The Capacity of Periodontal Gel to Occupy the Spaces Inside the Periodontal Pockets Using Computational Fluid Dynamic
Abstract
:1. Introduction
- Mathematical modeling (partial differential equations)
- Numerical methods (discretization and solution techniques)
- Software tools (solvers, pre- and post-processing utilities)
- -
- Tetracycline: a broad-spectrum naphthacene antibiotic, which binds to the 30 S ribosomal subunit, inhibiting protein synthesis [3]. In dentistry, it can be found in the form of tetracycline fiber (Periodontal Plus AB). This product is effective, easy to place, and has few side effects and adverse consequences. However, further long-term studies are needed to evaluate the regular use of this product [4].
- -
- Minocycline: a derivative of tetracycline with excellent absorption and tissue penetration that is used for several bacterial infections. It is a slow release product of good consistency and with antibacterial activity [5]. In dentistry, it can be found in the form of microspheres (Arestin) and ointment (Periocline). According to Matesanz-Pérez et al., the different studies that were used in their meta-analysis suggested a positive effect of minocycline on the reduction of probing depth, but not on the onset [6].
- -
- Metronidazole: derived from nitronidazole and has antibacterial activities. Non-ionized metronidazole is absorbed by obligate anaerobic organisms and is then reduced by transport proteins into an active intermediate product. Thus, it inhibits DNA synthesis and bacterial cell growth [7]. Elyzol can also be found in dentistry and it is a gel that is composed of a solid suspension of metronidazole benzoate with a moniglyceride and a triglyceride.
- -
- Doxycycline: a broad-spectrum antibiotic with antimicrobial activity. It binds to the 30 S ribosomal subunit and blocks the binding of aminoacyl-tRNA to the mRNA-ribosome complex, thereby inhibiting protein synthesis. In addition, this agent has exhibited inhibition of collagenase activity. Ligosan can be found in dentistry and it appears to have two powerful effects: (1) if administered with a systemic antibiotic, it increases its effectiveness; (2) maximum effectiveness occurs when the periodontal pocket is treated in the active phase with bleeding in the probing and/or pus [8]. Ligosan complements the standard non-surgical procedure (SRP) for periodontal pocket treatment. The product remains in the correct position and after 12 days it undergoes a spontaneous process of biodegradation and bio-absorption which does not require removal [9].
- -
- Chlorhexidine Gluconate: an antiseptic agent with topical antibacterial activity. It is positively charged and reacts with the negatively charged microbial cell, thus destroying the cell membrane. For this reason, Gram-positive bacteria, which have a more negative charge, are more sensitive to this product [10]. Periochip, which is used in dentistry, has been found to reduce pocket depth after causal therapy [11].
- -
- There are viscous solutions of sulfates that are added to sulfuric acid which make use of the drying properties of the polysaccharide matrix of the biofilm cells. By absorbing the water from the biofilm, the bacteria, and their matrix, this causes a clot and therefore, the collapse of the internal structure of the plaque [12]. HybenX can be found in dentistry and the liquid is placed in the pocket, kept for a minute, and then rinsed. Tooth sensitivity during application has occurred [12].
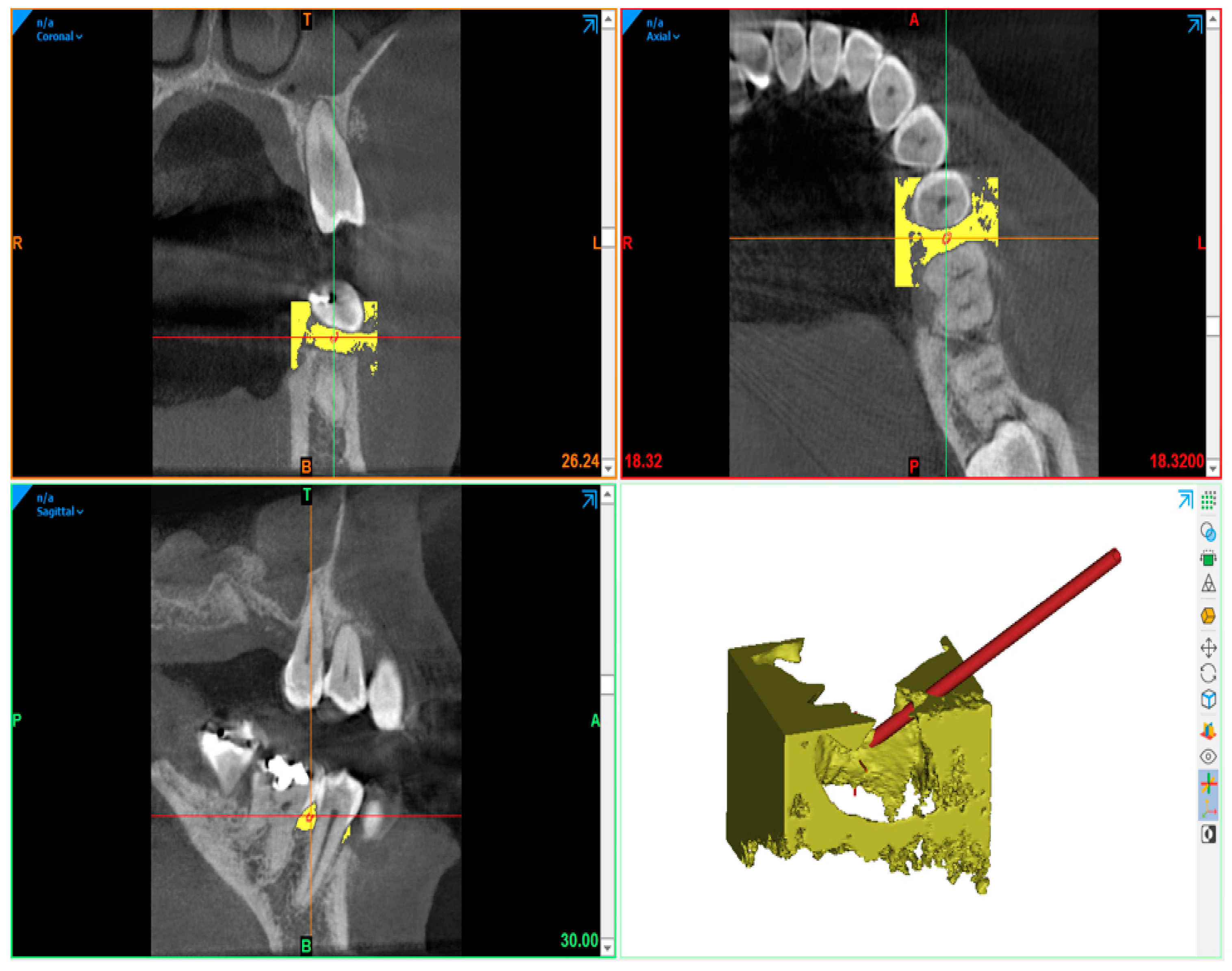
2. Materials and Methods
- Poly Ethylene Oxide (PEO): a resorbable medical polymer with high molecular weight. It provides rheological properties, longer degradation times, and physical stability to the gel [18].
- Hydroxypropyl Methylcellulose (HPMC): is a resorbable medical polymer derived from cellulose. It provides rheological properties and high hydrophilicity for the gel [19].
- Type I Collagen: of equine origin, needed for the consistency of the product, and has hydrophilic and haemostatic characteristics [20].
- Vitamin C: is an antioxidant agent that is synthesized by plants and most animals [21]. According to the study of Sànchez-Najera, Vitamin C has important bactericidal activity against Streptococcus mutans, Staphylococcus aureus, Porphyromonas gingivalis, Candida albicans, and Enterococcus faecalis [22]. The action of Vitamin C on the polymerization of the polymers maintains the rheological properties both before and after sterilization, ensuring fluidity of the gel and prolonged times of reabsorption (at least 14 days). It presents: visco-elasticity, mucus-adhesiveness (mucosal adhesiveness, gums), physical stability, and the ability to remain stable in a liquid environment (saliva, blood).
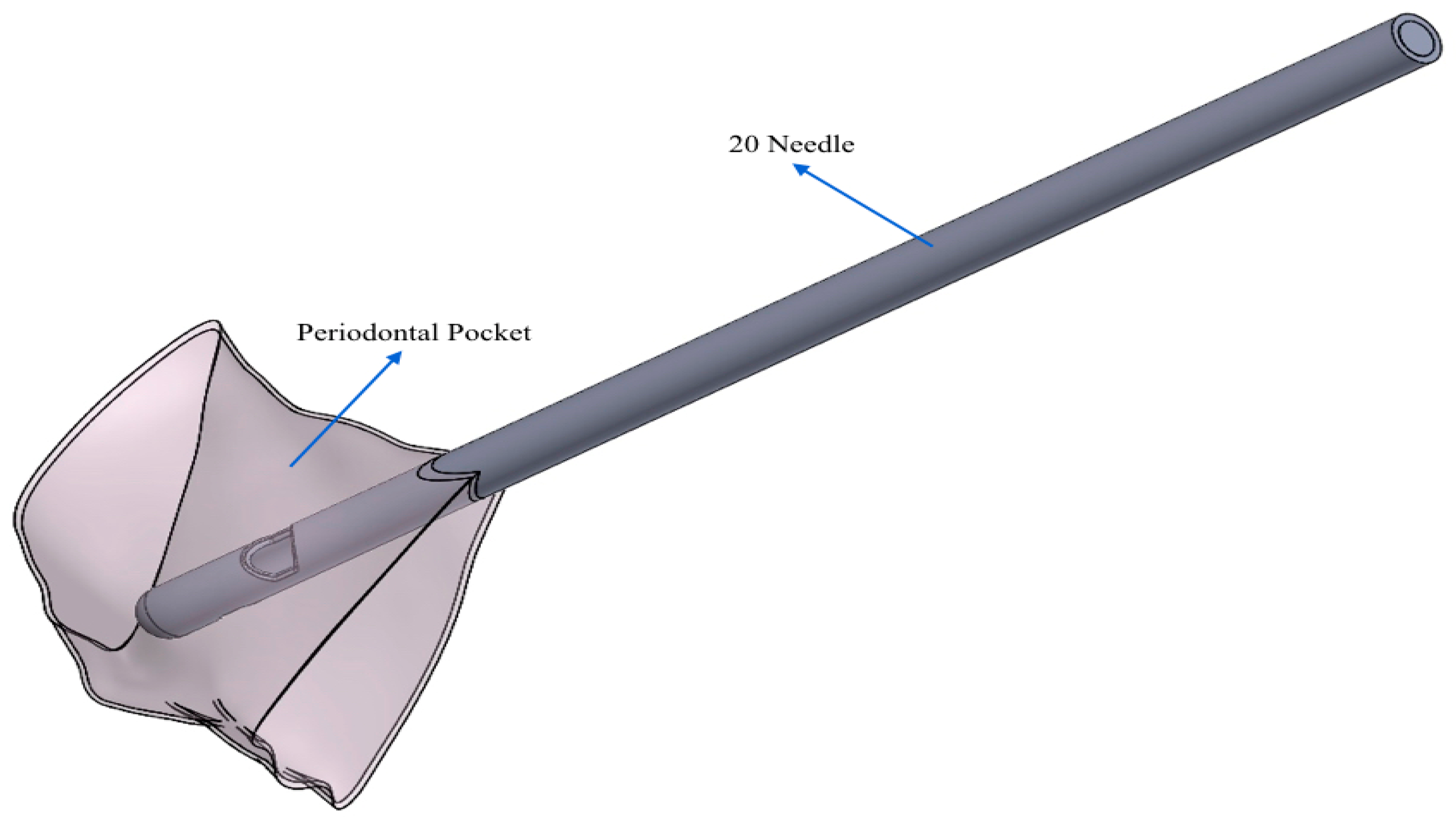
- 20 G needle (test gel): closed spherical tip and two exits;
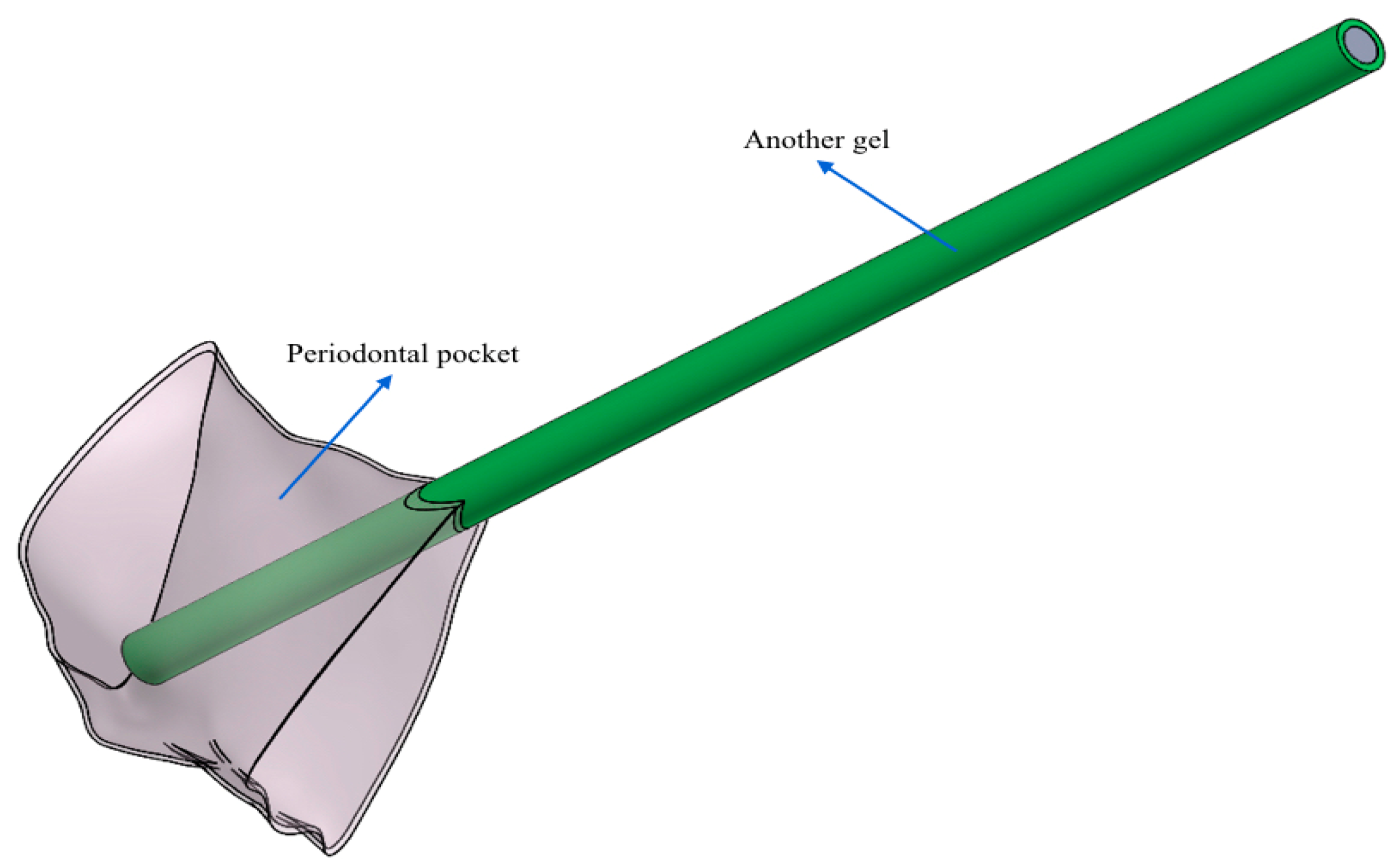
- Another needle (control gel): flat open tip and one exit.
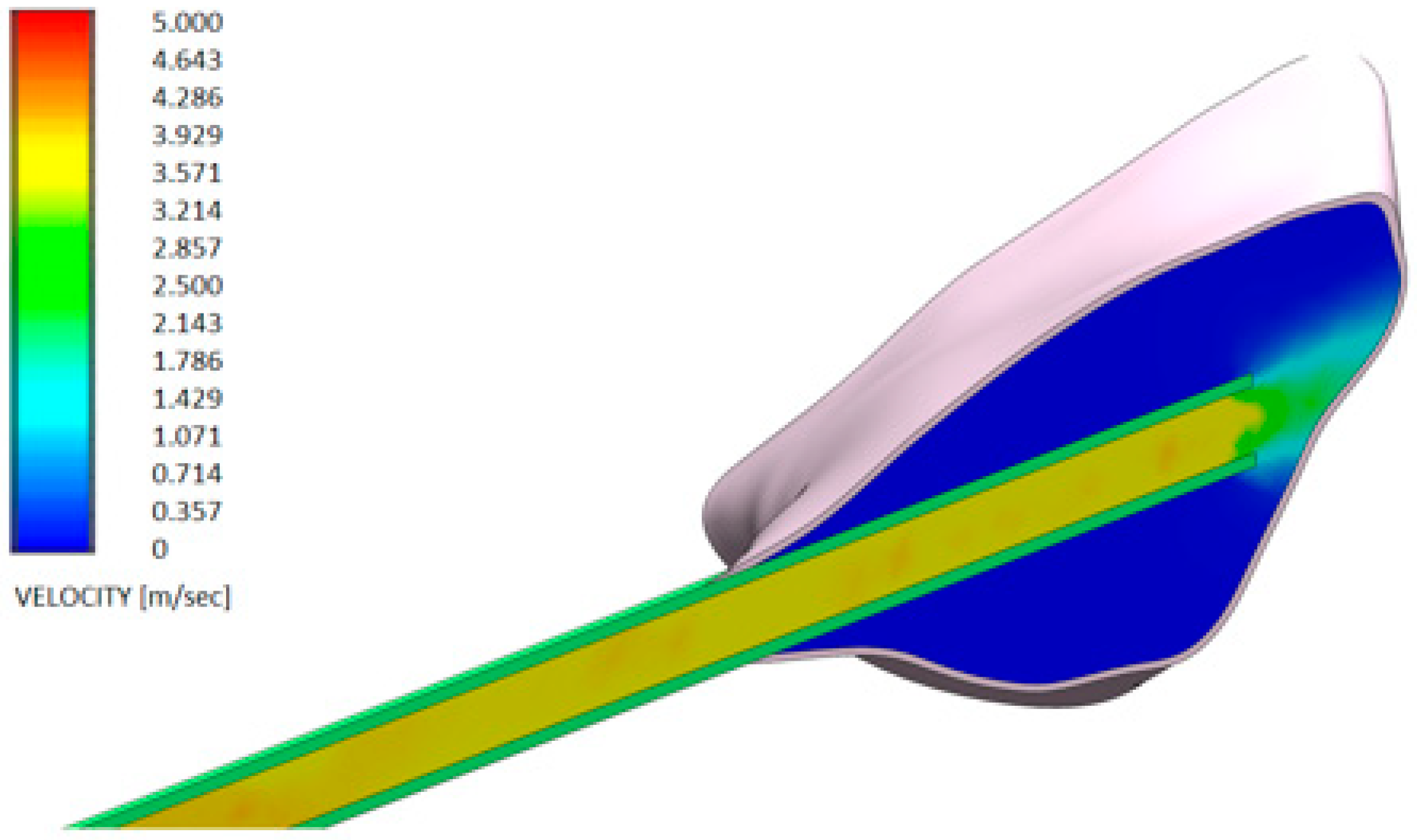
3. Results
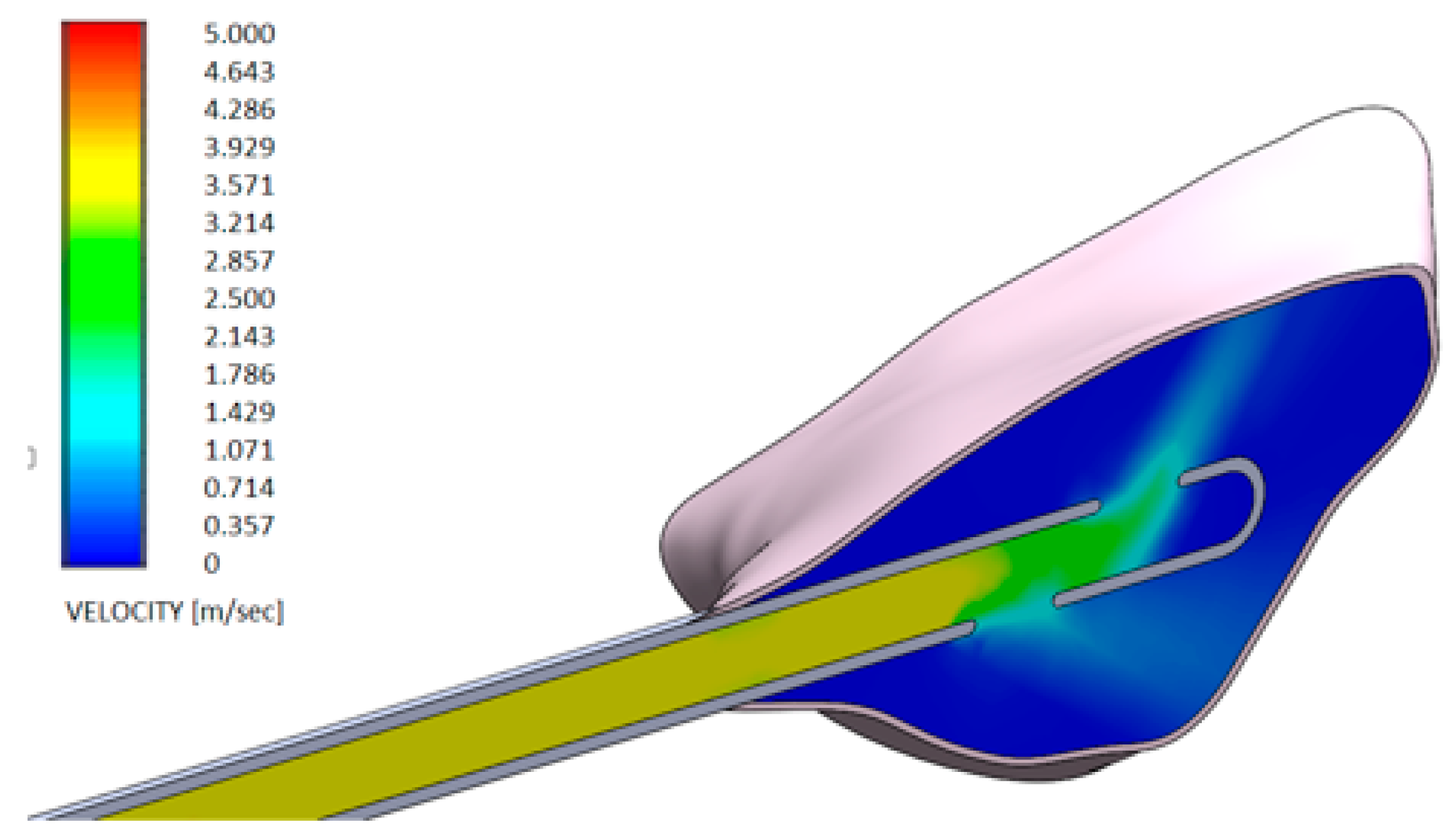
- Test gel + 20 G needle: the gel exits uniformly without speed peaks, both from the first and second exit, thanks to the rheological properties of the gel and the geometry of the needle;
- Control gel + 20 G needle: the liquid penetrates inside the pocket only from the first exit;
- Test gel + Flat Point needle and Another Gel + Flat Point needle: the gel remains confined to the tip, so it has an uneven distribution within the periodontal pocket.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, D.A.; Tannehill, J.C.; Pletcher, R.H. Computational Fluid Mechanics and Heat Transfer; Hemisphere: New York, NY, USA, 1984. [Google Scholar]
- Arcilla, A.S.; Hauser, J.; Eiseman, P.R.; Thompson, J.F. Numerical Grid Generation in Computational Fluid Dynamics and Related Fields; North-Holland: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Forsberg, K.H.; Patel, S.; Wencewicz, T.A.; Dantas, G. The Tetracycline Destructases: A Novel Family of Tetracycline-Inactivating Enzymes. Chem. Biol. 2015, 22, 888–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, S.; Kumar, S.; Dagli, N.; Dagli, R.J. Effect of tetracycline HCl in the treatment of chronic periodotitis—A clinical study. J. Int. Soc. Prev. Community Dent. 2014, 4, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hokari, T.; Morozumi, T.; Komatsu, Y.; Shimizu, T.; Yoshino, T.; Tanaka, M.; Tanaka, Y.; Nohno, K.; Kubota, T.; Yoshie, H. Effects of Antimicrobial Photodynamic Therapy and Local Administration of Minocycline on Clinical, Microbiological, and Inflammatory Markers of Periodontal Pockets: A Pilot Study. Int. J. Dent. 2018, 2018, 1748584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matesanz-Pérez, P.I.; García-Gargallo, M.; Figuero, E.; Bascones-Martínez, A.; Sanz, M.; Herrera, D. A systematic review on the effects of local antimicrobials as adjuncts to subgingival debridement, compared with subgingival debridement alone, in the treatment of chronic periodontitis. J. Clin. Periodontol. 2013, 40, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Gatto, R.; Petrucci, A.; Monaco, A. Effectiveness of systemic amoxicillin/metronidazole as adjunctive therapy to scaling and root planing in the treatment of chronic periodontitis: A systemic review and meta-analysis. J. Periodontol. 2012, 83, 1257–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.S.; Lee, S.H.; Eickholz, P.; Zimmer, H.; Kim, C.K. Systemic detection of doxycycline after local administration. Acta Odontol. Scand. 2009, 67, 289–296. [Google Scholar] [CrossRef]
- Ratka-Kruger, P.; Schacher, B.; Burklin, T.; Boddinghaus, B.; Holle, R.; Renggli, H.H.; Eickholz, P.; Kim, T.S. Non-surgical periodontal therapy with adjunctive topical doxyxycline: A double-masked, randomized, controlled multicenter study. II. Microbiological results. J. Periodontol. 2005, 76, 66–74. [Google Scholar] [CrossRef]
- Nicolosi, L.N.; del Carmen, R.M.; Martinez, C.D.; González, N.N.; Cruz, M.E. Effect of oral hygiene and 0.12% chlorhexidine gluconate oral rinse in preventing ventilator-associated pneumonia after cardiovascular surgery. Respir. Care 2014, 59, 504–509. [Google Scholar] [CrossRef] [Green Version]
- Navjot, J.; Daljit, K.; Rachna, J. Comparison of Periochip (chlorhexidine gluconate 2.5 mg) and Arestin (Minocycline hydrochloride 1 mg) in the management of chronic periodontitis. Indian J. Dent. 2015, 6, 20–26. [Google Scholar]
- Irokawa, D.; Makino-Oi, A.; Fujita, T.; Yamamoto, S.; Tomita, S.; Saito, A. Adjunct antimicrobial therapy and periodontal surgery to trat generalized aggressive periodontitis: A case report. Bull. Tokyo Dent. Coll. 2016, 57, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Konde, S.; Jairam, L.S.; Peethambar, P.; Noojady, S.R.; Kumar, N.C. Antibiotic overusage and resistance: A cross-sectional survey among pediatric dentists. J. Indian Pedod. Prev. Dent. 2016, 34, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.G.M.; Seers, C.A.; Sabri, A.N.; Reynolds, E.C. Dental plaque bacteria with reduced susceptibility to chlorhexidine are multidrug resistant. BMC Microbiol. 2016, 16, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viganò, L.; Nosotti, M.G.; Orlova, N.; Casu, C. Use of chlorhexidine, side effects and antibiotic resistance. Biointerface Res. Appl. Chem. 2018, 8, 3265–3266. [Google Scholar]
- Rahman, M.N.A.; Qader, A.J.A.; Sukmasari, S.; Ismali, A.F.; Doolaanea, A.A. Rheological Characterization of Different Gelling Polymers for Dental Gel Formulation. Pharm. Sci. Res. 2017, 9, 2633–2640. [Google Scholar]
- Popa, L.; Ghica, M.V.; Dinu-Pirvu, C.E. Periodontal chitosan-gels designed for improved local intra-pocket drug delivery. Farmacia 2013, 61, 240–250. [Google Scholar]
- Bag, M.A.; Valenzuela, L.M. Impact of the Hydration States of Polymers on Their Hemocompatibility for Medical Applications: A Review. Int. J. Mol. Sci. 2017, 18, 1422. [Google Scholar] [CrossRef] [Green Version]
- Martis, C.S.; Leitao, R.F.C.; Costa, D.V.S.; Melo, I.M.; Santos, G.S.; Lima, V.; Baldim, V.; Wong, D.V.T.; Bonfim, L.E.; Melo, C.B.; et al. Topical HPMC/S-Nitrosoglutathione Solution Decreases Inflammation and Bone Resorption in Experimental Periodontal Disease in Rats. PLoS ONE 2016, 11, e0153716. [Google Scholar]
- Fiocco, L.; Stevens, M.M.; Bernardo, E.; Jones, J.R. Biocompatibility and bioactivity of porous polymer-derived Ca-Mg silicate ceramics. Acta Biomater. 2017, 1, 56–67. [Google Scholar] [CrossRef]
- Ruamrak, C.; Lourith, N.; Natakankitkul, S. Comparison of clinical efficacies of sodium ascorbyl phosphate, retinol and their combination in acne treatment. Int. J. Cosmet. Sci. 2009, 31, 41–46. [Google Scholar] [CrossRef]
- Isela, N.-N.R.; Sergio, N.-C.; José, M.-S.J.; Rene, H.-D.; Claudio, C.-R. Ascorbic acid on oral microbial growth and biofilm formation. Pharma Innov. 2013, 2, 103–109. [Google Scholar]
- Arvand, A.; Hormes, M.; Reul, H. A validated computational UID dynamics model to estimate hemolysis in a rotary blood pump. Artif. Organs 2005, 29, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J. Digital DICOM in Dentistry. Open Dent. J. 2015, 9, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DenOtter, T.D.; Schubert, J. Hounsfield Unif. StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Mohan, R.; Singh, A.; Gundappa, M. Three-dimensional imaging in periodontal diagnosis—Utilization of cone beam computed tomography. J. Indian Soc. Periodontol. 2011, 15, 11–17. [Google Scholar] [PubMed]
- Jervøe-Storm, P.M.; Hagner, M.; Neugebauer, J.; Ritter, L.; Zöller, J.E.; Jepsen, S.; Frentzen, M. Comparison of cone-beam computerized tomography and intraoral radiographs for deter-mination of the periodontal ligament in a variable phantom. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 109, e95–e101. [Google Scholar] [CrossRef]
- Soni, S.; Aggarwal, P.K.; Sharma, N.; Chander, S. Coenzyme Q10 and periodontal health: A review. Int. J. Oral Maxillofac. Pathol. 2012, 3, 21–26. [Google Scholar]
- Tòthovà, L.; Celec, P. Oxidative stress and antioxidants in the diagnosis and therapy of periodontitis. Front. Physiol. 2017, 8, 1055. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, L.; Cervino, G.; Herford, A.S.; Lauritano, F.; D’Amico, C.; Lo Giudice, R.; Laino, L.; Troiano, G.; Crimi, S.; Cicciù, M. Interferon crevicular fluid profile and correlation with periodontal disease and wound healing: A systemic review of recent data. Int. J. Mol. Sci. 2018, 19, 1908. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [Green Version]




| Global Mesh Settings |
| Automatic initial mesh: On |
| Result resolution level: 4 |
| Advanced narrow channel refinement: On |
| Refinement in solid region: Off |
| Number of elements: 46,150 (elements used for two configurations) |
| Geometry Resolution |
| Evaluation of the minimum gap size: Manual |
| Minimum gap size: 5.000 × 10−4 m |
| Evaluation of the minimum wall thickness: Automatic |
| Initial Conditions |
| Thermodynamic parameters |
| Static Pressure: 101,325.00 Pa |
| Temperature: 293.20 K |
| Velocity parameters |
| Velocity vector |
| Velocity in X direction: 0 m/s |
| Velocity in Y direction: 0 m/s |
| Velocity in Z direction: 0 m/s |
| Material Settings |
| Fluids |
| GEL H42®–Other GEL |
| Boundary Conditions |
| Environment Pressure 1 |
| Type: Environment Pressure |
| Faces: Real pocket-1/Cavity1//Face |
| Coordinate system: Global coordinate system |
| Reference axis: X |
| Thermodynamic parameters |
| Environment pressure: 101,325.00 Pa |
| Temperature type: Temperature of the initial components |
| Temperature: 293.20 K |
| Inlet Velocity 1 |
| Type: Inlet Velocity |
| Faces: LID1-1/Imported1//Face |
| Coordinate system: Face Coordinate System |
| Reference axis: X |
| Flow parameters |
| Flow vectors direction: Normal to face |
| Velocity normal to face: 4.000 m/s |
| Thermodynamic parameters |
| Temperature type: Temperature of the initial components |
| Temperature: 293.20 K |
| Boundary layer parameters |
| Boundary layer type: Turbulent |
| Computational Domain |
| Size |
| X min: 0.026 m |
| X max: 0.045 m |
| Y min: 0.010 m |
| Y max: 0.030 m |
| Z min: 0.015 m |
| Z max: 0.035 m |
| X size: 0.019 m |
| Y size: 0.020 m |
| Z size: 0.020 m |
| Boundary Conditions |
| 2D plane flow: None |
| At X min: Default |
| At X max: Default |
| At Y min: Default |
| At Y max: Default |
| At Z min: Default |
| At Z max: Default |
| Physical Features |
| Heat conduction in solids: Off |
| Time dependent: Off |
| Gravitational effects: Off |
| Rotation: Off |
| Flow type: Laminar only |
| High Mach number flow: Off |
| Free surface: Off |
| Default roughness: 0 micrometer |
| Gel H42 | Another Gel |
|---|---|
|
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levrini, L.; Paracchini, L.; Nosotti, M.G. The Capacity of Periodontal Gel to Occupy the Spaces Inside the Periodontal Pockets Using Computational Fluid Dynamic. Dent. J. 2020, 8, 1. https://doi.org/10.3390/dj8010001
Levrini L, Paracchini L, Nosotti MG. The Capacity of Periodontal Gel to Occupy the Spaces Inside the Periodontal Pockets Using Computational Fluid Dynamic. Dentistry Journal. 2020; 8(1):1. https://doi.org/10.3390/dj8010001
Chicago/Turabian StyleLevrini, Luca, Luigi Paracchini, and Maria Giulia Nosotti. 2020. "The Capacity of Periodontal Gel to Occupy the Spaces Inside the Periodontal Pockets Using Computational Fluid Dynamic" Dentistry Journal 8, no. 1: 1. https://doi.org/10.3390/dj8010001







