3 months | Bacterial count BoP SoP Mean PPD | 35% phosphoric acid led to greater decontamination of the implant surface, but did not enhance clinical outcomes |
| Isehed et al. (2018) | PPD ≥ 5 mm BoP and/or SoP Angular bone loss ≥ 3 mm | 14 | Regenerative surgical treatment with adjunctive enamel matrix derivative (EMD) | Regenerative surgical treatment | 5 years | Implant loss MBL BoP Plaque SoP | Adjunctive EMD is positively associated with implant survival up to 5 years |
| Papadopoulos et al. (2015) | PPD ≥ 6 mm BoP SoP MBL ≥ 2 mm | 16 | Access flap Plastic curette Sterilised gauze soaked in saline | Access flap Plastic curette Diode Laser | 6 months | PPD CAL BoP PI | Surgical treatment leads to improvement of all clinical parameters; additional use of diode laser does not have beneficiary effect |
| de Waal et al. (2013) | PPD ≥ 5 mm Bone loss ≥ 2 mm BoP and/or SoP | 79 | Resective surgery with apically positioned flap Bone recontouring Debridement 0.12% CHX 0.05% CPC | Resective surgery with apically positioned flap Bone recontouring Debridement Placebo | 12 months | Bacterial Count PI BoP SoP PPD MBL | CHX + CPC leads to greater immediate suppression of bacterial load, but this does not translate into better clinical results |
| de Waal et al. (2015) | PPD ≥ 5 mm Bone loss ≥ 2 mm BoP and/or SoP | 102 | Resective surgery with apically positioned flap Bone recontouring Debridement 2% CHX | Resective surgery with apically positioned flap Bone recontouring Debridement 0.12% CHX 0.05% CPC | 12 months | BoP PI SoP PPD MBL Bacterial Count | 2% CHX does not lead to improved clinical, radiographic or microbiological results compared with a 0.12% CHX and 0.05% CPC solution |
| Study/Domain | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Overall |
|---|---|---|---|---|---|---|
| Arisan 2015 | Concerns | Low | Low | Low | Concerns | Concerns |
| Hentenaar 2017 | Low | Concerns | Low | Low | Low | Concerns |
| Isehed 2018 | Low | High | High | High | Low | High |
| John 2015 | Low | High | Low | Low | Low | High |
| Machtei 2012 | Low | Low | Low | Low | Low | Low |
| Papadopoulos 2015 | Concerns | High | High | Low | Low | High |
| Renvert 2011 | Low | Low | Low | Low | Low | Low |
| Roos-Jansaker 2017 | Concerns | High | Low | Concerns | Low | High |
| Sahm 2011 | Concerns | Concerns | Low | Low | Low | Concerns |
| De Waal 2013 | Low | Low | Low | Low | Low | Low |
| De Waal 2015 | Low | Low | Low | Low | Low | Low |
| Intervention | No. of Implants (Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Effect | Overall Certainty of Evidence |
|---|---|---|---|---|---|---|---|---|
| Non-surgical manual debridement of implant surfaces | 40 (2 Studies) | Very Serious 1 | Not serious | Not serious | Serious 3 | Suspected 4 | All studies report positive changes to clinical parameters surrounding peri-implantitis lesions | ⊕ VERY LOW |
| Surgical debridement of implant surfaces | 71 (4 studies) | Very Serious 1 | Not serious | Not serious | Serious 3 | Suspected 4 | All studies report positive changes to clinical parameters surrounding peri-implantitis lesions | ⊕ VERY LOW |
| Non-surgical debridement + Diode laser | 45 (2 studies) | Serious 2 | Not serious | Not serious | Serious 3 | Suspected 4 | All studies report positive changes to clinical parameters surrounding peri-implantitis lesions | ⊕ VERY LOW |
| Adjunctive Diode Laser Application with surgical debridement | 8 (1 study) | Very Serious 1 | Not serious | Not serious | Serious 3 | Suspected 4 | Single study reports positive changes to clinical parameters surrounding peri-implantitis lesions, however, additional usage of diode laser does not have significant benefit | ⊕ VERY LOW |
| Surgical Debridement + Phosphoric Acid Decontamination | 30 (1 study) | Serious 2 | Not serious | Not serious | Serious 3 | Suspected 4 | Single study reports greater decontamination of implant surface with phosphoric acid, however, no clinical benefit seen as compared to control | ⊕ VERY LOW |
| Surgical debridement + Enamel Matrix Derivative (EMD) | 9 (1 study) | Very Serious 1 | Not serious | Not serious | Serious 3 | Suspected 4 | Single study suggests that usage of adjunctive EMD is associated with greater implant survival up to 5 years | ⊕ VERY LOW |
| Non-surgical debridement + chlorhexidine chips | 40 (1 study) | Not serious | Not serious | Not serious | Serious 3 | Suspected 4 | Single study reports that CHX chips result in substantial improvement in sites with peri-implantitis | ⊕⊕ LOW |
| Non-surgical debridement + matrix chips | 33 (1 study) | Not serious | Not serious | Not serious | Serious 3 | Suspected 4 | Single study reports that matrix chips result in substantial improvement in sites with peri-implantitis | ⊕⊕ LOW |
| Non-surgical debridement + Air-abrasive device | 48 (3 studies) | Very Serious 1 | Not serious | Not serious | Serious 3 | Suspected 4 | All studies report positive changes to clinical parameters surrounding peri-implantitis lesions | ⊕ VERY LOW |
| Non-surgical debridement + chloramine gel | 16 (1 study) | Very Serious 1 | Not serious | Not serious | Serious 3 | Suspected 4 | Single study reports improvements in some clinical parameters surrounding peri-implantitis lesions, however, no group difference was found between conventional debridement and adjunctive use of chloramine | ⊕ VERY LOW |
| Non-surgical debridement + chlorhexidine application | 28 (2 studies) | Very Serious 1 | Not serious | Not serious | Serious 3 | Suspected 4 | All studies report positive changes to clinical parameters surrounding peri-implantitis lesions | ⊕ VERY LOW |
| Surgical resective treatment + 0.12% CHX and 0.05% CPC | 80 (2 studies) | Not serious | Not serious | Not serious | Serious 3 | Suspected 4 | All studies report positive changes across several indicators of peri-implantitis | ⊕⊕ LOW |
| Surgical resective treatment + 2% CHX | 49 (1 study) | Not serious | Not serious | Not serious | Serious 3 | Suspected 4 | Single study reported positive changes across several indicators of peri-implantitis, however, no significant difference between solutions of 2% CHX against 0.2% CHX + 0.05% CPC | ⊕⊕ LOW |
| The outcome of interest: resolution of peri-implantitis (for which a single pooled effect estimate was not available and only a narrative synthesis of the evidence was provided). | ||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.; Goyal, A.; Currell, S.D.; Sharma, D. Management of Peri-Implantitis Lesions without the Use of Systemic Antibiotics: A Systematic Review. Dent. J. 2020, 8, 106. https://doi.org/10.3390/dj8030106
Khan A, Goyal A, Currell SD, Sharma D. Management of Peri-Implantitis Lesions without the Use of Systemic Antibiotics: A Systematic Review. Dentistry Journal. 2020; 8(3):106. https://doi.org/10.3390/dj8030106
Chicago/Turabian StyleKhan, Ahsen, Ankit Goyal, Scott D. Currell, and Dileep Sharma. 2020. "Management of Peri-Implantitis Lesions without the Use of Systemic Antibiotics: A Systematic Review" Dentistry Journal 8, no. 3: 106. https://doi.org/10.3390/dj8030106
APA StyleKhan, A., Goyal, A., Currell, S. D., & Sharma, D. (2020). Management of Peri-Implantitis Lesions without the Use of Systemic Antibiotics: A Systematic Review. Dentistry Journal, 8(3), 106. https://doi.org/10.3390/dj8030106






