Role of Cone-Beam Computed Tomography in the Management of Periodontal Disease
Abstract
1. Introduction
1.1. Current Evidence of CBCT and Periodontal Therapies
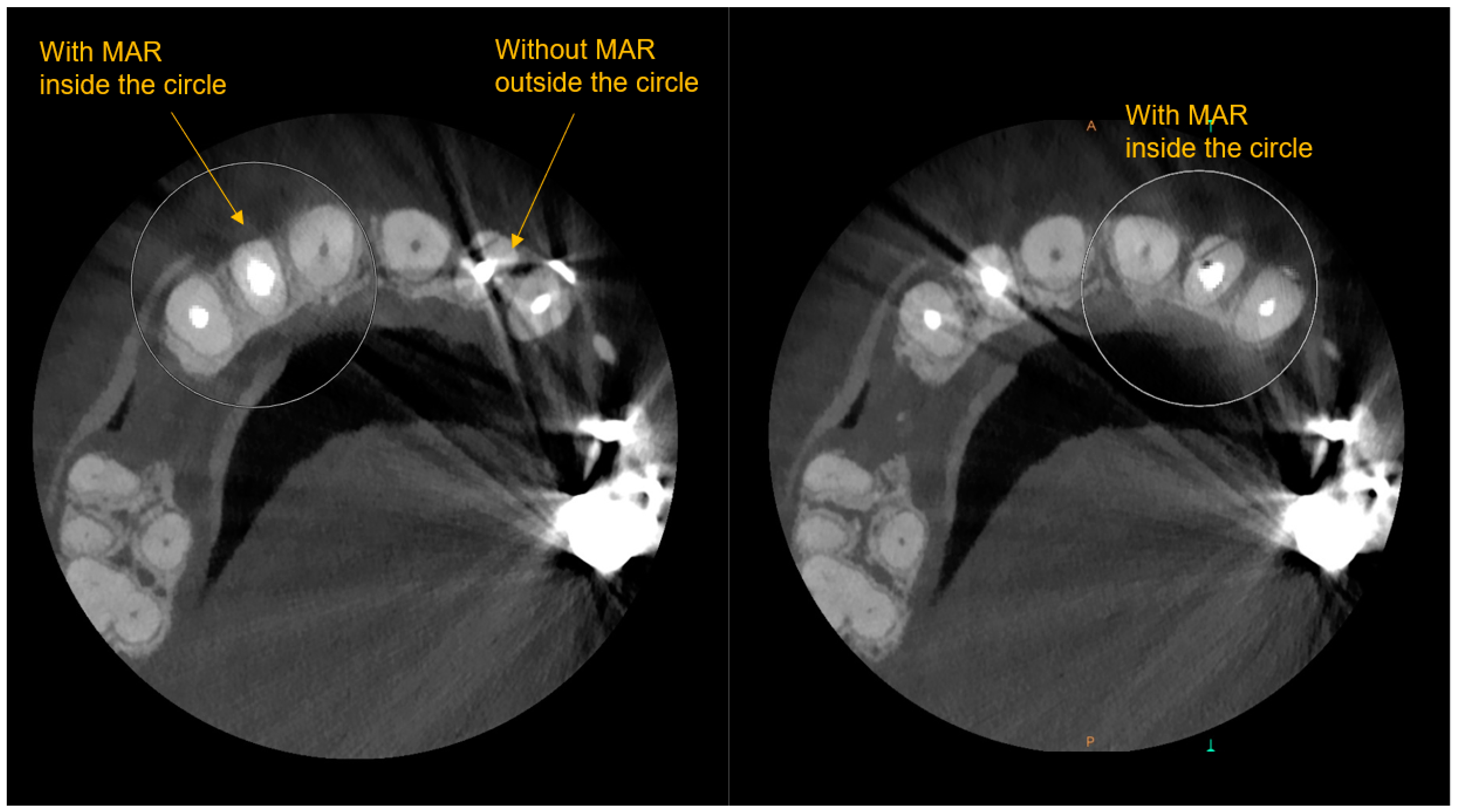
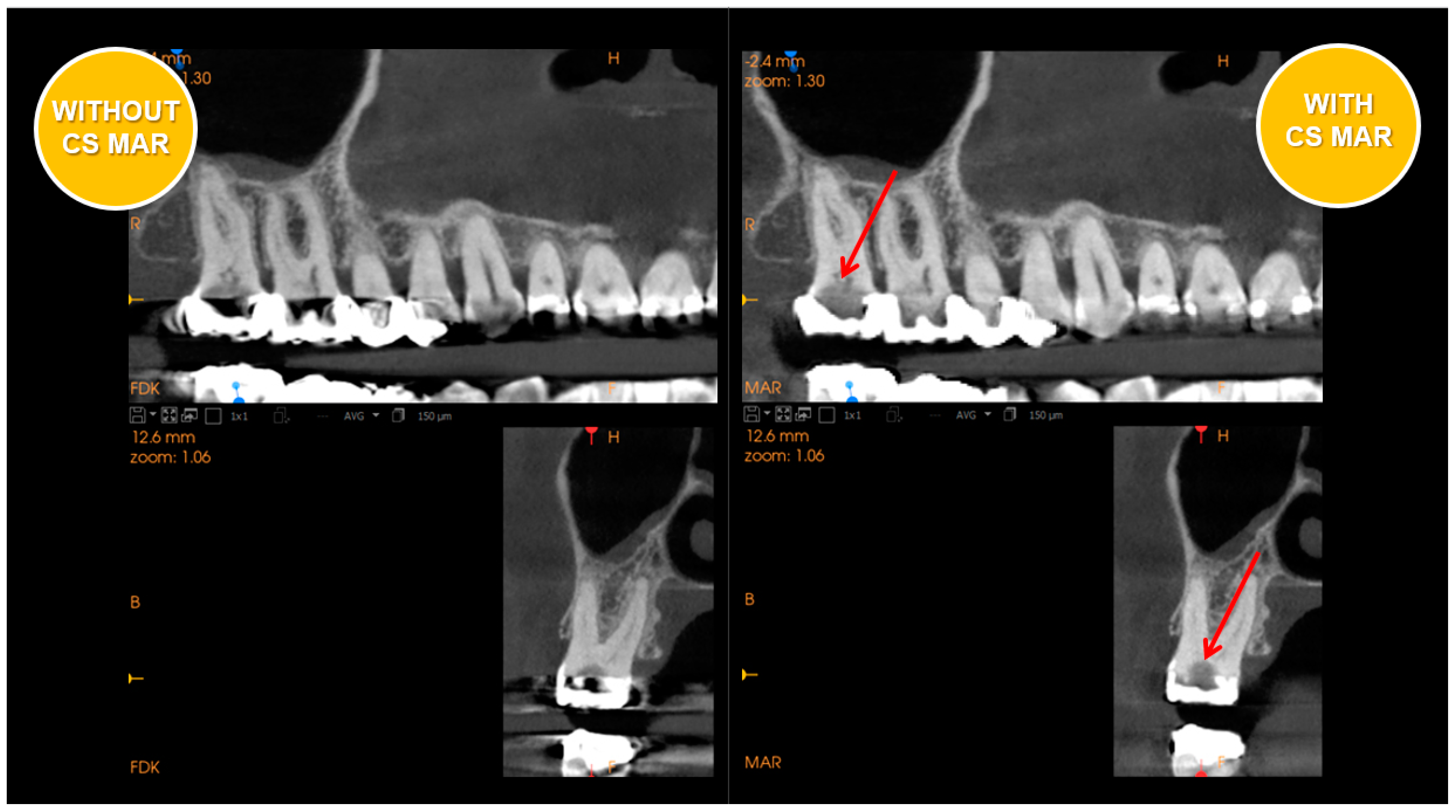
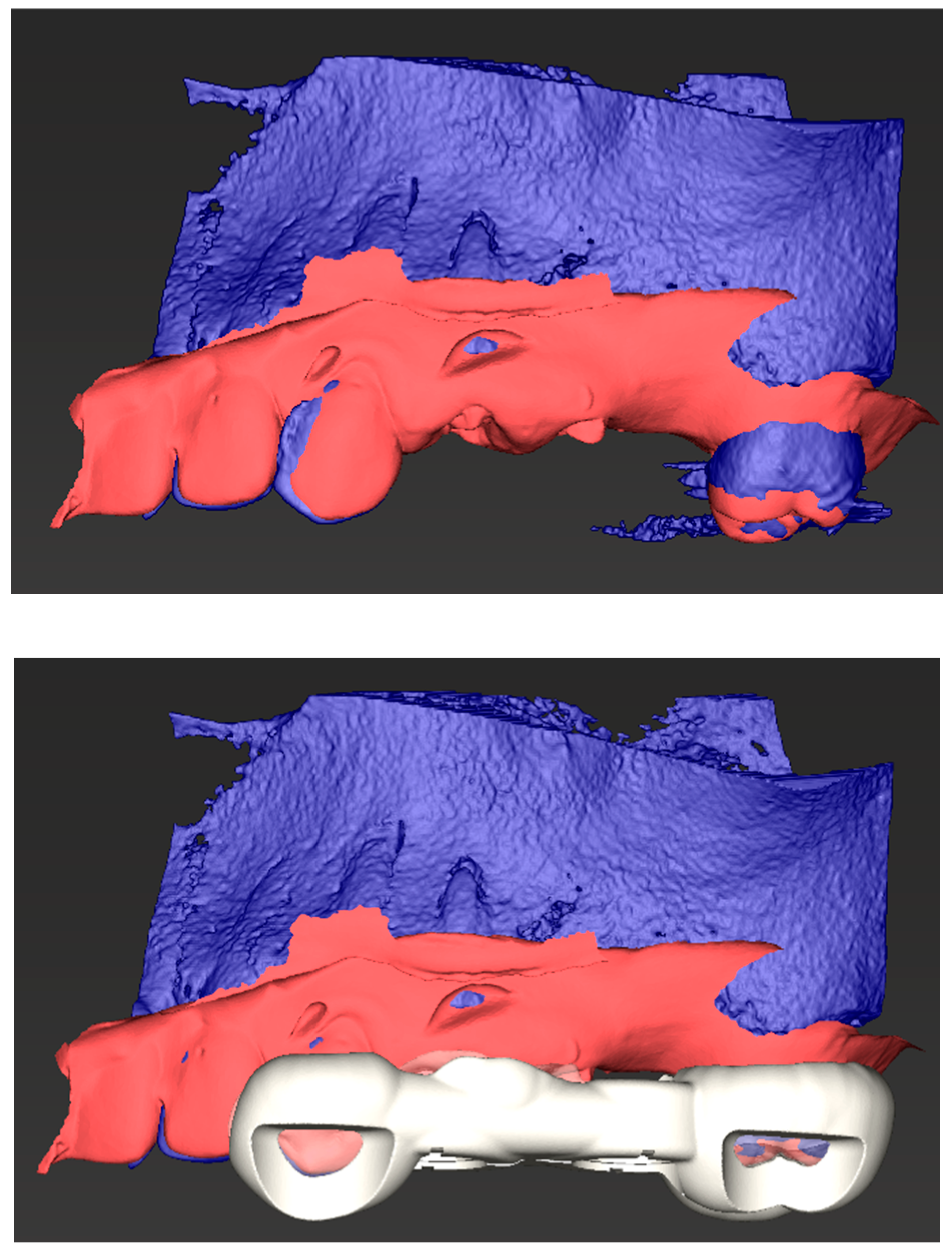
1.2. Technological Advances and Potential Uses of CBCT and Periodontal Therapies
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Eke, P.I.; Genco, R.J. CDC periodontal disease surveillance project: Background, objectives, and progress report. J. Periodontol. 2007, 7, 1366–1371. [Google Scholar] [CrossRef]
- Friedewald, V.E.; Kornman, K.S.; Beck, J.D.; Genco, R.; Goldfine, A.; Libby, P.; Offenbacher, S.; Ridker, P.M.; Van Dyke, T.E.; Roberts, W.C. American Journal of, Cardiology; Journal of Periodontology. Am. J. Cardiol. 2009, 104, 59–68. [Google Scholar] [CrossRef]
- Jeffcoat, M.K. Current concepts in periodontal disease testing. J. Am. Dent. Assoc. 1994, 125, 1071–1078. [Google Scholar] [CrossRef]
- Molander, B. Panoramic radiography in dental diagnostics. Swed. Dent. J. Suppl. 1996, 119, 1–26. [Google Scholar]
- Jeffcoat, M.K. Radiographic methods for the detection of progressive alveolar bone loss. J. Periodontol. 1992, 63, 367–372. [Google Scholar] [CrossRef]
- Reddy, M.S. Radiographic methods in the evaluation of periodontal therapy. J. Periodontol 1992, 63, 1078–1084. [Google Scholar] [CrossRef]
- Eshraghi, T.; McAllister, N.; McAllister, B. Clinical applications of digital 2-D and 3-D radiography for the periodontist. J Evid. Based Dent. Pract. 2012, 3, 36–45. [Google Scholar] [CrossRef]
- Bragger, U. Radiographic parameters: Biological significance and clinical use. Periodontology 2000, 39, 73–90. [Google Scholar] [CrossRef]
- Alamri, H.M.; Sadrameli, M.; Alshalhoob, M.A.; Sadrameli, M.; Alshehri, M.A. Applications of CBCT in dental practice: A review of the literature. Gen. Dent. 2012, 60, 390–400. [Google Scholar]
- Benn, D.K. A review of the reliability of radiographic measurements in estimating alveolar bone changes. J. Clin. Periodontol. 1990, 17, 14–21. [Google Scholar] [CrossRef]
- Misch, K.A.; Yi, E.S.; Sarment, D.P. Accuracy of cone beam computed tomography for periodontal defect measurements. J. Periodontol. 2006, 77, 1261–1266. [Google Scholar] [CrossRef]
- Braun, X.; Ritter, L.; Jervøe-Storm, P.-M.; Frentzen, M. Diagnostic accuracy of CBCT for periodontal lesions. Clin. Oral Investig. 2014, 18, 1229–1236. [Google Scholar] [CrossRef]
- Bagis, N.; Kolsuz, M.E.; Kursun, S.; Orhan, K. Comparison of intraoral radiography and cone-beam computed tomography for the detection of periodontal defects: An in vitro study. BMC Oral Health 2015, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, S.; Duan, J.; Zhang, Y.; Qiu, Y.; Sun, C.; Liu, D. The accuracy of cone-beam computed tomography in assessing maxillary molar furcation involvement. J. Clin. Periodontol. 2014, 41, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Suphanantachat, S.; Tantikul, K.; Tamsailom, S.; Kosalagood, P.; Nisapakultorn, K.; Tavedhikul, K. Comparison of clinical values between cone beam computed tomography and conventional intraoral radiography in periodontal and infrabony defect assessment. Dentomaxillofac. Radiol. 2017, 46, 20160461. [Google Scholar] [CrossRef]
- Bayat, S.; Talaeipour, A.R.; Sarlati, F. Detection of simulated periodontal defects using cone-beam CT and digital intraoral radiography. Dentomaxillofac. Radiol. 2016, 45, 20160030. [Google Scholar] [CrossRef]
- Walter, C.; Kaner, D.; Berndt, D.C.; Weiger, R.; Zitzmann, N.U. Three-dimensional imaging as a pre-operative tool in decision making for furcation surgery. J. Clin. Periodontol. 2009, 36, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Weiger, R.; Zitzmann, N.U. Accuracy of three-dimensional imaging in assessing maxillary molar furcation involvement. J. Clin. Periodontol. 2010, 37, 436–441. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Dommy, A.; Guru, S.R.; Joseph, A. Comparative evaluation of cone-beam computed tomography versus direct surgical measurements in the diagnosis of mandibular molar furcation involvement. Contemp. Clin. Dent. 2017, 8, 439. [Google Scholar]
- Banodkar, A.B.; Gaikwad, R.P.; Gunjikar, T.U.; Lobo, T.A. Evaluation of accuracy of cone beam computed tomography for measurement of periodontal defects: A clinical study. J. Indian Soc. Periodontol. 2015, 19, 285. [Google Scholar] [CrossRef]
- Vieira Feijó, C.; Granjeiro Feitosa de Lucena, J.; Mitsuo Kurita, L.; da Silva Pereira, S.L. Evaluation of cone beam computed tomography in the detection of horizontal periodontal bone defects: An in vivo study. Int. J. Periodontics Restor. Dent. 2012, 32, e162–e168. [Google Scholar]
- Zhao, H.; Wang, H.; Pan, Y.; Pan, C.; Jin, X. The relationship between root concavities in first premolars and chronic periodontitis. J. Periodontal res. 2014, 49, 213–219. [Google Scholar] [CrossRef]
- Nikolic-Jakoba, N.; Spin-Neto, R.; Wenzel, A. Cone-Beam Computed Tomography for Detection of Intrabony and Furcation Defects: A Systematic Review Based on a Hierarchical Model for Diagnostic Efficacy. J. Periodontol. 2016, 87, 630–644. [Google Scholar] [CrossRef] [PubMed]
- Waltrick, K.B.; de Abreu Junior, M.J.N.; Corrêa, M.; Zastrow, M.D.; D’Avila Dutra, V. Accuracy of linear measurements and visibility of the mandibular canal of cone-beam computed tomography images with different voxel sizes: An in vitro study. J. Periodontol. 2013, 84, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.G.; Boke, F.; Ayali, A. Cone-beam computed tomography evaluation of the soft tissue thickness and greater palatine foramen location in the palate. J. Clin. Periodontol. 2015, 42, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.L.; Leong, D.J.; Fu, J.H.; Yeh, C.Y.; Tatarakis, N.; Wang, H.L. The significance of the lingual nerve during periodontal/implant surgery. J. Periodontol. 2010, 81, 372–377. [Google Scholar] [CrossRef]
- Amid, R.; Mirakhori, M.; Safi, Y.; Kadkhodazadeh, M.; Namdari, M. Assessment of gingival biotype and facial hard/soft tissue dimensions in the maxillary anterior teeth region using cone beam computed tomography. Arch. Oral Biol. 2017, 79, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Batista Jr, E.L.; Moreira, C.C.; Batista, F.C.; de Oliveira, R.R.; Pereira, K.K. Altered passive eruption diagnosis and treatment: A cone beam computed tomography-based reappraisal of the condition. J. Clin. Periodontol. 2012, 39, 1089–1096. [Google Scholar] [CrossRef]
- Cao, J.; Hu, W.-J.; Zhang, H.; Liu, D.-G.; Le, D.; Yang, G.; Chung, K.H. A novel technique for measurement of dentogingival tissue by cone beam computed tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2015, 119, e82–e87. [Google Scholar] [CrossRef] [PubMed]
- Frost, N.A.; Mealey, B.L.; Jones, A.A.; Huynh-Ba, G. Periodontal biotype: Gingival thickness as it relates to probe visibility and buccal plate thickness. J. Periodontol. 2015, 86, 1141–1149. [Google Scholar] [CrossRef]
- Fu, J.H.; Yeh, C.Y.; Chan, H.L.; Tatarakis, N.; Leong, D.J.; Wang, H.L. Tissue biotype and its relation to the underlying bone morphology. J. Periodontol. 2010, 81, 569–574. [Google Scholar] [CrossRef]
- Fuentes, R.; Flores, T.; Navarro, P.; Salamanca, C.; Beltrán, V.; Borie, E. Assessment of buccal bone thickness of aesthetic maxillary region: A cone-beam computed tomography study. J. Periodontal Implant Sci. 2015, 45, 162–168. [Google Scholar] [CrossRef]
- Gambarini, G.; Miccoli, G.; Gaimari, G.; Pompei, D.; Pilloni, A.; Piasecki, L.; Al-Sudani, D.; Di Nardo, D.; Testarelli, L. Detection of Bone Defects Using CBCT Exam in an Italian Population. Int. J. Dent. 2017, 2017, 7523848. [Google Scholar] [CrossRef] [PubMed]
- Mandelaris, G.A.; Neiva, R.; Chambrone, L. Cone-beam computed tomography and interdisciplinary dentofacial therapy: An American Academy of Periodontology best evidence review focusing on risk assessment of the dentoalveolar bone changes influenced by tooth movement. J. Periodontol. 2017, 88, 960–977. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Monje, F.; González-García, R.; Galindo-Moreno, P.; Rodriguez-Salvanes, F.; Wang, H.L. Comparison between microcomputed tomography and cone-beam computed tomography radiologic bone to assess atrophic posterior maxilla density and microarchitecture. Clin. Oral Implants Res. 2014, 25, 723–728. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Kubota, T.; Nohno, K.; Nezu, A.; Morozumi, T.; Yoshie, H. Clinical and CBCT Evaluation of Combined Periodontal Regenerative Therapies Using Enamel Matrix Derivative and Deproteinized Bovine Bone Mineral with or Without Collagen Membrane. Int. J. Periodontics Restor. Dent. 2018, 38, 373–381. [Google Scholar] [CrossRef]
- Siddiqui, Z.R.; Jhingran, R.; Bains, V.K.; Srivastava, R.; Madan, R.; Rizvi, I. Comparative evaluation of platelet-rich fibrin versus beta-tri-calcium phosphate in the treatment of Grade II mandibular furcation defects using cone-beam computed tomography. Eur. J. Dent. 2016, 10, 496. [Google Scholar] [PubMed]
- Pajnigara, N.; Kolte, A.; Kolte, R.; Pajnigara, N.; Lathiya, V. Diagnostic accuracy of cone beam computed tomography in identification and postoperative evaluation of furcation defects. J. Indian Soc. Periodontol. 2016, 20, 386. [Google Scholar]
- Benic, G.I.; Thoma, D.S.; Jung, R.E.; Sanz-Martin, I.; Unger, S.; Cantalapiedra, A.; Hämmerle, C.H. Guided bone regeneration with particulate vs. block xenogenic bone substitutes: A pilot cone beam computed tomographic investigation. Clin. Oral Implants Res. 2017, 28, e262–e270. [Google Scholar] [CrossRef] [PubMed]
- Fleiner, J.; Hannig, C.; Schulze, D.; Stricker, A.; Jacobs, R. Digital method for quantification of circumferential periodontal bone level using cone beam CT. Clin. Oral Investig. 2013, 17, 389–396. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Ge, Z.-P.; Ma, R.-H.; Hou, J.-X.; Li, G. A six-site method for the evaluation of periodontal bone loss in cone-beam CT images. Dentomaxillofac. Radiol. 2015, 45, 20150265. [Google Scholar] [CrossRef]
- Reddy, M.S.; Aichelmann-Reidy, M.E.; Avila-Ortiz, G.; Klokkevold, P.R.; Murphy, K.G.; Rosen, P.S.; Schallhorn, R.G.; Sculean, A.; Wang, H.L. Periodontal regeneration—furcation defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S131–S133. [Google Scholar] [CrossRef] [PubMed]
- Mandelaris, G.A.; Scheyer, E.T.; Evans, M.; Kim, D.; McAllister, B.; Nevins, M.L.; Rios, H.F.; Sarment, D. American Academy of Periodontology Best Evidence Consensus Statement on Selected Oral Applications for Cone-Beam Computed Tomography. J. Periodontol. 2017, 88, 939–945. [Google Scholar] [CrossRef]
- Kim, D.M.; Bassir, S.H. When Is Cone-Beam Computed Tomography Imaging Appropriate for Diagnostic Inquiry in the Management of Inflammatory Periodontitis? An American Academy of Periodontology Best Evidence Review. J. Periodontol. 2017, 88, 978–998. [Google Scholar] [CrossRef]
- McAllister, B.S.; Eshraghi, V.T. Commentary: Cone-Beam Computed Tomography: An Essential Technology for Management of Complex Periodontal and Implant Cases. J. Periodontol. 2017, 88, 937–938. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshraghi, V.T.; Malloy, K.A.; Tahmasbi, M. Role of Cone-Beam Computed Tomography in the Management of Periodontal Disease. Dent. J. 2019, 7, 57. https://doi.org/10.3390/dj7020057
Eshraghi VT, Malloy KA, Tahmasbi M. Role of Cone-Beam Computed Tomography in the Management of Periodontal Disease. Dentistry Journal. 2019; 7(2):57. https://doi.org/10.3390/dj7020057
Chicago/Turabian StyleEshraghi, V. Thomas, Kyle A. Malloy, and Mehrnaz Tahmasbi. 2019. "Role of Cone-Beam Computed Tomography in the Management of Periodontal Disease" Dentistry Journal 7, no. 2: 57. https://doi.org/10.3390/dj7020057
APA StyleEshraghi, V. T., Malloy, K. A., & Tahmasbi, M. (2019). Role of Cone-Beam Computed Tomography in the Management of Periodontal Disease. Dentistry Journal, 7(2), 57. https://doi.org/10.3390/dj7020057




