Reactivity and Bond Strength of Universal Dental Adhesives with Co-Cr Alloy and Zirconia
Abstract
1. Introduction
2. Materials and Methods
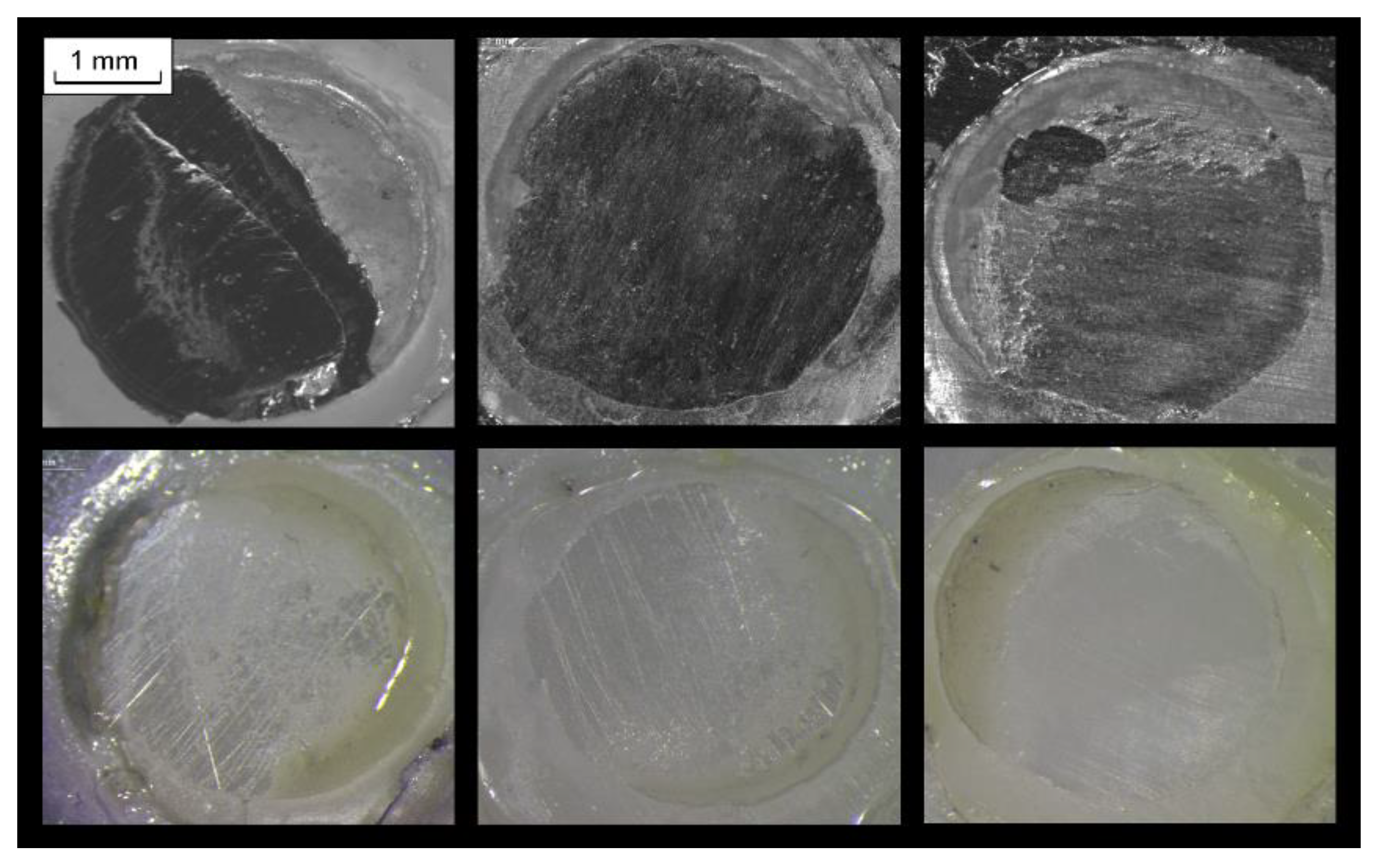
2.1. Reactivity with the Substrates
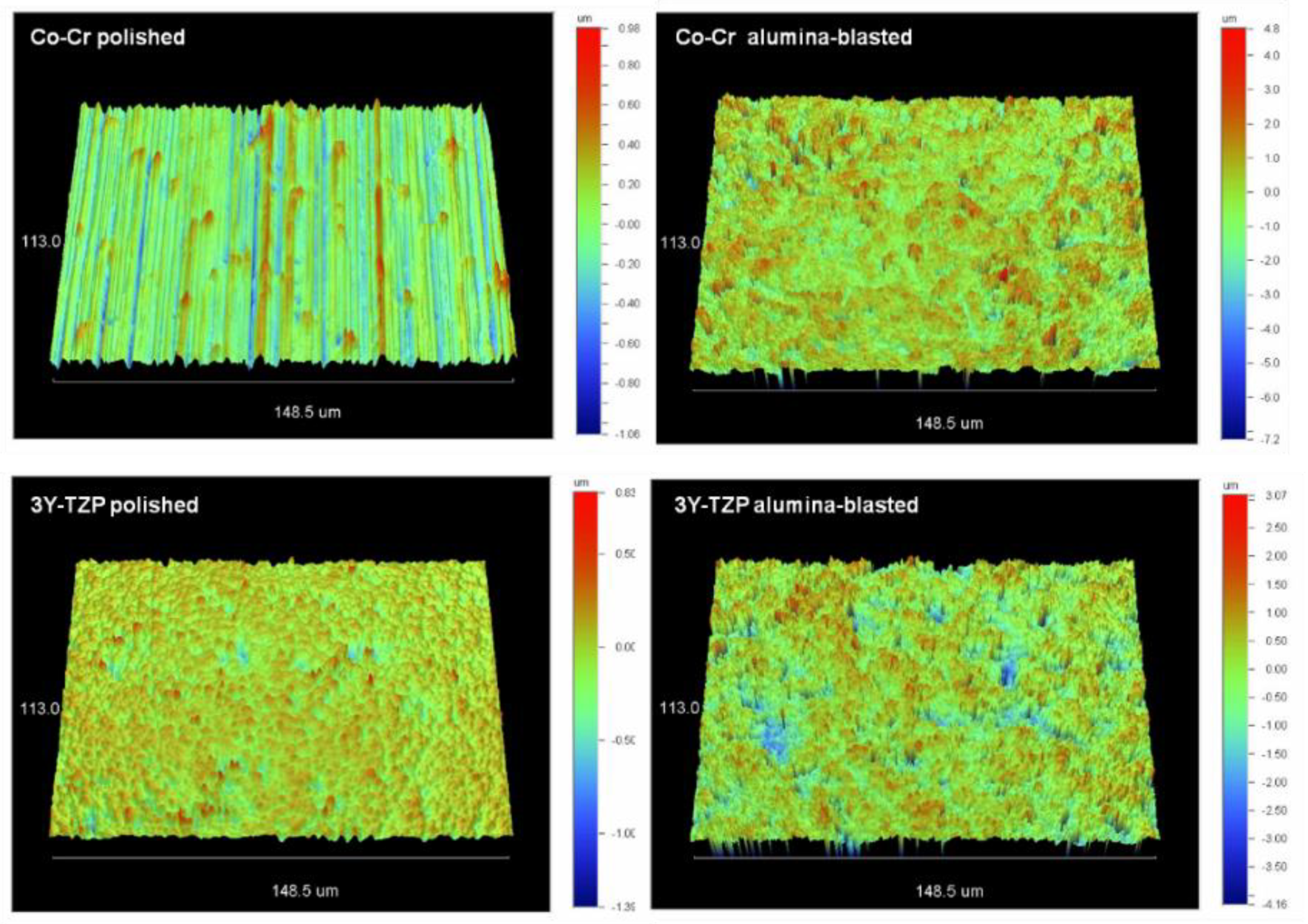
2.2. Roughness
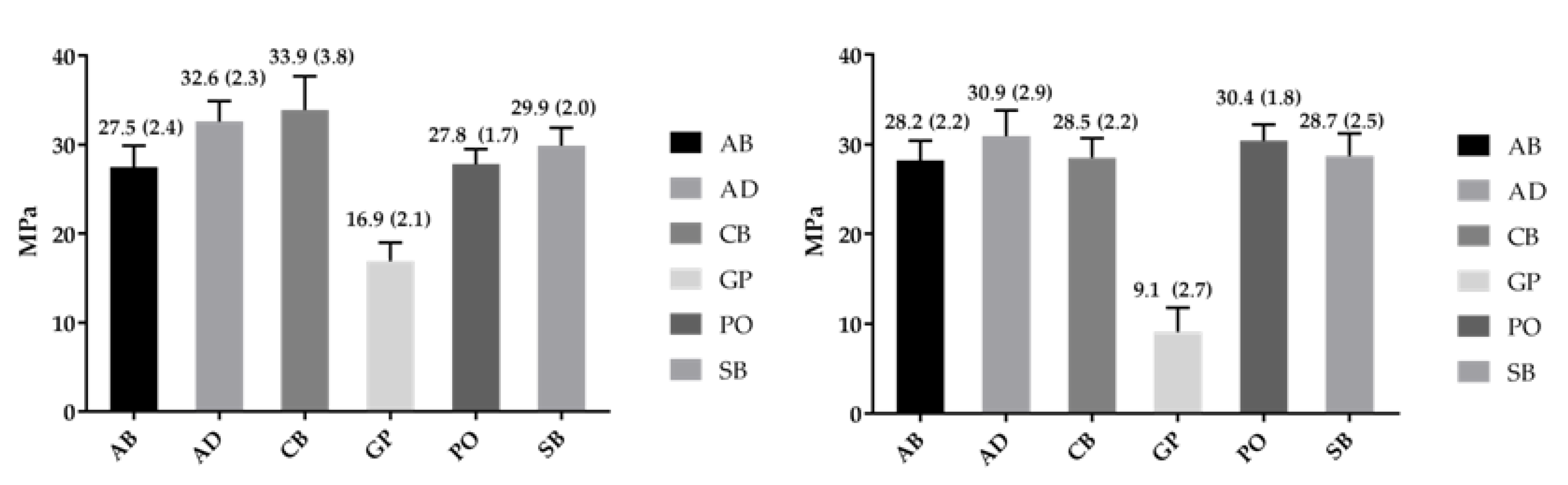
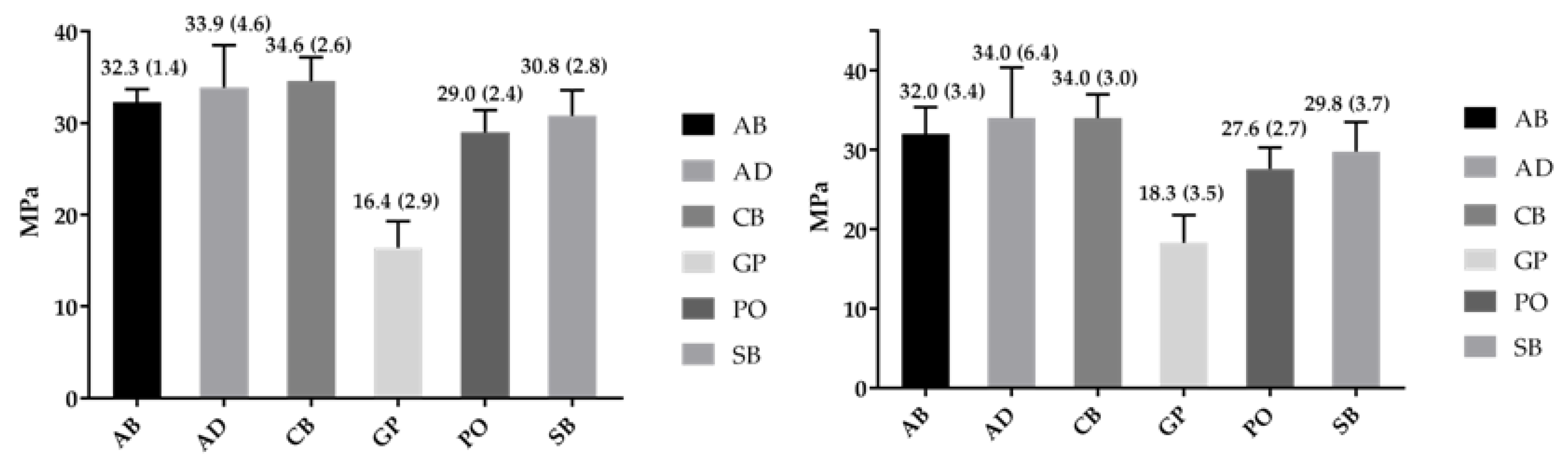
2.3. Bond Strength
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Chen, C.; Niu, L.N.; Xie, H.; Zhang, Z.Y.; Zhou, L.Q.; Jiao, K.; Chen, J.H.; Pashley, D.H.; Tay, F.R. Bonding of universal adhesives to dentine—Old wine in new bottles? J. Dent. 2015, 43, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Rosa, W.L.; Piva, E.; Silva, A.F. Bond strength of universal adhesives: A systematic review and meta-analysis. J. Dent. 2015, 43, 765–776. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Frankenberger, R. Editorial: What’s next after “universal” adhesives, “bioactive” adhesives? J. Adhes. Dent. 2017, 19, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Nagarkar, S.; Theis-Mahon, N.; Perdigao, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 34305, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Loguercio, A.D.; Munoz, M.A.; Luque-Martinez, I.; Hass, V.; Reis, A.; Perdigao, J. Does active application of universal adhesives to enamel in self-etch mode improve their performance? J. Dent. 2015, 43, 1060–1070. [Google Scholar] [CrossRef]
- Suzuki, S.; Takamizawa, T.; Imai, A.; Tsujimoto, A.; Sai, K.; Takimoto, M.; Barkmeier, W.W.; Latta, M.A.; Miyazaki, M. Bond durability of universal adhesive to bovine enamel using self-etch mode. Clin. Oral Investig. 2018, 22, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.E.; Meyers, E.J.; Guillory, V.L.; Vandewalle, K.S. Enamel Bond Strength of New Universal Adhesive Bonding Agents. Oper. Dent. 2015, 40, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Lee, M.G.; Woo, S.U.; Lee, C.O.; Yi, J.K.; Kim, D.S. Comparative study of the dentin bond strength of a new universal adhesive. Dent. Mater. J. 2016, 35, 606–612. [Google Scholar] [CrossRef]
- Munoz, M.A.; Luque-Martinez, I.; Malaquias, P.; Hass, V.; Reis, A.; Campanha, N.H.; Loguercio, A.D. In vitro longevity of bonding properties of universal adhesives to dentin. Oper. Dent. 2015, 40, 282–292. [Google Scholar] [CrossRef]
- Thanatvarakorn, O.; Prasansuttiporn, T.; Takahashi, M.; Thittaweerat, S.; Foxton, R.M.; Ichinose, S.; Tagami, J.; Nakajima, M. Effect of Scrubbing Technique with Mild Self-etching Adhesives on Dentin Bond Strengths and Nanoleakage Expression. J. Adhes. Dent. 2016, 18, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, K.; Gerula-Szymanska, A.; Smektala, T.; Safranow, K.; Lewusz, K.; Nowicka, A. Effects of different etching modes on the nanoleakage of universal adhesives: A systematic review and meta-analysis. J. Esthet. Restor. Dent. 2018, 30, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Takamizawa, T.; Barkmeier, W.W.; Tsujimoto, A.; Berry, T.P.; Watanabe, H.; Erickson, R.L.; Latta, M.A.; Miyazaki, M. Influence of different etching modes on bond strength and fatigue strength to dentin using universal adhesive systems. Dent. Mater. 2016, 32, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Amaral, M.; Belli, R.; Cesar, P.F.; Valandro, L.F.; Petschelt, A.; Lohbauer, U. The potential of novel primers and universal adhesives to bond to zirconia. J. Dent. 2014, 42, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Seabra, B.; Arantes-Oliveira, S.; Portugal, J. Influence of multimode universal adhesives and zirconia primer application techniques on zirconia repair. J. Prosthet. Dent. 2014, 112, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Li, Q.; Zhang, F.; Lu, Y.; Tay, F.R.; Qian, M.; Chen, C. Comparison of resin bonding improvements to zirconia between one-bottle universal adhesives and tribochemical silica coating, which is better? Dent. Mater. 2016, 32, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Hattan, M.A.; Pani, S.C.; Alomari, M. Composite bonding to stainless steel crowns using a new universal bonding and single-bottle systems. Int. J. Dent. 2013, 2013, 607405. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Kontham, U.R.; Kamath, A.; Kontham, R. Shear bond strength of composite resin bonded to preformed metal crowns for primary molars using a universal adhesive and two different surface treatments: An in vitro study. Eur. Arch. Paediatr. Dent. 2016, 17, 377–380. [Google Scholar] [CrossRef]
- ISO 29022:2013: Dentistry-Adhesion-Notched-Edge Shear Bond Strength; International Organization for Standardization: Geneva, Switzerland, 2013.
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Wilwerding, T.M.; Latta, M.A.; Miyazaki, M. Interfacial Characteristics and Bond Durability of Universal Adhesive to Various Substrates. Oper Dent. 2017, 42, E59–E70. [Google Scholar] [CrossRef]
- de Souza, G.; Hennig, D.; Aggarwal, A.; Tam, L.E. The use of MDP-based materials for bonding to zirconia. J. Prosthet. Dent. 2014, 112, 895–902. [Google Scholar] [CrossRef]
- Al Jeaidi, Z.A.; Alqahtani, M.A.; Awad, M.M.; Rodrigues, F.P.; Alrahlah, A.A. Bond strength of universal adhesives to air-abraded zirconia ceramics. J. Oral. Sci. 2017, 59, 565–570. [Google Scholar] [CrossRef]
- Hallmann, L.; Ulmer, P.; Reusser, E.; Hämmerle, C.H.F. Effect of blasting pressure, abrasive particle size and grade on phase transformation and morphological change of dental zirconia surface. Surf. Coat. Technol. 2012, 206, 4293–4302. [Google Scholar] [CrossRef]
- Chintapalli, R.K.; Marro, F.G.; Jimenez-Pique, E.; Anglada, M. Phase transformation and subsurface damage in 3Y-TZP after sandblasting. Dent. Mater. 2013, 29, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Yu, W.Q.; Zhang, F.Q.; Smales, R.J.; Zhang, Y.L.; Lu, C.H. Corrosion behaviour and surface analysis of a Co-Cr and two Ni-Cr dental alloys before and after simulated porcelain firing. Eur. J. Oral. Sci. 2011, 119, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ohno, H.; Araki, Y.; Sagara, M. The adhesion mechanism of dental adhesive resin to the alloy—Relationship between Co-Cr alloy surface structure analyzed by ESCA and bonding strength of adhesive resin. Dent. Mater. J. 1986, 5, 46–65. [Google Scholar] [CrossRef] [PubMed]
- Yamane, Y.; Ohno, H.; Endo, K. Mechanism of adhesion between 4-META resin and alloys based on Bolger’s acid-base interaction. Dent. Mater. J. 2001, 20, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.; Thompson, V.P. Sandblasting and silica-coating of dental alloys: Volume loss, morphology and changes in the surface composition. Dent. Mater. 1993, 9, 151–161. [Google Scholar] [CrossRef]
- Kern, M.; Thompson, V.P. Durability of resin bonds to a cobalt-chromium alloy. J. Dent. 1995, 23, 47–54. [Google Scholar] [CrossRef]
- Li, W.; Pierre-Louis, A.M.; Kwon, K.D.; Kubicki, J.D.; Strongin, D.R.; Phillips, B.L. Molecular level investigations of phosphate sorption on corundum (α-Al2O3) by 31P solid state NMR, ATR-FTIR and quantum chemical calculation. Geochim. Cosmochim. Acta 2013, 107, 252–266. [Google Scholar] [CrossRef]
- Nishigawa, G.; Maruo, Y.; Irie, M.; Maeda, N.; Yoshihara, K.; Nagaoka, N.; Matsumoto, T.; Minagi, S. Various Effects of Sandblasting of Dental Restorative Materials. PLoS ONE 2016, 11, e0147077. [Google Scholar] [CrossRef]
- Nagaoka, N.; Yoshihara, K.; Feitosa, V.P.; Tamada, Y.; Irie, M.; Yoshida, Y.; Van Meerbeek, B.; Hayakawa, S. Chemical interaction mechanism of 10-MDP with zirconia. Sci. Rep. 2017, 7, 45563. [Google Scholar] [CrossRef]
- Shimoe, S.; Hirata, I.; Otaku, M.; Matsumura, H.; Kato, K.; Satoda, T. Formation of chemical bonds on zirconia surfaces with acidic functional monomers. J. Oral. Sci. 2018, 60, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Pilo, R.; Dimitriadi, M.; Palaghia, A.; Eliades, G. Effect of tribochemical treatments and silane reactivity on resin bonding to zirconia. Dent. Mater. 2018, 34, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Soldera, F.A.; Gaillard, Y.; Gomila, M.A.; Muecklich, F. FIB-Tomography of Nanoindentation Cracks in Zirconia Polycrystals. Microsc. Microanal. 2007, 13, 1510–1511. [Google Scholar] [CrossRef]
- Yoshihara, K.; Nagaoka, N.; Okihara, T.; Kuroboshi, M.; Hayakawa, S.; Maruo, Y.; Nishigawa, G.; De Munck, J.; Yoshida, Y.; Van Meerbeek, B. Functional monomer impurity affects adhesive performance. Dent. Mater. 2015, 31, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhou, L.; Zhang, Z.; Niu, L.; Zhang, L.; Chen, C.; Zhou, J.; Yang, H.; Wang, X.; Fu, B.; et al. Paucity of Nanolayering in Resin-Dentin Interfaces of MDP-based Adhesives. J. Dent. Res. 2016, 95, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hanabusa, M.; Yoshihara, K.; Yoshida, Y.; Okihara, T.; Yamamoto, T.; Momoi, Y.; Van Meerbeek, B. Interference of functional monomers with polymerization efficiency of adhesives. Eur. J. Oral. Sci. 2016, 124, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Van Landuyt, K.L.; De Munck, J.; Snauwaert, J.; Coutinho, E.; Poitevin, A.; Yoshida, Y.; Inoue, S.; Peumans, M.; Suzuki, K.; Lambrechts, P.; et al. Monomer-solvent phase separation in one-step self-etch adhesives. J. Dent. Res. 2005, 84, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; De Munck, J.; Shirai, K.; Hikita, K.; Inoue, S.; Sano, H.; Lambrechts, P.; Van Meerbeek, B. Effect of evaporation of primer components on ultimate tensile strengths of primer-adhesive mixture. Dent. Mater. 2005, 21, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- DeHoff, P.H.; Anusavice, K.J.; Wang, Z. Three-dimensional finite element analysis of the shear bond test. Dent. Mater. 1995, 11, 126–131. [Google Scholar] [CrossRef]
- Gaintantzopoulou, M.; Rahiotis, C.; Eliades, G. Molecular characterization of one-step self-etching adhesives placed on dentin and inert substrate. J. Adhes. Dent. 2008, 10, 83–93. [Google Scholar]
- Papadogiannis, D.; Dimitriadi, M.; Zafiropoulou, M.; Gaintantzopoulou, M.D.; Eliades, G. Universal Adhesives: Setting Characteristics and Reactivity with Dentin. Materials 2019, 12, 1720. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, S.; Rawls, H.R.; Hotta, M. Relationship between thin-film bond strength as measured by a scratch test, and indentation hardness for bonding agents. Dent. Mater. 2016, 32, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kern, M. Bonding to oxide ceramics-laboratory testing versus clinical outcome. Dent. Mater. 2015, 31, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Sano, H.; Ciucchi, B.; Yoshiyama, M.; Carvalho, R.M. Adhesion testing of dentin bonding agents: A review. Dent. Mater. 1995, 11, 117–125. [Google Scholar] [CrossRef]
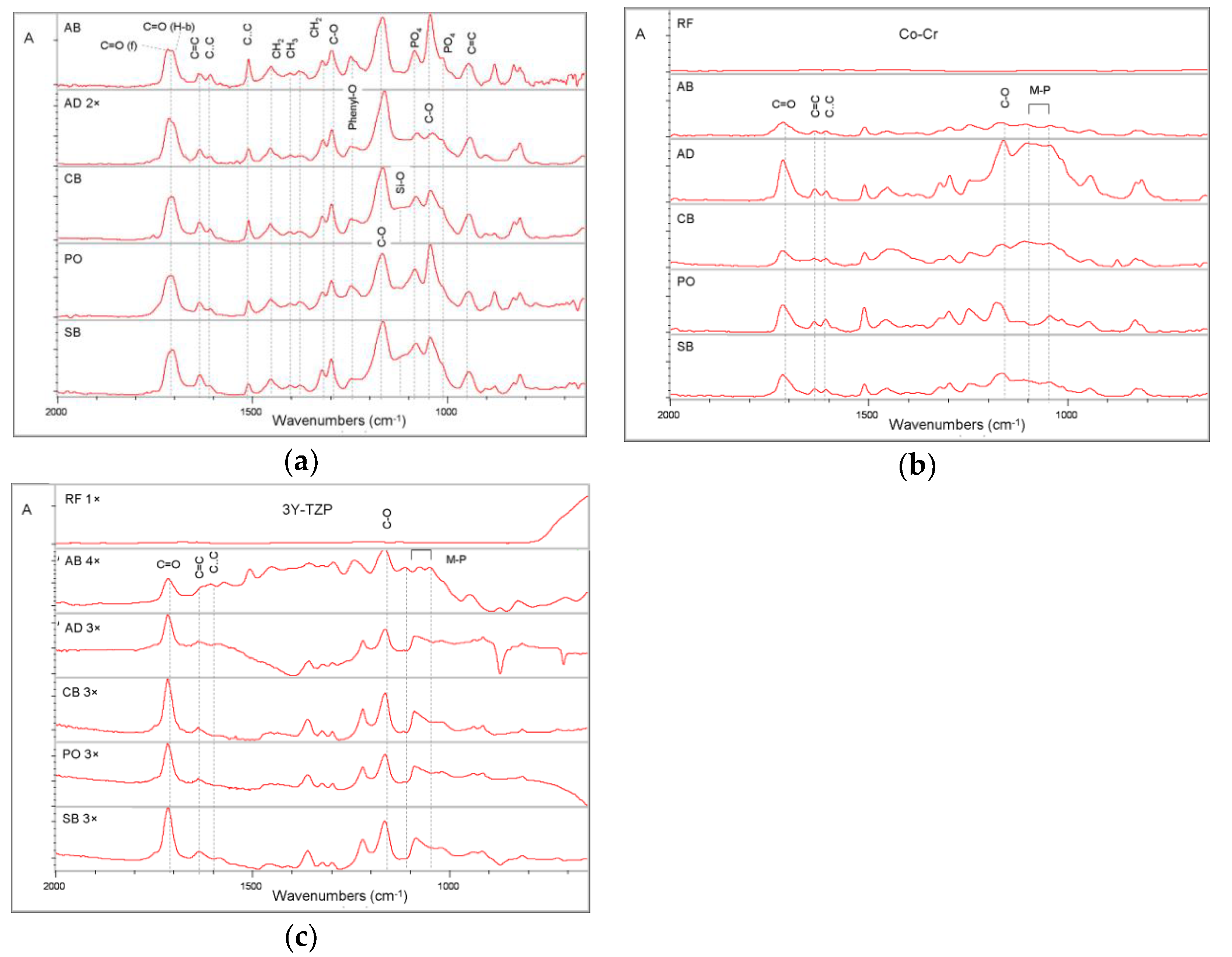
| Material | Composition * | Manufacturer |
|---|---|---|
| Adhese Universal (AD) | 10-MDP, 2-HEMA, BisGMA, MCAP, D3MA, highly dispersed silica, ethanol, water, photoinitiators (pH = 2.8) | Ivoclar-Vivadent, Schaan, Liechtenstein |
| All-Bond Universal (AB) | 10-MDP, 2-HEMA, BisGMA, ethanol, water, photoinitiator (pH = 3.1) | Bisco, Schaumburg, IL, USA |
| Clearfil Universal Bond (CB) | 10-MDP, 2-HEMA, BisGMA, hydrophilic aliphatic dimethacrylate, MPTMS, colloidal silica, photoinitiators (pH = 2.3) | Kuraray Noritake Dental, Okayama, Japan |
| G-Premio Bond (GP) | 10-MDP, 4-MET, MTDP, methacrylic acid ester, silica, acetone, water, photoinitiators (pH = 1.5) | GC Corp., Tokyo, Japan |
| Prelude One (PO) | 10-MDP, Methacryloyloxyalkyl acid carboxylate, 2-HEMA, BisGMA, ethanol (pH = 2.8) | Danville Materials, S. Ramon, CA, USA |
| Scotchbond Universal Adhesive (SB) | 10-MDP, 2-HEMA, BisGMA, DCDMA, MPTMS, VP-copolymer, fumed silica, ethanol, water, photoinitiators (pH = 2.7) | 3M ESPE, St. Paul, MN, USA |
| Substrate | Treatment | Sa (nm) | Sz (μm) | Sdr (%) | Sc (nm3/nm2) | Sv (nm3/nm2) |
|---|---|---|---|---|---|---|
| Co-Cr | Polished | 180 a (32) | 1.7 a (0.2) | 2.9 a (0.8) * | 232.2 a (23.4) | 14.9 a (2.4) * |
| Alumina Blasted | 652 b (71) ** | 5.6 b (0.5) ** | 77 b (5.6) ** | 822.6 b (58.9) | 98.4 b (8.7) ** | |
| 3Y-TZP | Polished | 83 a (11) | 1.2 a (0.3) | 1.5 a (0.3) * | 122.8 a (10.8) | 15.9 a (4) * |
| Alumina Blasted | 426 b (58) ** | 3.9 b (0.3) ** | 44 b (4.7) ** | 567.8 b (49.5) | 64.8 b (10.2) ** |
| Co-Cr | Weibull Parameter | AB | AD | CB | GP | PO | SB |
|---|---|---|---|---|---|---|---|
| Polished | Shape-β (95% CI) | 12.3 a,A (7.8–19.3) | 18.3 a,D (11.0–30.3) | 16.2 a,G (9.9–26.7) | 9.5 a,J (5.9–15.3) | 17.9 a,O (11.4–28.0) | 16.1 a,S (10.1–25.6) |
| Scale-σ0 (95% CI)/MPa | 28.6 a,B (27.1–30.2) | 33.6 b,E (32.4–34.8) | 35.1 b,H (33.7–36.5) | 17.8 c,L (16.6–19.2) | 28.6 a,P (27.6–29.7) | 30.9 a,T (29.6–32.3) | |
| σ0.05 (95% CI)/MPa | 22.5 a,C (19.6–25.8) | 28.5 b,F (25.8–31.5) | 29.2 b,I (26.1–32.6) | 13.0 c,N (10.8–15.7) | 24.2 a,b,R (22.0–26.6) | 25.7 a,b,U (23.1–28.6) | |
| r2 | 0.92 | 0.95 | 0.92 | 0.95 | 0.85 | 0.92 | |
| Alumina Blasted | Shape-β (95% CI) | 15.1 a,A (9.4–24.1) | 12.8 a,D (7.9–20.8) | 14.7 a,G (9.1–23.8) | 3.6 b,K (2.3–5.6) | 16.2 a,O (10.4–25.3) | 14.2 a,S (8.8–23.1) |
| Scale-σ0 (95% CI)/MPa | 29.1 a,B (27.9–30.4) | 32.2 b,E (30.6–33.9) | 29.4 a,b,H (28.2–30.8) | 10.0 c,M (8.4–12.0) | 31.3 a,b,Q (30.0–32.6) | 29.7 a,b,T (28.4–31.1) | |
| σ0.05 (95% CI)/MPa | 23.9 a,C (21.3–26.8) | 25.5 a,F (22.2–29.3) | 24.1 a,I (21.4–27.1) | 4.4 b,N (2.8–6.9) | 24.1 a,R (21.3–27.3) | 26.1 a,U (23.5–28.9) | |
| r2 | 0.94 | 0.94 | 0.92 | 0.84 | 0.68 | 0.94 |
| 3Y-TZP | Weibull Parameter | AB | AD | CB | GP | PO | SB |
|---|---|---|---|---|---|---|---|
| Polished | Shape-β | 30.3 a,A (17.9–51.4) | 10.3 b,E (6.1–17.4) | 16.8 a,b,H (10.3–27.2) | 6.7 b,K (4.2–10.7) | 13.7 b,N (8.5–21.9) | 11.2 b,Q (7.2–17.5) |
| Scale-σ0 (95% CI)/MPa | 32.9 a,C (32.3–33.7) | 35.8 b,F (33.8–38.1) | 35.7 b,I (34.4–37.1) | 17.5 d,L (15.9–19.4) | 30.0 c,O (28.6–31.5) | 32.0 a,b,R (30.2–34.0) | |
| σ0.05 (95%CI)/MPa | 29.9 a,D (28.1–31.8) | 26.8 a,b,G (22.3–32.1) | 29.9 a,J (26.9–33.2) | 11.2 c,M (8.7–14.8) | 24.2 b,P (21.3–27.5) | 24.6 b,S (21.2–28.5) | |
| r2 | 0.85 | 0.96 | 0.98 | 0.98 | 0.91 | 0.91 | |
| Alumina Blasted | Shape-β | 12.2 a,B (7.4–20.0) | 5.6 a,E (3.6–8.8) | 7.63 a,H (5.02–11.6) | 6.8 a,K (4.0–11.5) | 12.0 a,N (7.5–19.1) | 9.0 a,Q (5.7–14.5) |
| Scale-σ0 (95%CI)/MPa | 33.4 a,C (31.7–35.3) | 36.6 a,F (32.6–41.2) | 36.3 a,I (33.2–39.5) | 19.7 b,L (17.9–21.7) | 28.8 c,O (27.2–30.4) | 31.4 a,c,R (29.2–33.7) | |
| σ0.05 (95% CI)/MPa | 26.2 a,D (22.6–30.3) | 21.6 a,G (16.0–29.1) | 24.6 a,J (19.9–30.3) | 12.8 b,M (9.7–16.7) | 22.4 a,P (19.4–25.9) | 22.6 a,S (18.7–27.3) | |
| r2 | 0.97 | 0.91 | 0.66 | 0.94 | 0.93 | 0.95 |
| Treatment | Failure Mode | AB | AD | CB | GP | PO | SB | X2 | p |
|---|---|---|---|---|---|---|---|---|---|
| Co-Cr Polished | Type I | 7 | 6 | 7 | 8 | 7 | 6 | 1.31 | 0.93 |
| Type III | 3 | 4 | 3 | 2 | 3 | 4 | |||
| Co-Cr Alumina Blasted | Type I | 6 | 4 | 5 | 7 | 6 | 5 | 2.22 | 0.83 |
| Type III | 4 | 6 | 5 | 3 | 4 | 5 | |||
| X2 | 0.22 | 0.8 | 0.83 | 0.27 | 0.22 | 0.2 | |||
| p | 0.64 | 0.37 | 0.36 | 0.61 | 0.64 | 0.65 | |||
| 3Y-TZP Polished | Type I | 8 | 9 | 9 | 10 | 10 | 9 | 3.71 | 0.59 |
| Type III | 2 | 1 | 1 | 0 | 0 | 1 | |||
| 3Y-TZP Alumina Blasted | Type I | 7 | 8 | 7 | 10 | 10 | 8 | 6.72 | 0.24 |
| Type III | 3 | 2 | 3 | 0 | 0 | 2 | |||
| X2 | 0.27 | 0.39 | 1.25 | - | - | 0.39 | |||
| p | 0.61 | 0.53 | 0.27 | - | - | 0.53 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadogiannis, D.; Dimitriadi, M.; Zafiropoulou, M.; Gaintantzopoulou, M.-D.; Eliades, G. Reactivity and Bond Strength of Universal Dental Adhesives with Co-Cr Alloy and Zirconia. Dent. J. 2019, 7, 78. https://doi.org/10.3390/dj7030078
Papadogiannis D, Dimitriadi M, Zafiropoulou M, Gaintantzopoulou M-D, Eliades G. Reactivity and Bond Strength of Universal Dental Adhesives with Co-Cr Alloy and Zirconia. Dentistry Journal. 2019; 7(3):78. https://doi.org/10.3390/dj7030078
Chicago/Turabian StylePapadogiannis, Dimitris, Maria Dimitriadi, Maria Zafiropoulou, Maria-Dimitra Gaintantzopoulou, and George Eliades. 2019. "Reactivity and Bond Strength of Universal Dental Adhesives with Co-Cr Alloy and Zirconia" Dentistry Journal 7, no. 3: 78. https://doi.org/10.3390/dj7030078
APA StylePapadogiannis, D., Dimitriadi, M., Zafiropoulou, M., Gaintantzopoulou, M.-D., & Eliades, G. (2019). Reactivity and Bond Strength of Universal Dental Adhesives with Co-Cr Alloy and Zirconia. Dentistry Journal, 7(3), 78. https://doi.org/10.3390/dj7030078





