Evaluating the Mechanical Properties, and Calcium and Fluoride Release of Glass-Ionomer Cement Modified with Chicken Eggshell Powder
Abstract
1. Introduction
2. Materials and Methods
2.1. Eggshell Powder Preparation
- Group A: GIC without CESP (n = 12).
- Group B: GIC with 3% wt. CESP added to the powder component (n = 12).
- Group C: GIC with 5% wt. CESP added to the powder component (n = 12).
2.2. Compressive Strength
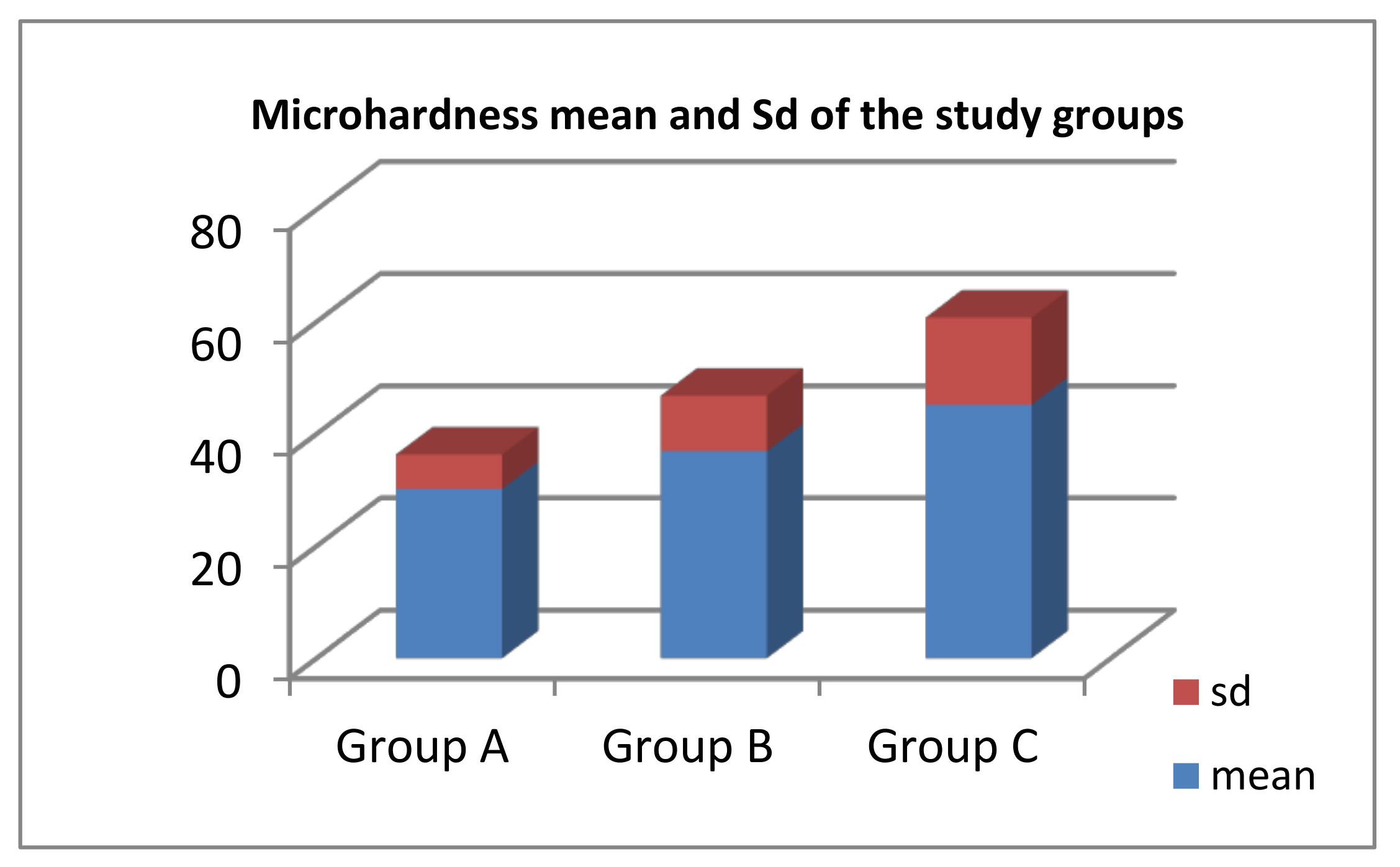
2.3. Microhardness Test (Vickers)
2.4. Fluoride and Calcium Ions Release
3. Results
3.1. Fluoride Release
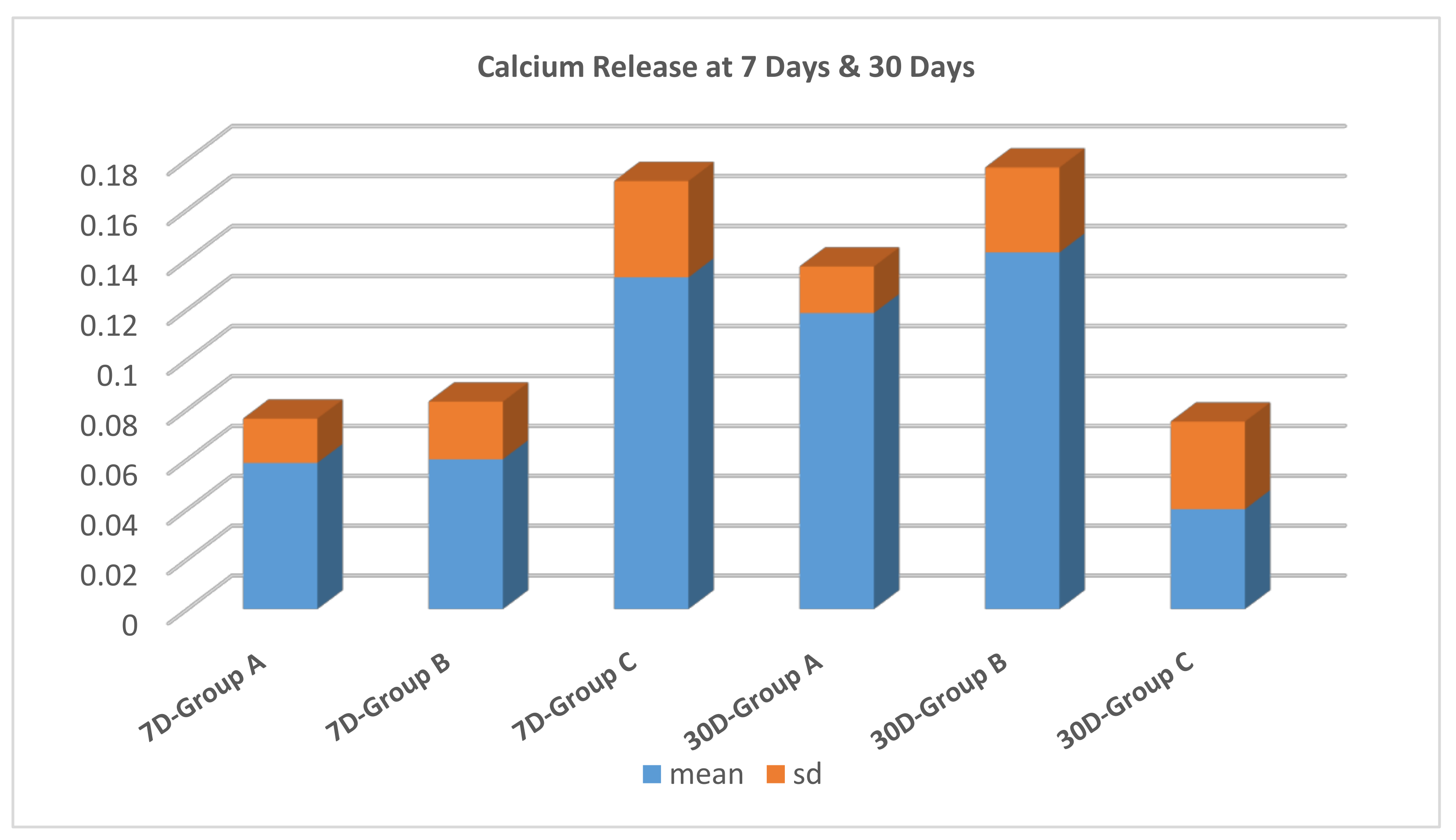
3.2. Calcium Release
4. Discussion
5. Conclusions
- The mechanical properties of conventional GIC were enhanced by the addition of CESP.
- GIC unique property of fluoride release is not compromised by adding CESP to its powder component.
- Calcium release was potentiated at 5% CESP concentration, which can enhance the remineralizing ability of GIC.
Author Contributions
Funding
Conflicts of Interest
References
- Wilson, A.D.; Kent, B.E. A new translucent cement for dentistry. Br. Dent. J. 1972, 132, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Hatton, P.V.; Hurrell-Gillingham, K.; Brook, I.M. Biocompatibility of glass-ionomer cements. J. Dent. 2006, 34, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Smales, R.J. Fluoride release/uptake of conventional and resin-modified glass ionomers, and compomers. J. Dent. 2001, 29, 30–36. [Google Scholar] [CrossRef]
- Attar, N.; Turgut, M.D. Fluoride release and uptake capacities of fluoride-releasing restorative materials. Oper. Dent. 2003, 28, 395–402. [Google Scholar] [PubMed]
- Vermeersch, G.; Leloup, G.; Vreven, J. Fluoride release from glass-ionomer cements, compomers, and resin composites. J. Oral Rehabil. 2001, 28, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Dong, D.R.; Huang, H.M.; Shih, Y.H. Fluoride ion diffusion from a glass-ionomer cement. J. Oral Rehabil. 2000, 27, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Lohbauer, U. Dental glassionomer cements as permanent filling materials?—Properties, limitations and future trends. Materials 2009, 3, 76–96. [Google Scholar] [CrossRef]
- McCabe, J.F.; Yan, Z.; Al Naimi, O.T.; Mahmoud, G.; Rolland, S.L. Smart materials in dentistry. Aust. Dent. J. 2011, 56, 3–10. [Google Scholar] [CrossRef] [PubMed]
- AbRahman, I.; Sam’an, M.M.; Luddin, N.; Shiekh, R.A. One-pot synthesis of hydroxyapatite–silica nanopowder composite for hardness enhancement of glass ionomer cement (GIC). Bull. Mater. Sci. 2014, 37, 213–219. [Google Scholar]
- Moshaverinia, A.; Ansari, S.; Moshaverinia, M.; Roohpour, N.; Darr, J.A.; Rehman, I. Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional Glass Ionomer Cements (GIC). Acta Biomater. 2008, 4, 432–440. [Google Scholar] [CrossRef] [PubMed]
- King’Ori, A.M. A review of the uses of poultry eggshell and shell membrane. Int. J. Poult. Sci. 2011, 10, 908–912. [Google Scholar]
- Kattimani, V.S.; Chakravarthi, P.S.; Kanumuru, N.R.; Subbarao, V.V.; Sidharthan, A.; Kumar, T.S.; Prasad, L.K. Eggshell derived hydroxyapatite as bone graft substitute in the healing of maxillary cystic bone defects: A preliminary report. J. Int. Oral Health 2014, 6, 15. [Google Scholar] [PubMed]
- Neunzehn, J.; Szuwart, T.; Wiesmann, H.-P. Eggshells as natural calcium carbonate source in combination with hyaluronan as beneficial additives for bone graft materials, an in vitro study. Head Face Med. 2015, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Rovensky, J.; Stancikova, M.; Masaryk, P.; Svik, K.; Istok, R. Egg shell calcium in the prevention and treatment of osteoporosis. Int. J. Clin. Pharmacol. Res. 2003, 23, 83–92. [Google Scholar] [PubMed]
- Haghgoo, R.; Mehran, M.; Ahmadvand, M.; Ahmadvand, M.J. Remineralization Effect of Eggshell versus Nano-hydroxyapatite on Caries-like Lesions in Permanent Teeth (In Vitro). J. Int. Oral Health 2016, 8, 435–439. [Google Scholar]
- Toro, P.; Quijada, R.; Yazdani-Pedram, M.; Arias, J.L. Eggshell, a new bio-filler for polypropylene composites. Mater. Lett. 2007, 61, 4347–4350. [Google Scholar] [CrossRef]
- Arias, J.L.; Fink, D.J.; Xiao, S.Q.; Heuer, A.H.; Caplan, A.I. Biomineralization and eggshells: Cell-mediated acellular compartments of mineralized extracellular matrix. Int. Rev. Cytol. 1993, 145, 217–250. [Google Scholar] [PubMed]
- Arias, J.L.; Fernandez, M.S. Biomimetic processes through the study of mineralized shells. Mater. Charact. 2003, 50, 189–195. [Google Scholar] [CrossRef]
- Fred, S.; Wang, P.Y.; Weatherspoon, J.; Mead, L. Method of Producing Eggshell Powder. US Patent 20060062857 A1, 23 March 2006. [Google Scholar]
- Shen, P.; Manton, D.J.; Cochrane, N.J.; Walker, G.D.; Yuan, Y.; Reynolds, C.; Reynolds, E.C. Effect of added calcium phosphate on enamel remineralization by fluoride in a randomized controlled in situ trial. J. Dent. 2011, 39, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.A.; Abo-Mosallam, H.A.; Lee, H.Y.; Kim, G.R.; Kim, H.W.; Lee, H.H. Biological and mechanical properties of experimental glass-ionomer cement modified by partial replacement of CaO with MgO or ZnO. J. Appl. Oral Sci. 2015, 23, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Moshaverinia, M.; Borzabadi-Farahani, A.; Sameni, A.; Moshaverinia, A.; Ansari, S. Effects of incorporation of nano-fluorapatite particles on microhardness, fluoride releasing properties, and biocompatibility of a conventional glass ionomer cement (GIC). Dent. Mater. J. 2016, 35, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Priyadarshini, B.M.; Neo, J.; Fawzy, A.S. Characterization of Chitosan/TiO2 Nano-Powder Modif|ed Glass-Ionomer Cement for Restorative Dental Applications. J. Esth. Restor. Dent. 2017, 29, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Baroudi, K.; Mahmoud, R.S.; Tarakji, B. Fluoride release of glass ionomer restorations after bleaching with two different bleaching materials. Eur. J. Dent. 2013, 7, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Shetty, V.; Hegde, A.M. Propylthiouracil (PROP)—A tool to determine taster status in relation to caries experience, streptococcus mutans levels and dietary preferences in children. J. Clin. Pediatr. Dent. 2006, 31, 113–117. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatric Dentistry. Pediatric restorative dentistry. Pediatr. Dent. 2017, 39, 312–324. [Google Scholar]
- John, M.J.; Thomas, S. Biofibres and Biocomposites. Carbohydr. Polym. 2008, 71, 343–364. [Google Scholar] [CrossRef]
- Raji, S.A.; Samuel, A.T. Egg shell as a fine aggregate in concrete for sustainable construction. Int. J. Sci. Technol. Res. 2015, 4, 8–13. [Google Scholar]
- Shiozawai, M.; Takahashi, H.; IwasakiI, N.; Motohiro, U.O. Effect of calcium chloride solution immersion on surface hardness of restorative glass ionomer cements. Dent. Mater. J. 2013, 32, 828–833. [Google Scholar] [CrossRef]
- Meredith, N.; Sherriff, M.; Setchell, D.J. Measurement of the microhardness and young,s modulus of human enamel and dentin using an indentation technique. Arch. Oral Boil. 1996, 41, 539–545. [Google Scholar] [CrossRef]
- Craig, R.G.; Powers, J.M. Restorative Dental Materials, 11th ed.; Mosby, The CV Mosby Co.: St. Louis, MO, USA, 2002; Chapter 4; pp. 102–106. [Google Scholar]
- Lubis, M.; Ginting, M.H.S.; Dalimunthe, N.F.; Hasibuan, D.M.T.; Sastrodihardjo, S. The Influence of Chicken Egg Shell as Fillers on Biocomposite Acrylic Resin for Denture Based. Mater. Sci. Eng. 2017, 180, 012008. [Google Scholar] [CrossRef]
- Rahmi; Marlina; Nisfayati. Effect of Eggshell on Mechanical Properties of Epichlorohydrin Cross-linked Chitosan/Eggshell Composites. Orient. J. Chem. 2017, 33, 478–482. [Google Scholar] [CrossRef]
- Van Duinen, R.N.; Davidson, C.L.; De Gee, A.J.; Feilzer, A.J. In situ transformation of glass-ionomer into an enamel-like material. Am. J. Dent. 2004, 17, 223–227. [Google Scholar] [PubMed]
- Preston, A.J.; Mair, L.H.; Agalamanyi, E.A.; Highham, S.M. Fluoride release from aesthetic dental materials. J. Oral Rehabili. 1999, 26, 123–129. [Google Scholar] [CrossRef]
- Karantakis, P.; Antonaides, M.H.; Pahini, S.T.; Papadogiannis, Y. Fluoride release from three glass ionomers, a compomer and a composite resin in water, artificial saliva and lactic acid. Oper. Dent. 2000, 25, 20–25. [Google Scholar] [PubMed]
- Kumar, R.S.; Ravikumar, N.; Kavitha, S.; Mahalaxmi, S.; Jayasree, R.; Kumar, T.S.; Haneesh, M. Nanochitosan modified glass ionomer cement with enhanced mechanical properties and fluoride release. Int. J. Biol. Macromol. 2017, 104, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Yli-Urpo, H.; Vallittu, P.K.; Närhi, T.O.; Forsback, A.P.; Väkiparta, M. Release of Silica, Calcium, Phosphorus, and Fluoride from Glass Ionomer Cement Containing Bioactive Glass. J. Biomater. Appl. 2004, 19, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, R.; Mondal, N.K.; Das, B.; Roy, P.; Pal, K.C.; Das, C.; Banerjee, A.; Datta, J.K. Eggshell Powder as an Adsorbent for Removal of Fluoride from Aqueous Solution: Equilibrium, Kinetic and Thermodynamic Studies. J. Chem. 2012, 9, 1457–1480. [Google Scholar] [CrossRef]
- Nasr, A.B.; Walha, K.; Puel, F.; Mangin, D.; Amar, R.B.; Charcosset, C. Precipitation and adsorption during fluoride removal from water by calcite in the presence of acetic acid. Desalin. Water Treat. 2013, 52, 2231–2240. [Google Scholar] [CrossRef]
| Test | A | B | C | p-Value |
|---|---|---|---|---|
| Compressive strength (MPa) | 52.45 a ± 1.8 | 75.46 b ± 13.9 | 71.43 b ± 16.7 | 0.001 |
| Test Interval | Group A | Group B | Group C | p-Value |
|---|---|---|---|---|
| 7 days | 2.03 aA ± 0.9 | 21.69 cA ± 0.6 | 4.51 bA ± 0.9 | 0.0001 |
| 30 days | 1.33 aA ± 0.6 | 12.2 bB ± 4.4 | 3.36 aA ± 1.4 | 0.0001 |
| p-value | 0.104 | 0.001 | 0.096 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allam, G.; Abd El-Geleel, O. Evaluating the Mechanical Properties, and Calcium and Fluoride Release of Glass-Ionomer Cement Modified with Chicken Eggshell Powder. Dent. J. 2018, 6, 40. https://doi.org/10.3390/dj6030040
Allam G, Abd El-Geleel O. Evaluating the Mechanical Properties, and Calcium and Fluoride Release of Glass-Ionomer Cement Modified with Chicken Eggshell Powder. Dentistry Journal. 2018; 6(3):40. https://doi.org/10.3390/dj6030040
Chicago/Turabian StyleAllam, Gehan, and Ola Abd El-Geleel. 2018. "Evaluating the Mechanical Properties, and Calcium and Fluoride Release of Glass-Ionomer Cement Modified with Chicken Eggshell Powder" Dentistry Journal 6, no. 3: 40. https://doi.org/10.3390/dj6030040
APA StyleAllam, G., & Abd El-Geleel, O. (2018). Evaluating the Mechanical Properties, and Calcium and Fluoride Release of Glass-Ionomer Cement Modified with Chicken Eggshell Powder. Dentistry Journal, 6(3), 40. https://doi.org/10.3390/dj6030040




