Effect of Dietary Fiber on the Composition of the Murine Dental Microbiome
Abstract
1. Introduction
2. Material and Methods
2.1. Dental Microbiome Sample Collection and Ethical Statement
2.2. Characterization of Dental Microbiome
2.3. Alpha Diversity Analyses
2.4. Beta Diversity Analyses
2.5. Differential Analysis of Microbial Taxa
2.6. Data Archive
3. Results
3.1. Experimental Setup
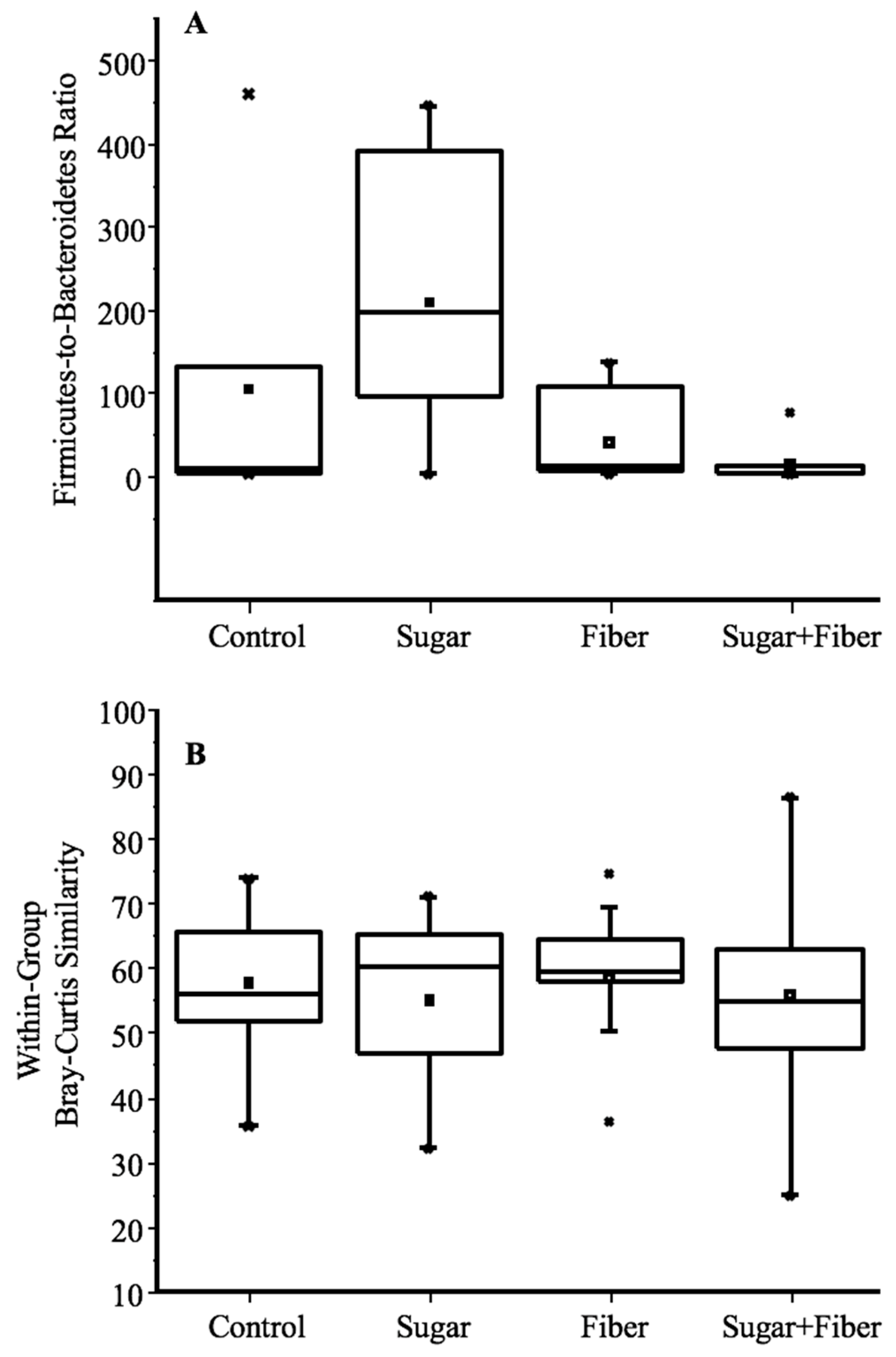
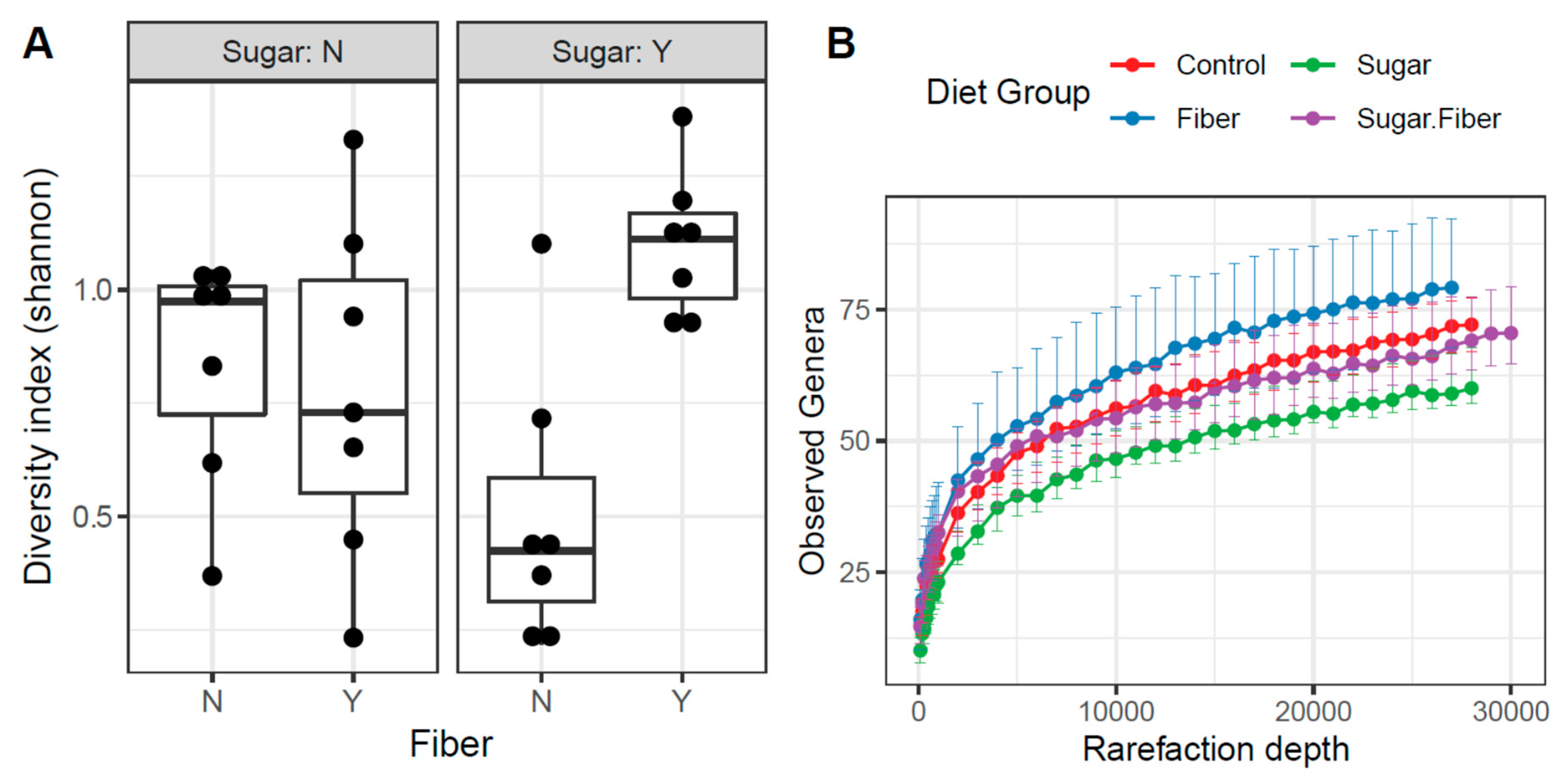
3.2. Diet-Induced Changes in Microbial Community Structure
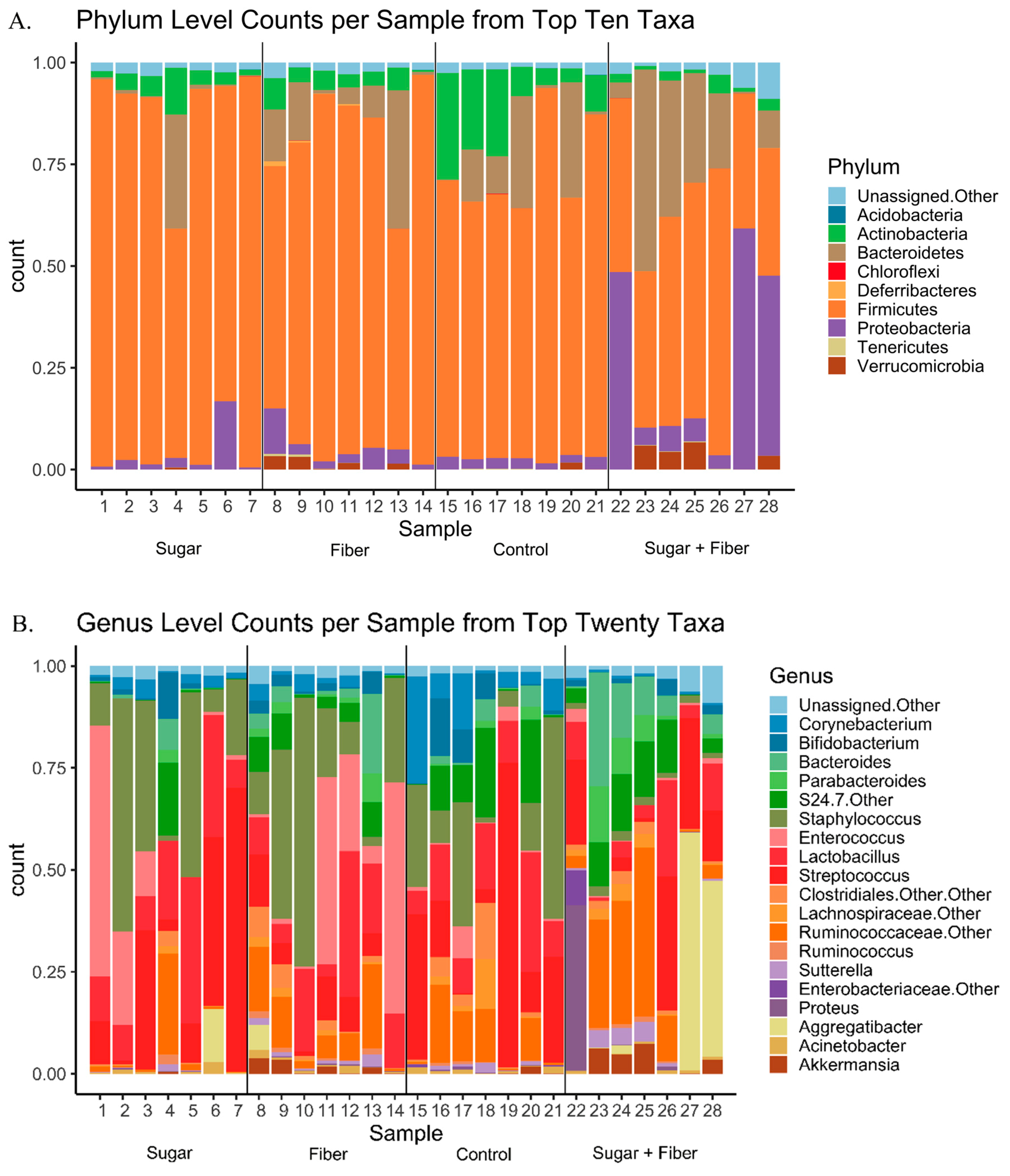
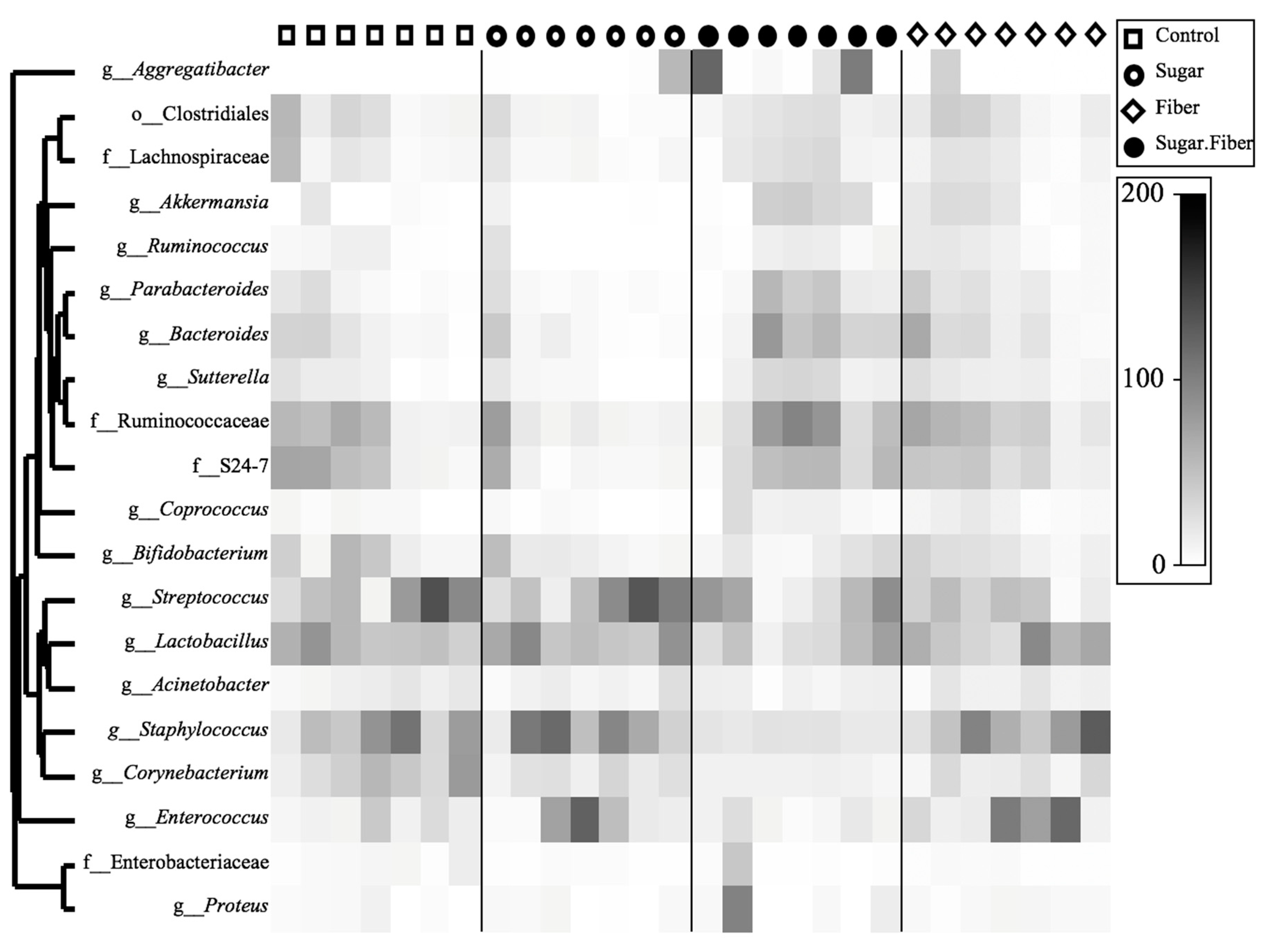
3.3. Taxonomic Abundance of Dietary Groups
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Kato, I.; Vasquez, A.; Moyerbrailean, G.; Land, S.; Djuric, Z.; Sun, J.; Lin, H.S.; Ram, J.L. Nutritional Correlates of Human Oral Microbiome. J. Am. Coll. Nutr. 2017, 36, 88–98. [Google Scholar] [CrossRef]
- Najeeb, S.; Zafar, M.S.; Khurshid, Z.; Zohaib, S.; Almas, K. The Role of Nutrition in Periodontal Health: An Update. Nutrients 2016, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.; Yang, Y.; Yooseph, S.; Hall, A.P.; Nguyen, D.D.; Dorrestein, P.C.; Nelson, K.E.; He, X.; Lux, R.; Shi, W.; et al. Meta-omics uncover temporal regulation of pathways across oral microbiome genera during in vitro sugar metabolism. ISME J. 2015, 9, 2605–2619. [Google Scholar] [CrossRef]
- Cornejo, O.E.; Lefebure, T.; Bitar, P.D.; Lang, P.; Richards, V.P.; Eilertson, K.; Do, T.; Beighton, D.; Zeng, L.; Ahn, S.J.; et al. Evolutionary and population genomics of the cavity causing bacteria Streptococcus mutans. Mol. Biol. Evol. 2013, 30, 881–893. [Google Scholar] [CrossRef]
- Cordain, L.; Eaton, S.B.; Sebastian, A.; Mann, N.; Lindeberg, S.; Watkins, B.A.; O’Keefe, J.H.; Brand-Miller, J. Origins and evolution of the Western diet: Health implications for the 21st century. Am. J. Clin. Nutr. 2005, 81, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. Pulling teeth from history: DNA from ancient teeth can help to yield information about our ancestors’ health, diet and diseases. EMBO Rep. 2014, 15, 923–925. [Google Scholar] [CrossRef]
- Carrera-Bastos, P.; Fontes-Villalba, M.; O’Keefe, J.H.; Lindeberg, S.; Cordain, L. The western diet and lifestyle and diseases of civilization. Res. Rep. Clin. Cardiol. 2011, 2, 15–35. [Google Scholar] [CrossRef]
- Hujoel, P. Dietary Carbohydrates and Dental-Systemic Diseases. J. Dent. Res. 2009, 88, 490–502. [Google Scholar] [CrossRef]
- Adler, C.J.; Dobney, K.; Weyrich, L.S.; Kaidonis, J.; Walker, A.W.; Haak, W.; Bradshaw, C.J.; Townsend, G.; Sołtysiak, A.; Alt, K.W.; et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 2013, 45, 450–455. [Google Scholar] [CrossRef]
- Crittenden, A.N.; Sorrentino, J.; Moonie, S.A.; Peterson, M.; Mabulla, A.; Ungar, P.S. Oral health in transition: The Hadza foragers of Tanzania. PLoS ONE 2017, 12, e0172197. [Google Scholar] [CrossRef] [PubMed]
- Bowden, G.; Ellwood, D.; Hamilton, I. Microbial ecology of the oral cavity. In Advances in Microbial Ecology; Alexander, M., Ed.; Plenum Press: New York, NY, USA, 1979; Volume 3, pp. 135–217. [Google Scholar]
- Theilade, E. Factors controlling the microflora of the health mouth. In Human Microbial Ecology; Hill, M.J., Marsh, P.D., Eds.; CRC Press: Boca Raton, FL, USA, 1990; pp. 2–48. [Google Scholar]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E.; Pedersen, A.M.L. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Woelber, J.P.; Bremer, K.; Vach, K.; Konig, D.; Hellwig, E.; Ratka-Kruger, P.; Al-Ahmad, A.; Tennert, C. An oral health optimized diet can reduce gingival and periodontal inflammation in humans—A randomized controlled pilot study. BMC Oral Health 2017, 17, 28. [Google Scholar] [CrossRef] [PubMed]
- Van Altena, F.; Beynen, A.C.; Altena, F.V.; Visser, E.A. Beneficial Effect of a Cellulose-Containing Chew Treat on Canine Periodontal Disease in a Double-Blind, Placebo-Controlled Trial. Am. J. Anim. Vet. Sci. 2010, 5, 192–195. [Google Scholar]
- Logan, E.I. Oral cleansing by dietary means: Results of six-month studies. In Proceedings of the Companion Animal Oral Health Conference, Lawrence, KS, USA, 1–3 March 1996; pp. 11–15. [Google Scholar]
- Logan, E.I.; Boyce, E.N.; Berg, M. Effects of dietary form on plaque and calculus and gingival health in cats—Methodology and results. In Proceedings of the World Veterinary Dental Congress, Birmingham, UK, 1–3 April 1997; pp. 28–32. [Google Scholar]
- Borges-Yanez, S.A.; Maupome, G.; Martinez-Gonzalez, M.; Cervantes-Turrubiates, L.; Gutierrez-Robledo, L.M. Dietary fiber intake and dental health status in urban, urban-marginal, and rural communities in Central Mexico. J. Nutr. Health Aging 2004, 8, 333–339. [Google Scholar] [PubMed]
- Schwartz, N.; Nunn, M.E.; Kaye, E.K.; Spiro, A.; Garcia, R. High-Fiber Foods Reduce Periodontal Disease Progression in Men Aged 65 and Older: The Veterans Affairs Normative Aging Study/Dental Longitudinal Study. J. Am. Geriatr. Soc. 2012, 60, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Trudel, L.; St-Amand, L.; Bareil, M.; Cardinal, P.; Lavoie, M.C. Bacteriology of the oral cavity of BALB/c mice. Can. J. Microbiol. 1986, 32, 673–678. [Google Scholar] [CrossRef]
- Rodrigue, L.; Barras, M.; Marcotte, H.; Lavoie, M. Bacterial colonization of the oral cavity of the BALB/c mouse. Microb. Ecol. 1993, 26, 267–275. [Google Scholar] [CrossRef]
- Gadbois, T.; Marcotte, H.; Rodrigue, L.; Coulombe, C.; Goyette, N.; Lavoie, M.C. Distribution of the Resident Oral Bacterial Populations in Different Strains of Mice. Microb. Ecol. Health 1993, 6, 245–251. [Google Scholar]
- Hyde, E.R.; Luk, B.; Cron, S.; Kusic, L.; McCue, T.; Bauch, T.; Kaplan, H.; Tribble, G.; Petrosino, J.F.; Bryan, N.S. Characterization of the rat oral microbiome and the effects of dietary nitrate. Free Radic. Boil. Med. 2014, 77, 249–257. [Google Scholar] [CrossRef]
- Abusleme, L.; Hong, B.-Y.; Hoare, A.; Konkel, J.E.; Diaz, P.I.; Moutsopoulos, N.M. Oral Microbiome Characterization in Murine Models. Bio-Protocol 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Kim, K.Y.; Lee, J.-H.; Choi, Y. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 2010, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Ge, Y.; Cheng, L.; Zeng, B.; Yu, J.; Peng, X.; Zhao, J.; Li, W.; Ren, B.; Li, M.; et al. Oral bacteria colonize and compete with gut microbiota in gnotobiotic mice. Int. J. Oral Sci. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Willcox, M.D.; Green, R.M.; Knox, K.W. Effect of different diets on oral bacteria and caries activity in Sprague-Dawley rats. Microbios 1997, 91, 105–120. [Google Scholar] [PubMed]
- Murtaza, N.; Burke, L.M.; Vlahovich, N.; Charlesson, B.; O’Neill, H.M.; Ross, M.L.; Campbell, K.L.; Krause, L.; Morrison, M. Analysis of the Effects of Dietary Pattern on the Oral Microbiome of Elite Endurance Athletes. Nutrients 2019, 11, 614. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Xu, X.; He, J.; Xue, J.; Wang, Y.; Li, K.; Zhang, K.; Guo, Q.; Liu, X.; Zhou, Y.; Cheng, L.; et al. Oral cavity contains distinct niches with dynamic microbial communities. Environ. Microbiol. 2015, 17, 699–710. [Google Scholar] [CrossRef]
- Green, S.J.; Venkatramanan, R.; Naqib, A. Deconstructing the Polymerase Chain Reaction: Understanding and Correcting Bias Associated with Primer Degeneracies and Primer-Template Mismatches. PLoS ONE 2015, 10, e0128122. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- McDonald, D.; Clemente, J.C.; Kuczynski, J.; Rideout, J.R.; Stombaugh, J.; Wendel, D.; Wilke, A.; Huse, S.; Hufnagle, J.; Meyer, F.; et al. The Biological Observation Matrix (BIOM) format or: How I learned to stop worrying and love the ome-ome. GigaScience 2012, 1, 7. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 22 May 2019).
- Wickham, H.; Sievert, C. ggplot2 Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Brown, M.E.; Chang, M.C. Exploring bacterial lignin degradation. Curr. Opin. Chem. Boil. 2014, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Duran-Pinedo, A.E.; Frias-Lopez, J. Beyond microbial community composition: Functional activities of the oral microbiome in health and disease. Microb. Infect. 2015, 17, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Scannapieco, F.A. The oral microbiome: Its role in health and in oral and systemic infections. Clin. Microbiol. Newsl. 2013, 35, 163–169. [Google Scholar] [CrossRef]
- Keller, M.K.; Kressirer, C.A.; Belstrøm, D.; Twetman, S.; Tanner, A.C.R. Oral microbial profiles of individuals with different levels of sugar intake. J. Oral Microbiol. 2017, 9, 1355207. [Google Scholar] [CrossRef]
- Gomez, A.; Espinoza, J.L.; Harkins, D.M.; Leong, P.; Saffery, R.; Bockmann, M.; Torralba, M.; Kuelbs, C.; Kodukula, R.; Inman, J.; et al. Host Genetic control of the oral microbiome in Health and Disease. Cell Host Microbe 2017, 22, 269.e3–278.e3. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A.; James, W.P. Diet and Dental Caries: The Pivotal Role of Free Sugars Reemphasized. J. Dent. Res. 2015, 94, 1341–1347. [Google Scholar] [CrossRef]
- Derrien, M.; Belzer, C.; De Vos, W.M. Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 2017, 106, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.Y.; Kostopoulos, I.; De Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef] [PubMed]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Mark Welch, J.; Rossetti, B.; Rieken, C.; Dewhirst, F.; Borisky, G. Biogeography of a human oral microbiome at the micron scale. Proc. Natl. Acad. Sci. USA 2016, 113, E791–E800. [Google Scholar] [CrossRef] [PubMed]
| Beta Diversity at the Phylum Level | Beta Diversity at the Genus Level | |||||||
|---|---|---|---|---|---|---|---|---|
| ADONIS | ANOSIM | ADONIS | ANOSIM | |||||
| Factor | R2 | Pr (>F) | R | p | R2 | Pr (>F) | R | p |
| Sugar | 0.03 | NS | 0.03 | NS | 0.03 | 0.331 | 0.02 | 0.225 |
| Fiber | 0.32 | <0.01 | 0.38 | <0.01 | 0.18 | < 0.01 | 0.20 | <0.01 |
| Sugar + Fiber | 0.04 | NS | NA | NA | 0.07 | 0.082 | NA | NA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedghi, L.; Byron, C.; Jennings, R.; Chlipala, G.E.; Green, S.J.; Silo-Suh, L. Effect of Dietary Fiber on the Composition of the Murine Dental Microbiome. Dent. J. 2019, 7, 58. https://doi.org/10.3390/dj7020058
Sedghi L, Byron C, Jennings R, Chlipala GE, Green SJ, Silo-Suh L. Effect of Dietary Fiber on the Composition of the Murine Dental Microbiome. Dentistry Journal. 2019; 7(2):58. https://doi.org/10.3390/dj7020058
Chicago/Turabian StyleSedghi, Lea, Craig Byron, Ryan Jennings, George E. Chlipala, Stefan J. Green, and Laura Silo-Suh. 2019. "Effect of Dietary Fiber on the Composition of the Murine Dental Microbiome" Dentistry Journal 7, no. 2: 58. https://doi.org/10.3390/dj7020058
APA StyleSedghi, L., Byron, C., Jennings, R., Chlipala, G. E., Green, S. J., & Silo-Suh, L. (2019). Effect of Dietary Fiber on the Composition of the Murine Dental Microbiome. Dentistry Journal, 7(2), 58. https://doi.org/10.3390/dj7020058





