Adjunctive Effects of a Sub-Antimicrobial Dose of Doxycycline on Clinical Parameters and Potential Biomarkers of Periodontal Tissue Catabolism
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Study Design
2.3. Gingival Crevicular Fluid (GCF) Sampling
2.4. MPO, MMP-9, MMP-13, OPG, and TRAP-5 in GCF by Enzyme-Linked Immunosorbent Assay (ELISA) and MMP-8 by Immunofluorometric Assay (IFMA) Analysis
3. Statistical Analysis
4. Results
4.1. Patient Disposition and Demographics
4.2. Clinical Results (Whole Mouth)
4.3. Clinical Results (Sampling Sites)
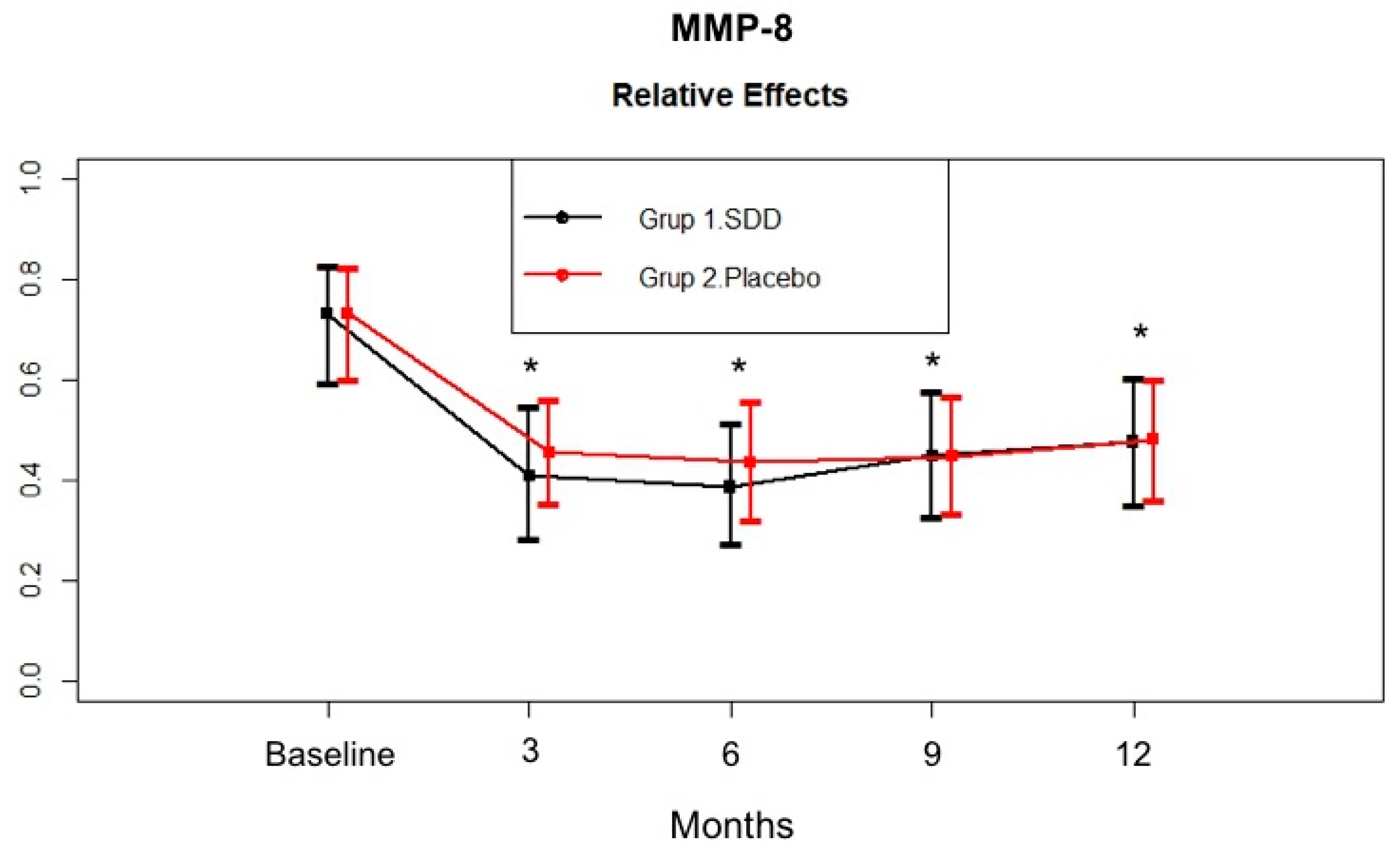
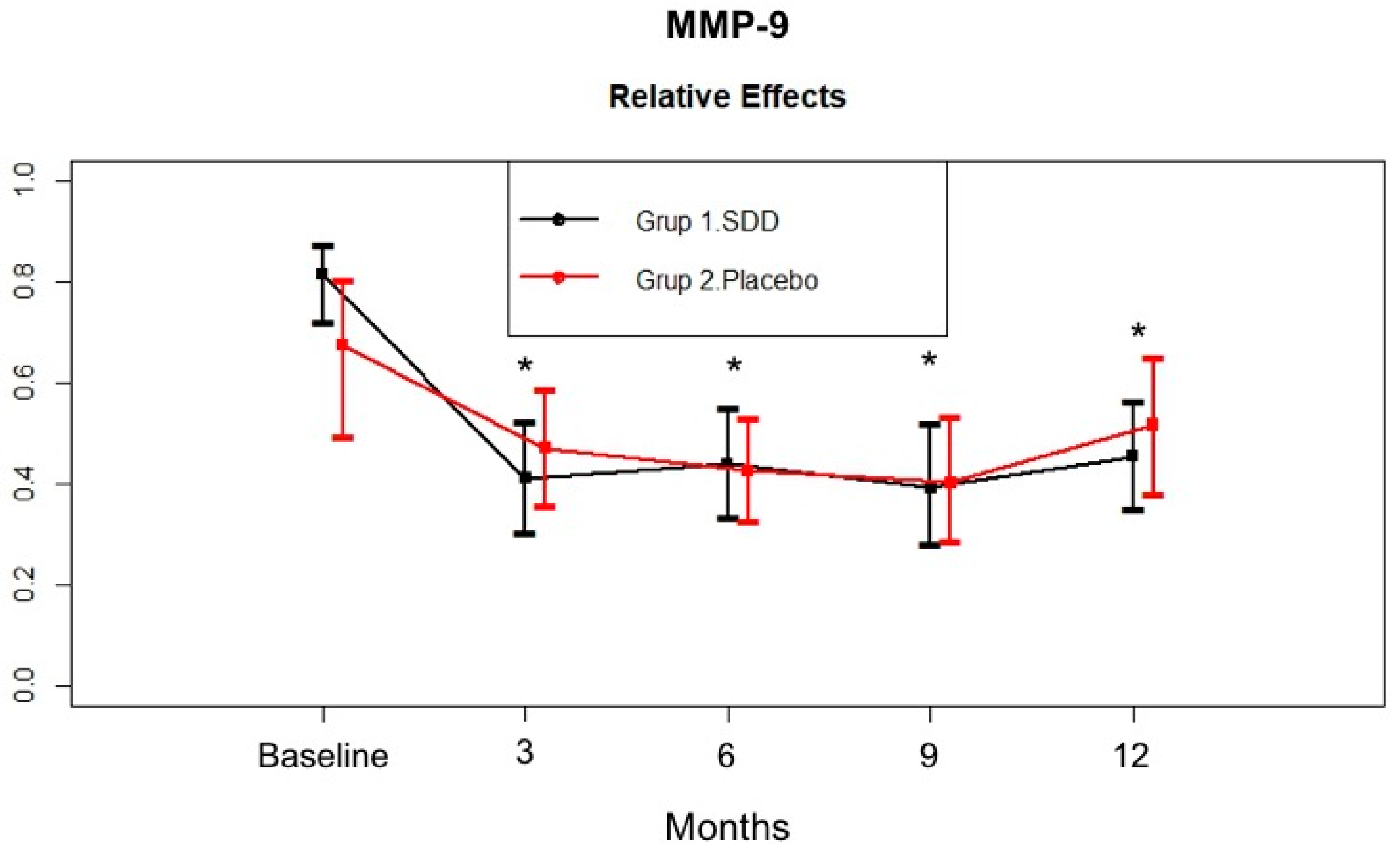
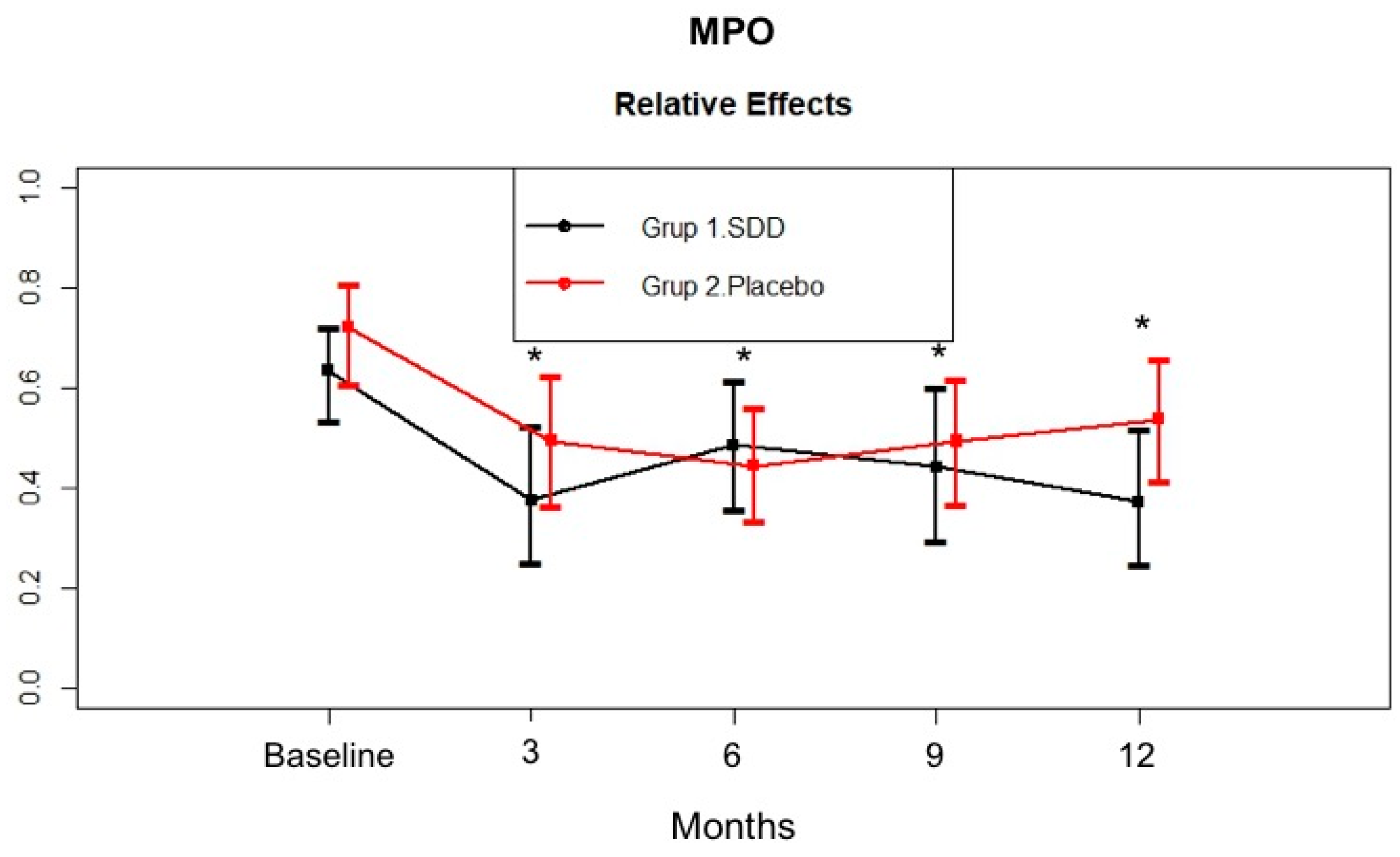
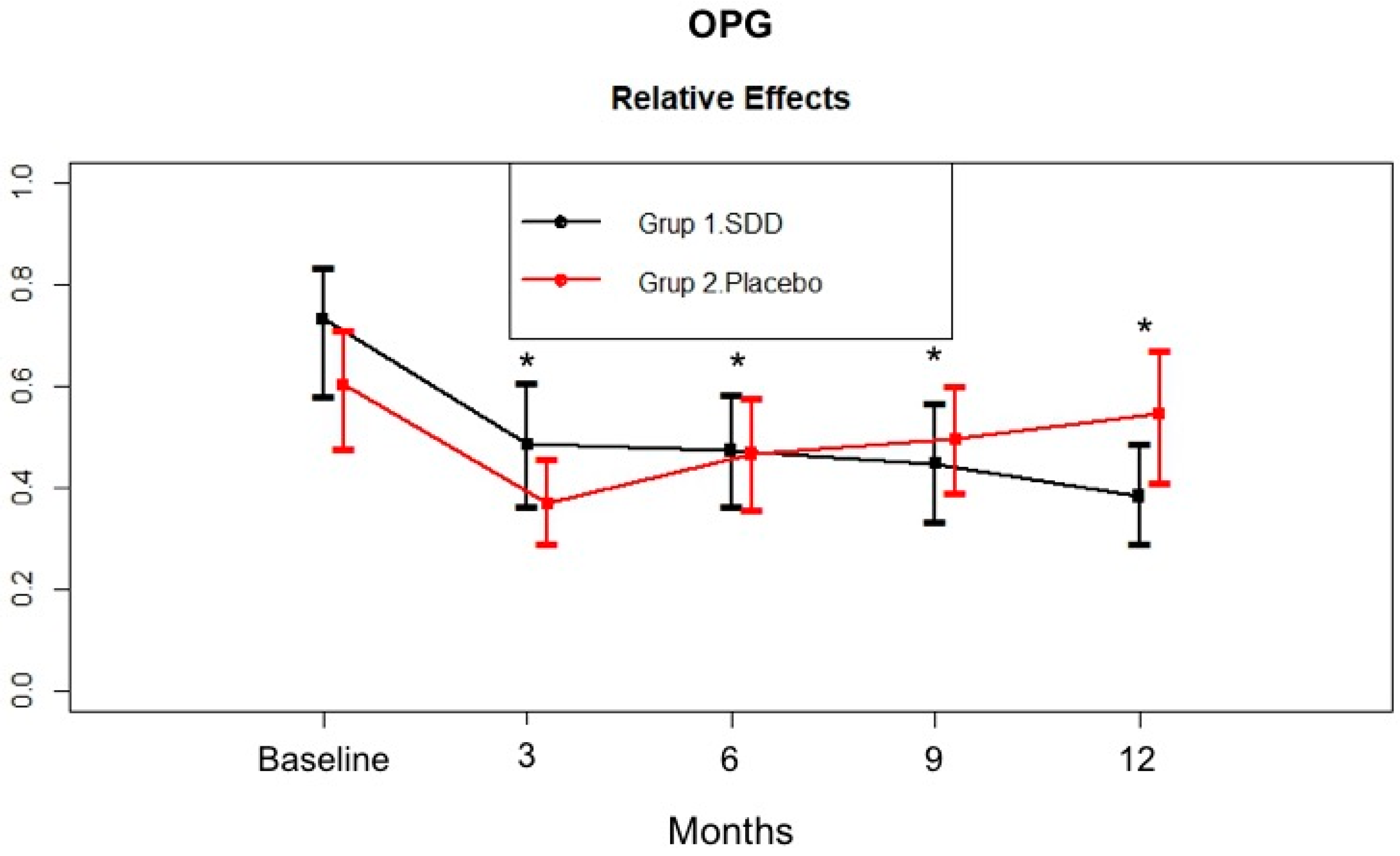
4.4. GCF MMP-8, MMP-9, MPO, and OPG Levels
5. Discussion
Author Contributions
Funding
Conflicts of Interest
Appendix A
| SDD Group | Placebo Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | 12 months | Baseline | 3 months | 6 months | 9 months | 12 months | |
| PD (mm) | 4.03 ± 0.82 | 2.65 ± 0.40 * | 2.53 ± 0.39 *¶ | 2.45 ± 0.52 *¶ | 2.42 ± 0.55 *¶ | 4.15 ± 0.80 | 2.86 ± 0.46 * | 2.85 ± 0.57 * | 2.88 ± 0.61 * | 2.89 ± 0.62 * |
| CAL (mm) | 4.71 ± 1.14 | 4.13 ± 1.03 * | 4.16 ± 0.98 *¶ | 4.09 ± 1.03 *¶ | 4.16 ± 1.00 * | 5.19 ± 1.20 | 4.73 ± 1.01 * | 4.86 ± 1.05 | 4.84 ± 1.06 | 4.84 ± 1.10 |
| GI | 1.82 ± 0.40 | 0.76 ± 0.33 *¶ | 0.83 ± 0.25 *¶ | 0.70 ± 0.29 *¶ | 0.70 ± 0.35 * | 1.89 ± 0.29 | 1.00 ± 0.30 * | 1.02 ± 0.28 * | 0.97 ± 0.33 * | 0.92 ± 0.33 * |
| PI | 3.84 ± 0.89 | 1.96 ± 0.74 * | 1.97 ± 0.80 * | 1.69 ± 0.97 * | 1.41 ± 0.73 * | 4.18 ± 0.75 | 2.07 ± 0.72 * | 1.95 ± 0.72 * | 1.89 ± 0.73 * | 1.87 ± 0.70 * |
| SDD Group | Placebo Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | 12 months | Baseline | 3 months | 6 months | 9 months | 12 months | |
| PD (mm) | 7.05 ± 0.72 | 3.66 ± 1.00 * | 3.16 ± 0.75 * | 2.94 ± 1.10 * | 2.90 ± 1.04 * | 6.68 ± 1.15 | 3.63 ± 1.37 * | 3.58 ± 1.46 * | 3.38 ± 1.37 * | 3.55 ± 1.40 * |
| CAL (mm) | 7.58 ± 1.49 | 5.53 ± 2.31 * | 5.26 ± 1.81 * | 5.21 ± 1.84 * | 4.90 ± 2.16 * | 7.90 ± 1.69 | 6.35 ± 1.93 * | 6.53 ± 1.98 * | 6.13 ± 2.09 * | 6.35 ± 2.09 * |
| GI | 2.11 ± 0.49 | 0.50 ± 0.62 * | 0.61 ± 0.52 * | 0.32 ± 0.45 * | 0.26 ± 0.39 * | 1.78 ± 0.59 | 0.68 ± 0.49 * | 0.65 ± 0.67 * | 0.58 ± 0.65 * | 0.43 ± 0.61 * |
| PI | 3.68 ± 0.87 | 1.92 ± 0.92 * | 1.82 ± 1.26 * | 1.82 ± 1.38 * | 1.32 ± 0.84 * | 4.05 ± 1.18 | 1.85 ± 1.01 * | 1.78 ± 0.94 * | 2.00 ± 1.15 * | 2.18 ± 1.31 * |
| GCF (μl) | 1.12 ± 0.35 | 0.54 ± 0.22 * | 0.38 ± 0.16 * | 0.55 ± 0.26 * | 0.50 ± 0.24 * | 0.98 ± 0.40 | 0.42 ± 0.23 * | 0.48 ± 0.31 * | 0.49 ± 0.26 * | 0.53 ± 0.28 * |
References
- Hernández, M.; Dutzan, N.; García-Sesnich, J.; Abusleme, L.; Dezerega, A.; Silva, N.; González, F.E.; Vernal, R.; Sorsa, T.; Gamonal, J. Host-pathogen interactions in progressive chronic periodontitis. J. Dent. Res. 2011, 90, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Taylor, J.J. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J. Clin. Periodontol. 2011, 38, 60–84. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F. Regulators of tissue destruction and homeostasis as diagnostic aids in periodontology. Periodontol 2000 2000, 24, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Tjäderhane, L.; Konttinen, Y.T.; Lauhio, A.; Salo, T.; Lee, H.M.; Golub, L.M.; Brown, D.L.; Mäntylä, P. Matrix metalloproteinases: Contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Ann. Med. 2006, 38, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Overall, C.M.; McCulloch, C. Proteolytic host cell enzymes in gingival crevice fluid. Periodontol 2000 2003, 31, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Fields, G.B. Interstitial collagen catabolism. J. Biol. Chem. 2013, 288, 8785–8793. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Van den Steen, P.E.; Opdenakker, G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): The next decade. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 222–272. [Google Scholar] [CrossRef] [PubMed]
- Tardif, G.; Reboul, P.; Pelletier, J.P.; Martel-Pelletier, J. Ten years in the life of an enzyme: The story of the human MMP-13 (collagenase-3). Mod. Rheumatol. 2004, 14, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hernández Ríos, M.; Sorsa, T.; Obregón, F.; Tervahartiala, T.; Valenzuela, M.A.; Pozo, P.; Dutzan, N.; Lesaffre, E.; Molas, M.; Gamonal, J. Proteolytic roles of matrix metalloproteinase (MMP)-13 during progression of chronic periodontitis: Initial evidence for MMP-13/MMP-9 activation cascade. J. Clin. Periodontol. 2009, 36, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; Gamonal, J.; Tervahartiala, T.; Mäntylä, P.; Rivera, O.; Dezerega, A.; Dutzan, N.; Sorsa, T. Associations between matrix metalloproteinase-8 and -14 and myeloperoxidase in gingival crevicular fluid from subjects with progressive chronic periodontitis: A longitudinal study. J. Periodontol. 2010, 81, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, U.K.; Könönen, E.; Pradhan-Palikhe, P.; Tervahartiala, T.; Pussinen, P.J.; Suominen-Taipale, L.; Sorsa, T. Salivary MMP-8, TIMP-1, and ICTP as markers of advanced periodontitis. J. Clin. Periodontol. 2010, 37, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Ejeil, A.L.; Igondjo-Tchen, S.; Ghomrasseni, S.; Pellat, B.; Godeau, G.; Gogly, B. Expression of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in healthy and diseased human gingiva. J. Periodontol. 2003, 74, 188–195. [Google Scholar] [CrossRef]
- Sorsa, T.; Hernández, M.; Leppilahti, J.; Munjal, S.; Netuschil, L.; Mäntylä, P. Detection of gingival crevicular fluid MMP-8 levels with different laboratory and chair-side methods. Oral Dis. 2010, 16, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Gursoy, U.K.; Nwhator, S.; Hernández, M.; Tervahartiala, T.; Leppilahti, J.; Gursoy, M.; Könönen, E.; Emingil, G.; Pussinen, P.J.; et al. Analysis of matrix metalloproteinases, especially MMP-8, in gingival crevicular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontol 2000 2016, 70, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Sorsa, T.; Gieselmann, D.; Arweiler, N.B.; Hernández, M. A quantitative point-of-care test for periodontal and dental peri-implant diseases. Nat. Rev. Dis. Primers 2017, 3, 17069. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.F.; Smith, Q.T. Crevicular fluid myeloperoxidase at healthy, gingivitis and periodontitis sites. J. Clin. Periodontol. 1989, 16, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.F.; Ho, K.Y.; Ho, Y.P.; Wu, Y.M.; Yang, Y.H.; Tsai, C.C. The investigation of glutathione peroxidase, lactoferrin, myeloperoxidase and interleukin-1beta in gingival crevicular fluid: Implications for oxidative stress in human periodontal diseases. J. Period. Res. 2004, 39, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Borges, I., Jr.; Moreira, E.A.; Filho, D.W.; de Oliveira, T.B.; da Silva, M.B.; Fröde, T.S. Proinflammatory and oxidative stress markers in patients with periodontal disease. Mediat. Inflamm. 2007, 2007, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.J.; Peppin, G.J. Collagenolytic metalloenzymes of the human neutrophil. Characteristics, regulation and potential function in vivo. Biochem. Pharmacol. 1986, 35, 3189–3197. [Google Scholar] [CrossRef]
- Vissers, M.C.; Winterbourn, C.C. Myeloperoxidase-dependent oxidative inactivation of neutrophil neutral proteinases and microbicidal enzymes. Biochem. J. 1987, 245, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Saari, H.; Suomalainen, K.; Lindy, O.; Konttinen, Y.T.; Sorsa, T. Activation of latent human neutrophil collagenase by reactive oxygen species and serine proteases. Biochem. Biophys. Res. Commun. 1990, 171, 979–987. [Google Scholar] [CrossRef]
- Wang, Y.; Rosen, H.; Madtes, D.K.; Shao, B.; Martin, T.R.; Heinecke, J.W.; Fu, X. Myeloperoxidase inactivates TIMP-1 by oxidizing its N-terminal cysteine residue: An oxidative mechanism for regulating proteolysis during inflammation. J. Biol. Chem. 2007, 282, 31826–31834. [Google Scholar] [CrossRef] [PubMed]
- Leppilahti, J.M.; Hernández-Ríos, P.A.; Gamonal, J.A.; Tervahartiala, T.; Brignardello-Petersen, R.; Mantyla, P.; Sorsa, T.; Hernández, M. Matrix metalloproteinases and myeloperoxidase in gingival crevicular fluid provide site-specific diagnostic value for chronic periodontitis. J. Clin. Periodontol. 2014, 41, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, B.; Chambers, T.J.; Fuller, K. Secretion of tartrate-resistant acid phosphatase by osteoclasts correlates with resorptive behavior. J. Cell. Biochem. 2006, 98, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Halleen, J.M. Tartrate-resistant acid phosphatase 5B is a specific and sensitive marker of bone resorption. Anticancer Res. 2003, 23, 1027–1029. [Google Scholar] [PubMed]
- Hienz, S.A.; Paliwal, S.; Ivanovski, S. Mechanisms of Bone Resorption in Periodontitis. J. Immunol. Res. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Jin, Q.; Cirelli, J.A.; Park, C.H.; Sugai, J.V.; Taba, M., Jr.; Kostenuik, P.J.; Giannobile, W.V. RANKL inhibition through osteoprotegerin blocks bone loss in experimental periodontitis. J. Periodontol. 2007, 78, 1300–1308. [Google Scholar] [CrossRef]
- Page, R.C. Periodontal therapy: Prospects for the future. J. Periodontol. 1993, 64, 744–753. [Google Scholar] [CrossRef]
- Loesche, W.J. The antimicrobial treatment of periodontal disease: Changing the treatment paradigm. Crit. Rev. Oral Biol. Med. 1999, 10, 245–275. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Williams, R.C.; Siciliano, V.I.; Alibrandi, A.; Cordasco, G.; Ramaglia, L. The effects of a desiccant agent in the treatment of chronic periodontitis: A randomized, controlled clinical trial. Clin. Oral Investig. 2018, 22, 791–800. [Google Scholar] [CrossRef]
- Tabenski, L.; Moder, D.; Cieplik, F.; Schenke, F.; Hiller, K.A.; Buchalla, W.; Schmalz, G.; Christgau, M. Antimicrobial photodynamic therapy vs. local minocycline in addition to non-surgical therapy of deep periodontal pockets: A controlled randomized clinical trial. Clin. Oral Investig. 2017, 21, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Matarese, G.; Ramaglia, L.; Cicciù, M.; Cordasco, G.; Isola, G. The Effects of Diode Laser Therapy as an Adjunct to Scaling and Root Planing in the Treatment of Aggressive Periodontitis: A 1-Year Randomized Controlled Clinical Trial. Photomed. Laser Surg. 2017, 35, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Lang, N.P. Host modulation in the management of periodontal diseases. J. Clin. Periodontol. 2005, 32, 108–129. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Lee, H.M.; Ryan, M.E.; Giannobile, W.V.; Payne, J.; Sorsa, T. Tetracyclines inhibit connective tissue breakdown by multiple non-antimicrobial mechanisms. Adv. Dent. Res. 1998, 12, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Ciancio, S.G.; Blieden, T.M.; Bradshaw, M.; Crout, R.J.; Hefti, A.F.; Massaro, J.M.; Polson, A.M.; Thomas, J.; Walker, C. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J. Periodontol. 2000, 71, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Emingil, G.; Attila, G.; Sorsa, T.; Luoto, H.; Kırılmaz, L.; Baylas, H. The effect of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid matrix metalloproteinase-8 levels in chronic periodontitis. J. Periodontol. 2004, 75, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Hefti, A.F.; Jepsen, S.; Etienne, D.; Walker, C.; Bradshaw, M.H. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. J. Clin. Periodontol. 2004, 31, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Metheny, R.J.; Karakiozis, J.M.; Wetzel, J.M.; Crout, R.J. Long-term sub-antimicrobial doxycycline (Periostat®) as adjunctive management in adult periodontitis: Effects on subgingival bacterial population dynamics. Adv. Dent. Res. 1998, 12, 32–39. [Google Scholar] [CrossRef]
- Walker, C.; Thomas, J.; Nango, S.; Lennon, J.; Wetzel, J.; Powala, C. Long-term treatment with subantimicrobial dose doxycycline exerts no antibacterial effect on the subgingival microflora associated with adult periodontitis. J. Periodontol. 2000, 71, 1465–1471. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172. [Google Scholar] [CrossRef]
- Löe, H.; Silness, J. Periodontal disease in pregnancy. Prevalence and severity. Acta Odontol. Scand. 1963, 21, 532–551. [Google Scholar] [CrossRef]
- Turesky, S.; Gilmore, N.D.; Glickman, I. Reduced plaque formation by the chloromethyl analogue of Vitamin C. J. Periodontol. 1970, 41, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Badersten, A.; Nilveus, R.; Egelberg, J. Effect of non-surgical periodontal therapy (IV). Operator variability. J. Clin. Periodontol. 1985, 12, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Lamster, I.B.; Hartley, L.J.; Oshrain, R.L. Evaluation and modification of spectrophotometric procedures for analysis of lactate dehydrogenase, beta-glucuronidase and arylsulphatase in human gingival crevicular fluid collected with filter-paper strips. Arch. Oral Biol. 1985, 30, 235–242. [Google Scholar] [CrossRef]
- The American Academy of Periodontology. Parameter on periodontal maintenance. J. Periodontol. 2000, 71, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Non-surgical pocket therapy: Mechanical. Ann. Periodontol. 1996, 1, 443–490. [Google Scholar] [CrossRef]
- Greenstein, G. Periodontal response to mechanical non-surgical therapy: A Review. J. Periodontol. 1992, 63, 118–130. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Effect of therapy on periodontal infections. J. Periodontol. 1993, 64, 754–759. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, Y.M.; Shin, S.Y.; Seol, Y.J.; Ku, Y.; Rhyu, I.C.; Chung, C.P.; Han, S.B. Effect of subantimicrobial dose doxycycline as an effective adjunct to scaling and root planing. J. Periodontol. 2004, 75, 1500–1508. [Google Scholar] [CrossRef]
- Golub, L.M.; McNamara, T.F.; Ryan, M.E.; Kohut, B.; Blieden, T.; Payonk, G.; Sipos, T.; Baron, H.J. Adjunctive treatment with subantimicrobial doses of doxcycline: Effects on gingival fluid collagenase activity and attachment loss in adult periodontitis. J. Clin. Periodontol. 2001, 28, 146–156. [Google Scholar] [CrossRef]
- Golub, L.M.; Lee, H.M.; Greenwald, R.A.; Ryan, M.E.; Sorsa, T.; Salo, T.; Giannobile, W.V. A matrix metalloproteinase inhibitor reduces bone-type collagen degradation fragments and specific collagenases in gingival crevicular fluid during adult periodontitis. Inflamm. Res. 1997, 46, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Golub, L.M.; Cianci, S.; Ramamurthy, N.S.; Leung, M.; McNamara, T.F. Low-dose doxycycline therapy: Effect on gingival and crevicular fluid collagenase activity in humans. J. Periodont. Res. 1990, 25, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Demirel, K.; Baer, P.N.; McNamara, T.F. Topical application of doxycycline on periodontally involved root surfaces in vitro: Comparative analysis of substantivity on cementum and dentin. J. Periodontol. 1991, 62, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Aras, H.; Cağlayan, F.; Güncü, G.N.; Berberoğlu, A.; Kilinç, K. Effect of systemically administered naproxen sodium on clinical parameters and myeloperoxidase and elastase-like activity levels in gingival crevicular fluid. J. Periodontol. 2007, 78, 868–873. [Google Scholar] [CrossRef]
- Dagar, M.; Deepa, D.K.; Molly, M.; Sharma, A.; Khattak, B.P. Effect of nonsurgical periodontal therapy on salivary myeloperoxidase levels: A biochemical study. J. Indian Soc. Periodontol. 2015, 19, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Marcaccini, A.M.; Meschiari, C.A.; Zuardi, L.R.; de Sousa, T.S.; Taba, M., Jr.; Teofilo, J.M.; Jacob-Ferreira, A.L.; Tanus-Santos, J.E.; Novaes, A.B., Jr.; Gerlach, R.F. Gingival crevicular fluid levels of MMP-8, MMP-9, TIMP-2, and MPO decrease after periodontal therapy. J. Clin. Periodontol. 2010, 37, 180–190. [Google Scholar] [CrossRef]
- Nizam, N.; Gümüş, P.; Pitkänen, J.; Tervahartiala, T.; Sorsa, T.; Buduneli, N. Serum and salivary matrix metalloproteinases, neutrophil elastase, myeloperoxidase in patients with chronic or aggressive periodontitis. Inflammation 2014, 37, 1771–1778. [Google Scholar] [CrossRef]
- Buchmann, R.; Hasilik, A.; Nunn, M.E.; Van Dyke, T.E.; Lange, D.E. PMN responses in chronic periodontal disease: Evaluation by gingival crevicular fluid enzymes and elastase-alpha-1-proteinase inhibitor complex. J. Clin. Periodontol. 2002, 29, 563–572. [Google Scholar] [CrossRef]
- Suomalainen, K.; Sorsa, T.; Golub, L.M.; Ramamurthy, N.; Lee, H.M.; Uitto, V.J.; Saari, H.; Konttinen, Y.T. Specificity of the anticollagenase action of tetracyclines: Relevance to their anti-inflammatory potential. Antimicrob. Agents Chemother. 1992, 36, 227–229. [Google Scholar] [CrossRef]
- Emingil, G.; Atilla, G.; Sorsa, T.; Tervahartiala, T. The effect of adjunctive subantimicrobial dose doxycycline therapy on GCF EMMPRIN levels in chronic periodontitis. J. Periodontol. 2008, 79, 469–476. [Google Scholar] [CrossRef]
- Emingil, G.; Gürkan, A.; Atilla, G.; Berdeli, A.; Cinarcik, S. Adjunctive low-dose doxycycline therapy effect on clinical parameters and gingival crevicular fluid tissue plasminogen activator levels in chronic periodontitis. Inflamm. Res. 2006, 55, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Emingil, G.; Atilla, G.; Sorsa, T.; Savolainen, P.; Baylas, H. Effectiveness of adjunctive low-dose doxycycline therapy on clinical parameters and gingival crevicular fluid laminin-5 gamma2 chain levels in chronic periodontitis. J. Periodontol. 2004, 75, 1387–1396. [Google Scholar] [CrossRef]
- Emingil, G.; Gürkan, A.; Atilla, G.; Kantarci, A. Subantimicrobial-dose doxycycline and cytokine-chemokine levels in gingival crevicular fluid. J. Periodontol. 2011, 82, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Gürkan, A.; Çınarcık, S.; Hüseyinov, A. Adjunctive subantimicrobial dose doxycycline: Effect on clinical parameters and gingival crevicular fluid transforming growth factor- beta levels in severe, generalized chronic periodontitis. J. Clin. Periodontol. 2005, 32, 244–253. [Google Scholar] [CrossRef]
- Choi, D.H.; Moon, I.S.; Choi, B.K.; Paik, J.W.; Kim, Y.S.; Choi, S.H.; Kim, C.K. Effects of sub-antimicrobial dose doxycycline therapy on crevicular fluid MMP-8, and gingival tissue MMP-9, TIMP-1 and IL-6 levels in chronic periodontitis. J. Period. Res. 2004, 39, 20–26. [Google Scholar] [CrossRef]
- Shibutani, T.; Murahashi, Y.; Tsukada, E.; Iwayama, Y.; Heersche, J.N. Experimentally induced periodontitis in beagle dogs causes rapid increases in osteoclastic resorption of alveolar bone. J. Periodontol. 1997, 68, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Crotti, T.; Smith, M.D.; Hirsch, R.; Soukoulis, S.; Weedon, H.; Capone, M.; Ahern, M.J.; Haynes, D. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J. Period. Res. 2003, 38, 380–387. [Google Scholar] [CrossRef]
- Da Costa, T.A.; Silva, M.J.; Alves, P.M.; Chica, J.E.; Barcelos, E.Z.; Giani, M.A.; Garlet, G.P.; da Silva, J.S.; Rodrigues Júnior, V.; Rodrigues, D.B.; et al. Inflammation Biomarkers of Advanced Disease in Nongingival Tissues of Chronic Periodontitis Patients. Mediat. Inflamm. 2015, 2015, 1–10. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Bostanci, N.; Ilgenli, T.; Emingil, G.; Afacan, B.; Han, B.; Töz, H.; Atilla, G.; Hughes, F.J.; Belibasakis, G.N. Gingival crevicular fluid levels of RANKL and OPG in periodontal diseases: Implications of their relative ratio. J. Clin. Periodontol. 2007, 34, 370–376. [Google Scholar] [CrossRef]
- Mogi, M.; Otogoto, J.; Ota, N.; Togari, A. Differential expression of RANKL and osteoprotegerin in gingival crevicular fluid of patients with periodontitis. J. Dent. Res. 2004, 83, 166–169. [Google Scholar] [CrossRef] [PubMed]
| SDD Group | Placebo Group | |
|---|---|---|
| Male/Female | 11:4 | 10:5 |
| Mean age, years | 48.9 ± 6.6 | 49.2 ± 7.3 |
| Age range | 38–59 | 40–61 |
| Smokers (n) | 7 | 6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emingil, G.; Gürkan, A.; Tervahartiala, T.; Hernandez, M.; Özgül, S.; Sorsa, T.; Alassiri, S. Adjunctive Effects of a Sub-Antimicrobial Dose of Doxycycline on Clinical Parameters and Potential Biomarkers of Periodontal Tissue Catabolism. Dent. J. 2019, 7, 9. https://doi.org/10.3390/dj7010009
Emingil G, Gürkan A, Tervahartiala T, Hernandez M, Özgül S, Sorsa T, Alassiri S. Adjunctive Effects of a Sub-Antimicrobial Dose of Doxycycline on Clinical Parameters and Potential Biomarkers of Periodontal Tissue Catabolism. Dentistry Journal. 2019; 7(1):9. https://doi.org/10.3390/dj7010009
Chicago/Turabian StyleEmingil, Gülnur, Ali Gürkan, Taina Tervahartiala, Marcela Hernandez, Semiha Özgül, Timo Sorsa, and Saeed Alassiri. 2019. "Adjunctive Effects of a Sub-Antimicrobial Dose of Doxycycline on Clinical Parameters and Potential Biomarkers of Periodontal Tissue Catabolism" Dentistry Journal 7, no. 1: 9. https://doi.org/10.3390/dj7010009
APA StyleEmingil, G., Gürkan, A., Tervahartiala, T., Hernandez, M., Özgül, S., Sorsa, T., & Alassiri, S. (2019). Adjunctive Effects of a Sub-Antimicrobial Dose of Doxycycline on Clinical Parameters and Potential Biomarkers of Periodontal Tissue Catabolism. Dentistry Journal, 7(1), 9. https://doi.org/10.3390/dj7010009






