Implant Diameter, Length, and the Insertion Torque/Depth Integral: A Study Using Polyurethane Foam Blocks
Abstract
1. Introduction
2. Materials and Methods
Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Szmukler-Moncler, S.; Salama, H.; Reingewirtz, Y.; Dubruille, J.H. Timing of loading and effect of micromotion on bone-dental implant interface: Review of experimental literature. J. Biomed. Mater. Res. 1998, 43, 192–203. [Google Scholar] [CrossRef]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implants Res. 2009, 20, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef]
- Javed, F.; Ahmed, H.B.; Crespi, R.; Romanos, G.E. Role of primary stability for successful osseointegration of dental implants: Factors of influence and evaluation. Interv. Med. Appl. Sci. 2013, 5, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, F.; Hasan, I.; Bourauel, C.; Biffar, R.; Mundt, T. Bone stability around dental implants: Treatment related factors. Ann. Anat. 2015, 199, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontol 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.; Gallucci, G.; Lin, W.S.; Pjetursson, B.; Polido, W.; Roehling, S.; Sailer, I.; Aghaloo, T.; Albera, H.; Bohner, L.; et al. Group 2 ITI Consensus Report: Prosthodontics and implant dentistry. Clin. Oral Implants Res. 2018, 29 (Suppl. 16), 215–223. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Primary stability determination by means of insertion torque and RFA in a sample of 4135 implants. Clin. Implant Dent. Relat. Res. 2012, 14, 501–507. [Google Scholar] [CrossRef]
- Mesa, F.; Muñoz, R.; Noguerol, B.; de Dios Luna, J.; Galindo, P.; O’Valle, F. Multivariate study of factors influencing primary dental implant stability. Clin. Oral Implants Res. 2008, 19, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Ostman, P.O.; Hellman, M.; Wendelhag, I.; Sennerby, L. Resonance frequency analysis measurements of implants at placement surgery. Int. J. Prosthodont. 2006, 19, 77–83. [Google Scholar]
- Aragoneses, J.M.; Suárez, A.; Brugal, V.A.; Gómez, M. Frequency Values and Their Relationship with the Diameter of Dental Implants. Prospective Study of 559 Implants. Implant Dent. 2019, 28, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Shiffler, K.; Lee, D.; Rowan, M.; Aghaloo, T.; Pi-Anfruns, J.; Moy, P.K. Effect of length, diameter, intraoral location on implant stability. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2016, 122, e193–e198. [Google Scholar] [CrossRef] [PubMed]
- Friberg, B.; Sennerby, L.; Meredith, N.; Lekholm, U. A comparison between cutting torque and resonance frequency measurements of maxillary implants. A 20-month clinical study. Int. J. Oral Maxillofac. Surg. 1999, 28, 297–303. [Google Scholar] [CrossRef]
- Aparicio, C. The use of the Periotest value as the initial success criteria of an implant: 8-year report. Int. J. Periodontics Restor. Dent. 1997, 17, 150–161. [Google Scholar]
- Bischof, M.; Nedir, R.; Szmukler-Moncler, S.; Bernard, J.P.; Samson, J. Implant stability measurement of delayed and immediately loaded implants during healing. Clin. Oral Implants Res. 2004, 15, 529–539. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A.; Carinci, F. Evaluation of factors influencing resonance frequency analysis values, at insertion surgery, of implants placed in sinus-augmented and nongrafted sites. Clin. Implant Dent. Relat. Res. 2007, 9, 144–149. [Google Scholar] [CrossRef]
- Karl, M.; Graef, F.; Heckmann, S.; Krafft, T. Parameters of resonance frequency measurement values: A retrospective study of 385 ITI dental implants. Clin. Oral Implants Res. 2008, 19, 214–218. [Google Scholar] [CrossRef]
- Alsabeeha, N.H.; De Silva, R.K.; Thomson, W.M.; Payne, A.G. Primary stability measurements of single implants in the midline of the edentulous mandible for overdentures. Clin. Oral Implants Res. 2010, 21, 563–566. [Google Scholar] [CrossRef]
- Sim, C.P.; Lang, N.P. Factors influencing resonance frequency analysis assessed by Osstell mentor during implant tissue integration: I. Instrument positioning, bone structure, implant length. Clin. Oral Implants Res. 2010, 21, 598–604. [Google Scholar] [CrossRef]
- González-García, R.; Monje, F.; Moreno-García, C. Predictability of the resonance frequency analysis in the survival of dental implants placed in the anterior non-atrophied edentulous mandible. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e664–e669. [Google Scholar] [CrossRef]
- Pommer, B.; Hof, M.; Fädler, A.; Gahleitner, A.; Watzek, G.; Watzak, G. Primary implant stability in the atrophic sinus floor of human cadaver maxillae: Impact of residual ridge height, bone density, and implant diameter. Clin. Oral Implants Res. 2014, 25, e109–e113. [Google Scholar] [CrossRef] [PubMed]
- Maiorana, C.; Farronato, D.; Pieroni, S.; Cicciu, M.; Andreoni, D.; Santoro, F. A Four-Year Survival Rate Multicenter Prospective Clinical Study on 377 Implants: Correlations between Implant Insertion Torque, Diameter, and Bone Quality. J. Oral Implantol. 2015, 41, e60–e65. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Carrascal, N.; Salomó-Coll, O.; Gilabert-Cerdà, M.; Farré-Pagés, N.; Gargallo-Albiol, J.; Hernández-Alfaro, F. Effect of implant macro-design on primary stability: A prospective clinical study. Med. Oral. Patol. Oral Cir. Bucal 2016, 21, e214–e221. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, Z.; Shao, X.; Wismeijer, D.; Sun, P.; Wang, J.; Wu, G. Multivariate linear regression analysis to identify general factors for quantitative predictions of implant stability quotient values. PLoS ONE 2017, 12, e0187010. [Google Scholar] [CrossRef]
- Sennerby, L.; Meredith, N. Implant stability measurements using resonance frequency analysis: Biological and biomechanical aspects and clinical implications. Periodontol 2000 2008, 47, 51–66. [Google Scholar] [CrossRef]
- Hsu, J.T.; Shen, Y.W.; Kuo, C.W.; Wang, R.T.; Fuh, L.J.; Huang, H.L. Impacts of 3D bone-to- implant contact and implant diameter on primary stability of dental implant. J. Formos. Med. Assoc. 2017, 116, 582–590. [Google Scholar] [CrossRef]
- Vayron, R.; Nguyen, V.H.; Lecuelle, B.; Haiat, G. Evaluation of dental implant stability in bone phantoms: Comparison between a quantitative ultrasound technique and resonance frequency analysis. Clin. Implant Dent. Relat. Res. 2018, 20, 470–478. [Google Scholar] [CrossRef]
- Park, K.J.; Kwon, J.Y.; Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H.; Lee, S.J.; Kim, T.H.; Kim, M.J. The relationship between implant stability quotient values and implant insertion variables: A clinical study. J. Oral Rehabil. 2012, 39, 151–159. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, S.J.; Cho, I.S.; Kim, S.K.; Kim, T.W. Rotational resistance of surface-treated mini-implants. Angle Orthod. 2009, 79, 899–907. [Google Scholar] [CrossRef]
- Degidi, M.; Daprile, G.; Piattelli, A.; Iezzi, G. Development of a new implant primary stability parameter: Insertion torque revisited. Clin. Implant Dent. Relat. Res. 2013, 15, 637–644. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, G.A. Patient selection and preparation. In Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Brånemark, P.I., Zarb, G.A., Albrektsson, T., Eds.; Quintessence Publishing Co.: Chicago, IL, USA, 1985; pp. 199–209. [Google Scholar]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of underpreparation on primary stability of implants inserted in poor quality bone sites: An in vitro study. J. Oral Maxillofac. Surg. 2015, 73, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Orlando, F.; Arosio, F.; Arosio, P.; Di Stefano, D.A. Bone Density and Implant Primary Stability. A Study on Equine Bone Blocks. Dent. J. (Basel) 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed]
- Capparé, P.; Vinci, R.; Di Stefano, D.A.; Traini, T.; Pantaleo, G.; Gherlone, E.F.; Gastaldi, G. Correlation between Initial BIC and the Insertion Torque/Depth Integral Recorded with an Instantaneous Torque-Measuring Implant Motor: An in vivo Study. Clin. Implant Dent. Relat. Res. 2015, 17 (Suppl. 2), e613–e620. [Google Scholar] [CrossRef]
- Iezzi, G.; Filippone, A.; Di Stefano, D.A.; Arosio, P.; Piattelli, A.; Scarano, A.; Perrotti, V. A site-specific intraoperative measurement of bone-to-implant contact during implant insertion: A study on bovine ribs using a computerized implant motor. J Dent. Sci. 2015, 10, 21–27. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Perrotti, V.; Iezzi, G.; Scarano, A.; Piattelli, A. Correlation between Implant Geometry, Bone Density, and the Insertion Torque/Depth Integral: A Study on Bovine Ribs. Dent. J. (Basel) 2019, 7, 25. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Perrotti, V.; Greco, G.B.; Cappucci, C.; Arosio, P.; Piattelli, A.; Iezzi, G. The effect of undersizing and tapping on bone to implant contact and implant primary stability: A histomorphometric study on bovine ribs. J. Adv. Prosthodont. 2018, 10, 227–235. [Google Scholar] [CrossRef]
- Andreasi Bassi, M.; Arosio, P.; Di Stefano, D.A. Evaluation of Peri-implant Bone Stress on D1 Bone Using a Computerized Torque-Measuring Implant Motor: A Study on Photoelastic Resin Blocks. Int. J. Oral Maxillofac. Implants 2018, 33, 770–778. [Google Scholar] [CrossRef]
- Arosio, P.; Greco, G.B.; Zaniol, T.; Iezzi, G.; Perrotti, V.; Di Stefano, D.A. Sinus augmentation and concomitant implant placement in low bone-density sites. A retrospective study on an undersized drilling protocol and primary stability. Clin. Implant Dent. Relat. Res. 2018, 20, 151–159. [Google Scholar] [CrossRef]
- ASTM—American Society for Testing and Materials. Standard ASTM F1839-08(2012); Standard Specification for Rigid Polyurethane Foam for Use as A Standard Material for Testing Orthopaedic Devices and Instruments; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Ku, H.H. Notes on the use of propagation of error formulas. Journal of Research of the National Bureau of Standards. J. Res. Natl. Bur. Stand. 1966, 70, 263–273. [Google Scholar]
- Bilhan, H.; Geckili, O.; Mumcu, E.; Bozdag, E.; Sünbüloğlu, E.; Kutay, O. Influence of surgical technique, implant shape and diameter on the primary stability in cancellous bone. J. Oral Rehabil. 2010, 37, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.C.; Heussen, N.; Elvers, D.; Steiner, T.; Hölzle, F.; Modabber, A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: A laboratory study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Delgado-Ruiz, R.A.; Sacks, D.; Calvo-Guirado, J.L. Influence of the implant diameter and bone quality on the primary stability of porous tantalum trabecular metal dental implants: An in vitro biomechanical study. Clin. Oral Implants Res. 2018, 29, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.A.V.; Garcia, J.J.; Caramês, J.M.M.; Quirynen, M.; da Silva Marques, D.N. Short implants versus bone grafting and standard-length implants placement: A systematic review. Clin. Oral Investig. 2018, 22, 69–80. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; De Souza, A.; Vazouras, K.; Gholami, H.; Pagni, S.; Weber, H.P. Survival rates of short dental implants (≤6 mm) compared with implants longer than 6 mm in posterior jaw areas: A meta-analysis. Clin. Oral Implants Res. 2018, 29 (Suppl. 16), 8–20. [Google Scholar] [CrossRef]
- Fan, T.; Li, Y.; Deng, W.W.; Wu, T.; Zhang, W. Short Implants (5 to 8 mm) Versus Longer Implants (>8 mm) with Sinus Lifting in Atrophic Posterior Maxilla: A Meta-Analysis of RCTs. Clin. Implant Dent. Relat. Res. 2017, 19, 207–215. [Google Scholar] [CrossRef]
- Weerapong, K.; Sirimongkolwattana, S.; Sastraruji, T.; Khongkhunthian, P. Comparative study of immediate loading on short dental implants and conventional dental implants in the posterior mandible: A randomized clinical trial. Int. J. Oral Maxillofac. Implants 2019, 34, 141–149. [Google Scholar] [CrossRef]
- Han, J.; Tang, Z.; Zhang, X.; Meng, H. A prospective, multi-center study assessing early loading with short implants in posterior regions. A 3-year post-loading follow-up study. Clin. Implant Dent. Relat. Res. 2018, 20, 34–42. [Google Scholar] [CrossRef]
- Anitua, E.; Flores, J.; Flores, C.; Alkhraisat, M.H. Long-term Outcomes of Immediate Loading of Short Implants: A Controlled Retrospective Cohort Study. Int. J. Oral Maxillofac. Implants 2016, 31, 1360–1366. [Google Scholar] [CrossRef]
- Felice, P.; Barausse, C.; Pistilli, V.; Piattelli, M.; Ippolito, D.R.; Esposito, M. Posterior atrophic jaws rehabilitated with prostheses supported by 6 mm long × 4 mm wide implants or by longer implants in augmented bone. 3-year post-loading results from a randomised controlled trial. Eur. J. Oral Implantol. 2018, 11, 175–187. [Google Scholar]
- Grant, J.A.; Bishop, N.E.; Götzen, N.; Sprecher, C.; Honl, M.; Morlock, M.M. Artificial composite bone as a model of human trabecular bone: The implant-bone interface. J. Biomech. 2007, 40, 1158–1164. [Google Scholar] [CrossRef] [PubMed]


| Block Density (g/cm3) | Implant Size (Thread Diameter × Length, mm) | Final Drill Diameter (mm) | Countersink Drill Diameter (mm) |
|---|---|---|---|
| 0.26 | 3.5 × 12.0 | 2.8 | None |
| 3.75 × 8.0 | 3.0 | 3.5 | |
| 3.75 × 12.0 | 3.0 | 3.5 | |
| 3.75 × 15.5 | 3.0 | 3.5 | |
| 5.0 × 12.0 | 3.8 | 4.3 | |
| 6.0 × 12.0 | 4.2 | 5.3 | |
| 0.33 | 3.5 × 12.0 | 2.8 | None |
| 3.75 × 8.0 | 2.8 | 3.7 | |
| 3.75 × 12.0 | 2.8 | 3.7 | |
| 3.75 × 15.5 | 2.8 | 3.7 | |
| 5.0 × 12.0 | 4.0 | 4.8 | |
| 6.0 × 12.0 | 4.8 | 6.0 | |
| 0.49 | 3.5 × 12.0 | 3.2 | 3.5 |
| 3.75 × 8.0 | 3.2 | 4.0 | |
| 3.75 × 12.0 | 3.2 | 4.0 | |
| 3.75 × 15.5 | 3.2 | 4.0 | |
| 5.0 × 12.0 | 4.2 | 5.0 | |
| 6.0 × 12.0 | 5.0 | 6.0 | |
| 0.65 | 3.5 × 12.0 | 3.2 | 3.5 |
| 3.75 × 8.0 | 3.2 | 4.0 | |
| 3.75 × 12.0 | 3.2 | 4.0 | |
| 3.75 × 15.5 | 3.2 | 4.0 | |
| 5.0 × 12.0 | 4.8 | 5.0 | |
| 6.0 × 12.0 | 5.0 | 6.0 |
| Measure | Symbol as Provided by the Micromotor | Unit of Measure | Information Provided |
|---|---|---|---|
| Average torque | Cm | Ncm | When recorded at probing, it is a quantification of bone density. |
| Peak torque | Cp | Ncm | When recorded at implant insertion, it is the maximum torque that was exerted by the micromotor during implant placement. In the present work, it has been indicated by the acronym IT (insertion torque). |
| Integral | I | Ncm2/100 | When measured at implant insertion, it provides the area bounded by the torque-depth curve (Figure 3). If the implant threads are evenly spaced and the rotation speed at insertion is constant (two conditions that are met in this study), the integral (I) is equal to the insertion energy, IE, multiplied by a constant factor. |
| Depth | p | mm/10 | Indicates the depth reached by the probe, when density is being measured, or by the implant when it is being placed. |
| Quantity | Formula |
|---|---|
| Undersizing coefficient, C | C = (1 − r) = 1 − rd/ri where rd: final (body) drill diameter ri: implant threads diameter |
| Normalized IT, IT* | IT* = IT/C |
| Normalized (I), (I)* | (I)* = I/C |
| Propagation of error formula [42] | σ2y = (df/dx1)2σ21 + … + (df/dxn)2σ2n where y = f(x1, … xn) is a function of multiple variables and σy is the standard deviation of y σi for (i = 1 … n) is the standard deviation of the xi independent variable |
| Standard deviation of IT*, σIT* | σIT* = σIT/C (being σc = 0) |
| Standard deviation of (I)*, σ(I)* | σ(I)* = σ(I)/C (being σc = 0) |
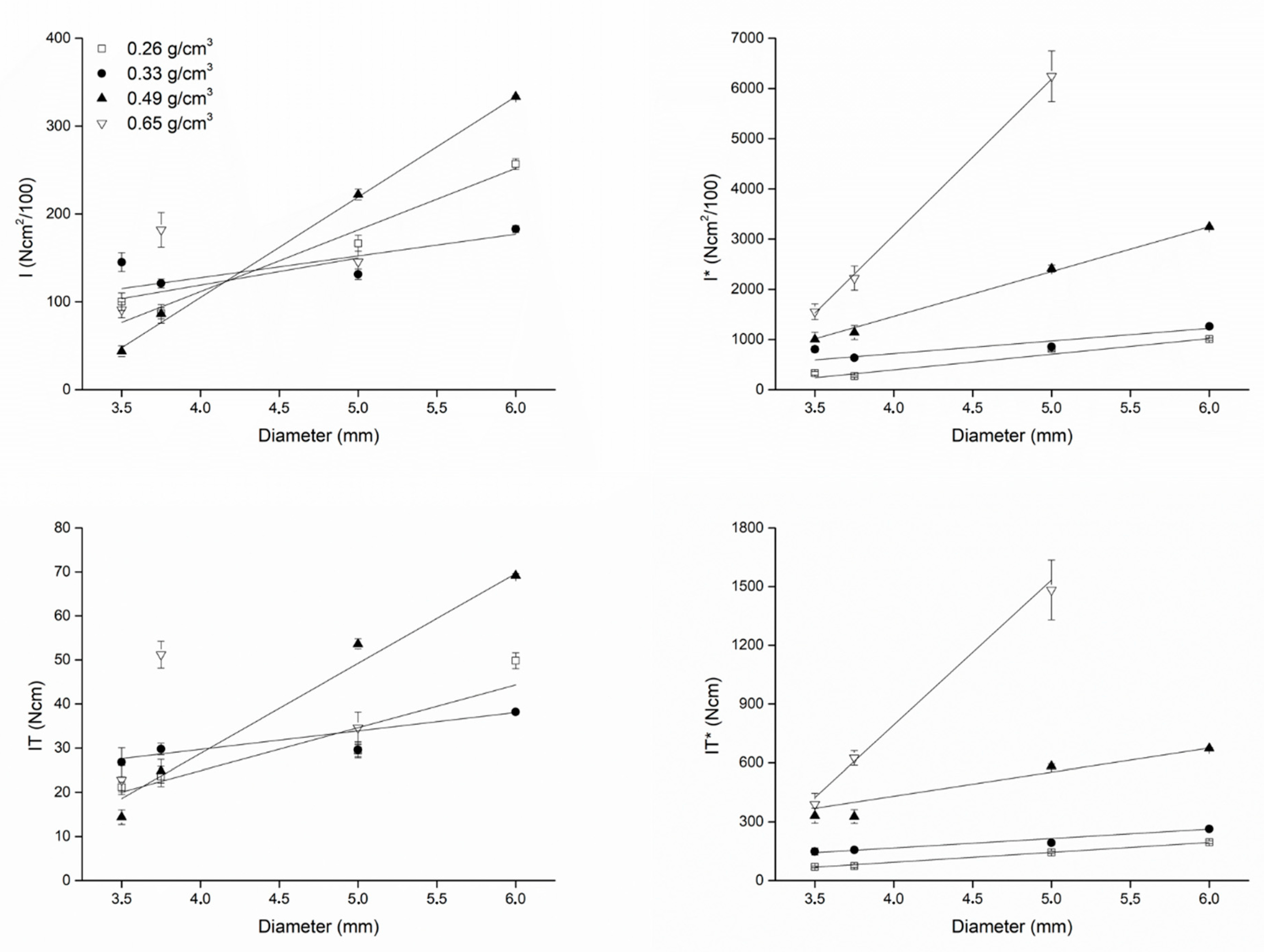
| Diameter (mm) | Bone Density (g/cm3) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.26 | 0.33 | 0.49 | 0.65 | |||||
| IT | IT* | IT | IT* | IT | IT* | IT | IT* | |
| 3.5 | 21.20 ± 1.10 | 26.80 ± 3.27 | 14.40 ± 1.67 | 22.80 ± 3.27 | 70.00 ± 3.62 | 148.89 ± 18.17 | 330.49 ± 38.40 | 389.27 ± 55.85 |
| 3.75 | 23.60 ± 2.30 | 29.80 ± 1.30 | 24.80 ± 2.68 | 51.20 ± 3.03 | 73.98 ± 7.22 | 156.84 ± 6.86 | 327.27 ± 35.41 | 625.24 ± 37.04 |
| 5.0 | 29.40 ± 1.34 | 29.60 ± 1.82 | 53.60 ± 1.14 | 34.60 ± 3.57 | 144.12 ± 6.58 | 193.04 ± 11.85 | 582.61 ± 12.39 | 1482.86 ± 152.80 |
| 6.0 | 49.80 ± 1.79 | 38.20 ± 0.45 | 69.20 ± 0.45 | -- | 195.29 ± 7.02 | 263.45 ± 3.08 | 673.30 ± 4.35 | -- |
| (I) | (I)* | (I) | (I)* | (I) | (I)* | (I) | (I)* | |
| 3.5 | 100.20 ± 9.98 | 145.20 ± 10.83 | 43.80 ± 6.10 | 91.00 ± 9.17 | 330.85 ± 32.97 | 806.67 ± 60.14 | 1005.25 ± 139.98 | 1553.66 ± 156.48 |
| 3.75 | 87.00 ± 6.28 | 121.00 ± 4.95 | 86.40 ± 10.53 | 182.00 ± 19.74 | 272.73 ± 19.70 | 636.84 ± 26.05 | 1140.18 ± 138.91 | 2222.52 ± 241.01 |
| 5.0 | 166.60 ± 9.13 | 131.40 ± 5.86 | 222.00 ± 6.32 | 145.70 ± 11.81 | 816.67 ± 44.74 | 856.96 ± 38.20 | 2413.04 ± 68.75 | 6244.29 ± 506.31 |
| 6.0 | 256.80 ± 6.14 | 182.80 ± 3.96 | 333.60 ± 1.14 | -- | 1007.06 ± 24.08 | 1260.69 ± 27.33 | 3245.84 ± 11.09 | -- |
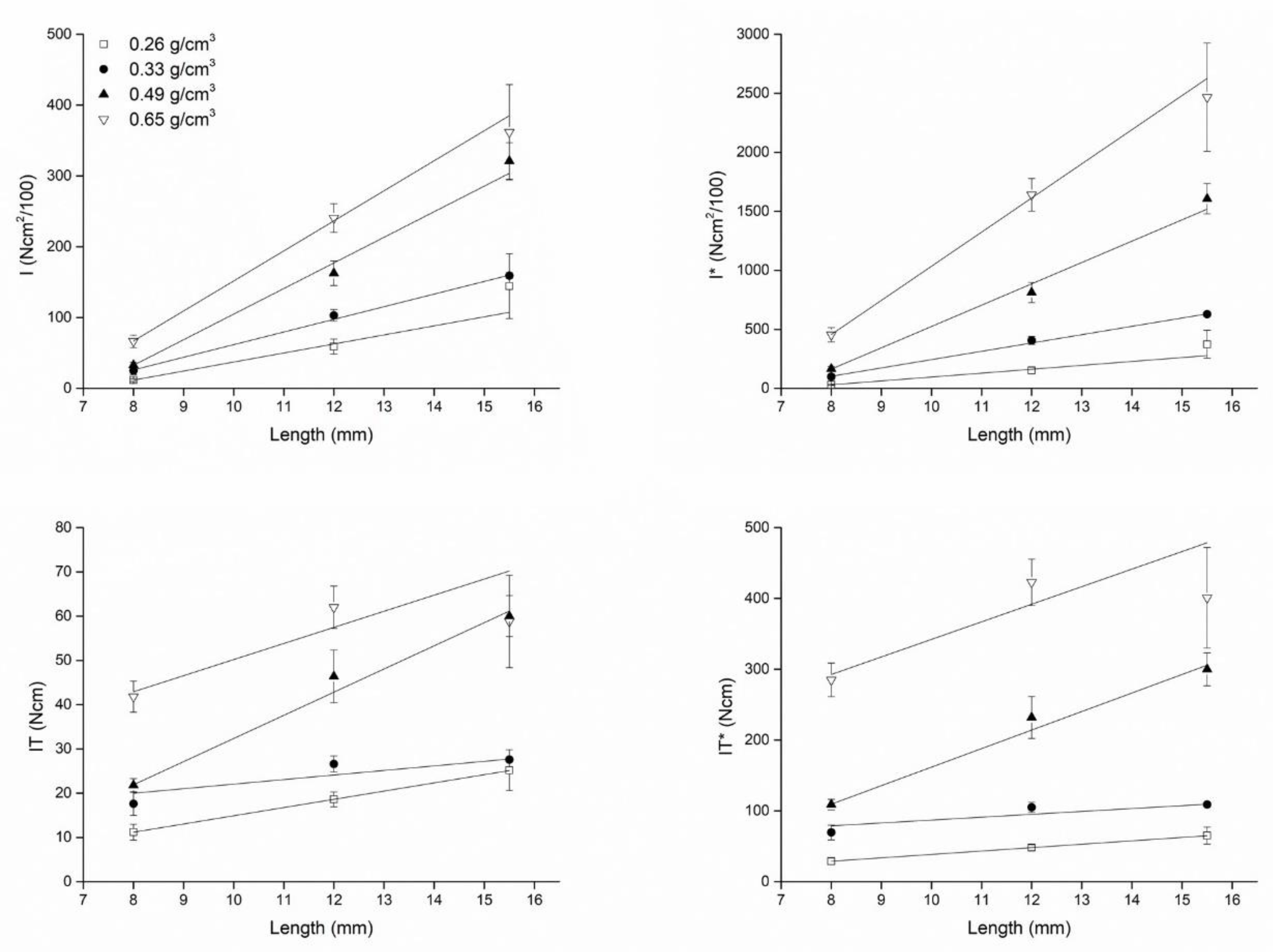
| Length (mm) | IT | IT* | IT | IT* | IT | IT* | IT | IT* |
| 8.0 | 11.20 ± 1.79 | 17.60 ± 2.61 | 21.80 ± 1.48 | 41.80 ± 3.49 | 28.97 ± 4.63 | 69.47 ± 10.29 | 109.00 ± 7.42 | 285.00 ± 23.81 |
| 12.0 | 18.60 ± 1.67 | 26.60 ± 1.82 | 46.40 ± 5.94 | 62.00 ± 4.80 | 48.10 ± 4.33 | 105.00 ± 7.17 | 232.00 ± 29.71 | 422.73 ± 32.70 |
| 15.5 | 25.20 ± 4.60 | 27.60 ± 0.55 | 60.00 ± 4.64 | 58.80 ± 10.43 | 65.17 ± 11.91 | 108.95 ± 2.16 | 300.00 ± 23.18 | 400.91 ± 71.09 |
| (I) | (I)* | (I) | (I)* | (I) | (I)* | (I) | (I)* | |
| 8.0 | 11.80 ± 1.79 | 25.00 ± 5.61 | 33.00 ± 3.74 | 66.60 ± 8.88 | 30.52 ± 4.63 | 98.68 ± 22.15 | 165.00 ± 18.71 | 454.09 ± 60.52 |
| 12.0 | 59.00 ± 10.75 | 103.00 ± 8.37 | 162.60 ± 17.18 | 240.60 ± 20.18 | 152.59 ± 27.79 | 406.58 ± 33.03 | 813.00 ± 85.92 | 1640.45 ± 137.60 |
| 15.5 | 144.40 ± 45.87 | 159.20 ± 4.44 | 321.20 ± 25.66 | 361.80 ± 67.34 | 373.45 ± 118.64 | 628.42 ± 17.52 | 1606.00 ± 128.28 | 2466.82 ± 459.14 |
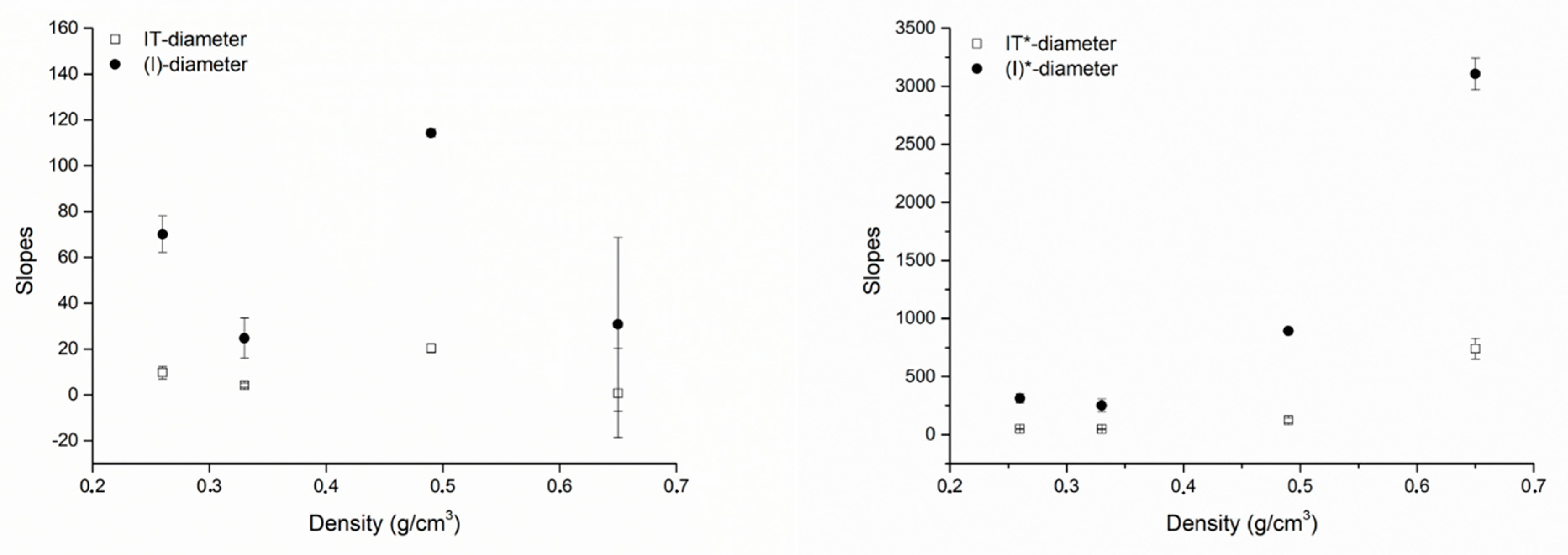
| Plot | Density (g/cm3) | m (Slope) | q (Intercept) | r (Pearson’s) |
|---|---|---|---|---|
| IT-Diameter | 0.26 | 9.71 ± 2.71 | −13.94 ± 12.16 | 0.93003 |
| 0.33 | 4.17 ± 0.99 | 13.03 ± 5.70 | 0.94774 | |
| 0.49 | 20.41 ± 1.90 | −52.86 ± 10.86 | 0.99148 | |
| 0.65 | 0.81 ± 19.45 | 33.83 ± 79.06 | 0.04176 | |
| IT*-Diameter | 0.26 | 50.49 ± 2.18 | −108.29 ± 9.28 | 0.99814 |
| 0.33 | 48.00 ± 4.15 | −25.38 ± 23.28 | 0.99261 | |
| 0.49 | 122.65 ± 20.63 | −60.48 ± 120.77 | 0.97285 | |
| 0.65 | 740.11 ± 89.34 | −2167.77 ± 333.79 | 0.99279 | |
| (I)-Diameter | 0.26 | 70.14 ± 7.86 | −168.86 ± 38.08 | 0.98767 |
| 0.33 | 24.75 ± 8.77 | 28.52 ± 44.72 | 0.89414 | |
| 0.49 | 114.34 ± 1.84 | −352.37 ± 10.85 | 0.99974 | |
| 0.65 | 30.82 ± 37.99 | −4.17 ± 155.17 | 0.63003 | |
| (I*)-Diameter | 0.26 | 311.62 ± 38.67 | −850.46 ± 178.62 | 0.98495 |
| 0.33 | 251.49 ± 54.47 | −285.72 ± 265.16 | 0.95615 | |
| 0.49 | 892.91 ± 28.47 | −2110.76 ± 169.51 | 0.99898 | |
| 0.65 | 3106.65 ± 137.61 | −9347.63 ± 506.67 | 0.99902 | |
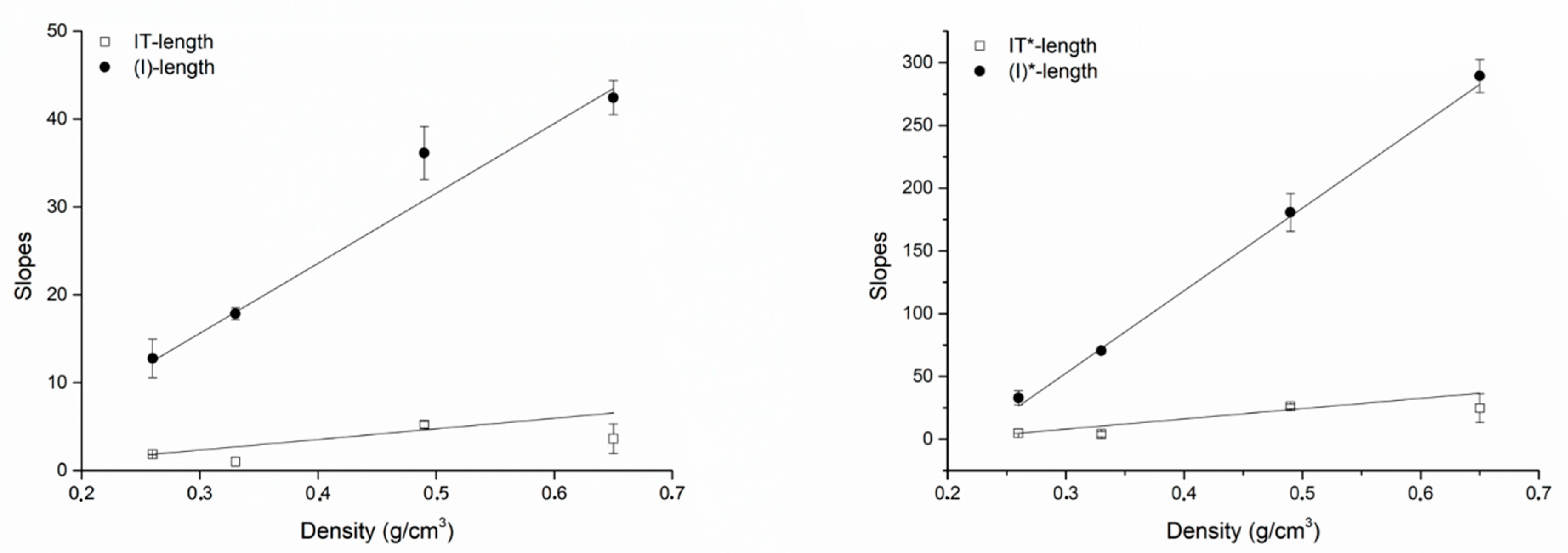
| IT-Length | 0.26 | 1.86 ± 0.01 | −3.67 ± 0.12 | 0.99998 |
| 0.33 | 1.03 ± 0.51 | 11.75 ± 7.62 | 0.89729 | |
| 0.49 | 5.23 ± 0.40 | −19.97 ± 3.66 | 0.99709 | |
| 0.65 | 3.64 ± 1.68 | 13.81 ± 16.97 | 0.90775 | |
| IT*-Length | 0.26 | 4.80 ± 0.03 | −9.49 ± 0.30 | 0.99998 |
| 0.33 | 4.07 ± 2.00 | 46.37 ± 30.07 | 0.89729 | |
| 0.49 | 26.17 ± 2.00 | −99.83 ± 18.30 | 0.99709 | |
| 0.65 | 24.81 ± 11.46 | 94.18 ± 115.71 | 0.90775 | |
| (I)-Length | 0.26 | 12.76 ± 2.19 | −90.35 ± 17.88 | 0.98554 |
| 0.33 | 17.85 ± 0.67 | −116.65 ± 8.74 | 0.99929 | |
| 0.49 | 36.15 ± 3.02 | −256.49 ± 25.43 | 0.99654 | |
| 0.65 | 42.43 ± 1.93 | −272.47 ± 17.15 | 0.99897 | |
| (I*)-Length | 0.26 | 33.01 ± 5.68 | −233.66 ± 46.25 | 0.98554 |
| 0.33 | 70.46 ± 2.66 | −460.47 ± 34.50 | 0.99929 | |
| 0.49 | 180.73 ± 15.08 | −1282.47 ± 127.15 | 0.99654 | |
| 0.65 | 289.28 ± 13.13 | −1857.72 ± 116.95 | 0.99897 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arosio, P.; Arosio, F.; Di Stefano, D.A. Implant Diameter, Length, and the Insertion Torque/Depth Integral: A Study Using Polyurethane Foam Blocks. Dent. J. 2020, 8, 56. https://doi.org/10.3390/dj8020056
Arosio P, Arosio F, Di Stefano DA. Implant Diameter, Length, and the Insertion Torque/Depth Integral: A Study Using Polyurethane Foam Blocks. Dentistry Journal. 2020; 8(2):56. https://doi.org/10.3390/dj8020056
Chicago/Turabian StyleArosio, Paolo, Federico Arosio, and Danilo Alessio Di Stefano. 2020. "Implant Diameter, Length, and the Insertion Torque/Depth Integral: A Study Using Polyurethane Foam Blocks" Dentistry Journal 8, no. 2: 56. https://doi.org/10.3390/dj8020056
APA StyleArosio, P., Arosio, F., & Di Stefano, D. A. (2020). Implant Diameter, Length, and the Insertion Torque/Depth Integral: A Study Using Polyurethane Foam Blocks. Dentistry Journal, 8(2), 56. https://doi.org/10.3390/dj8020056




