Paediatric Over-the-Counter (OTC) Oral Liquids Can Soften and Erode Enamel
Abstract
:1. Introduction
2. Results
2.1. pH Values
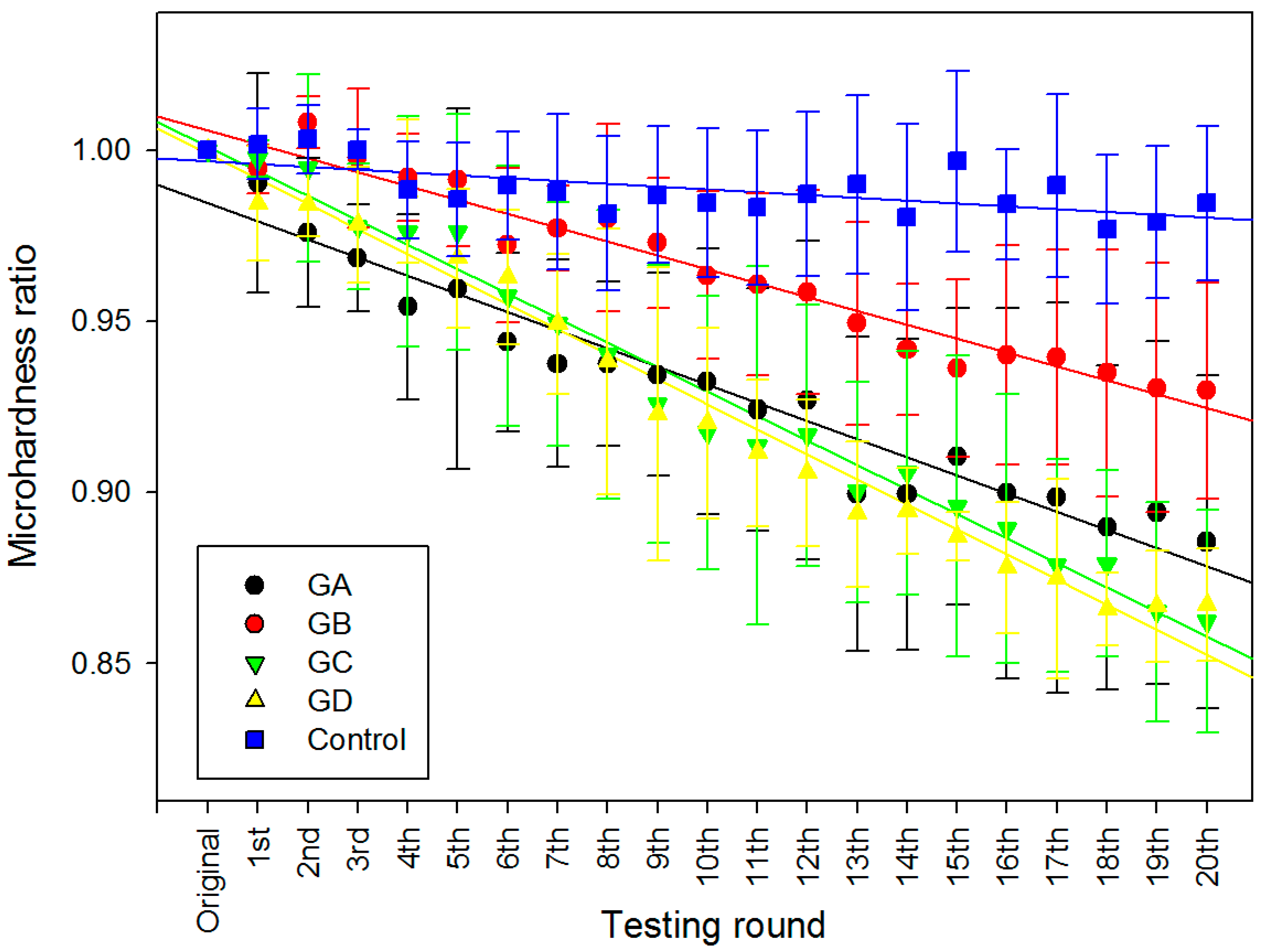
2.2. Surface Micro-Hardness
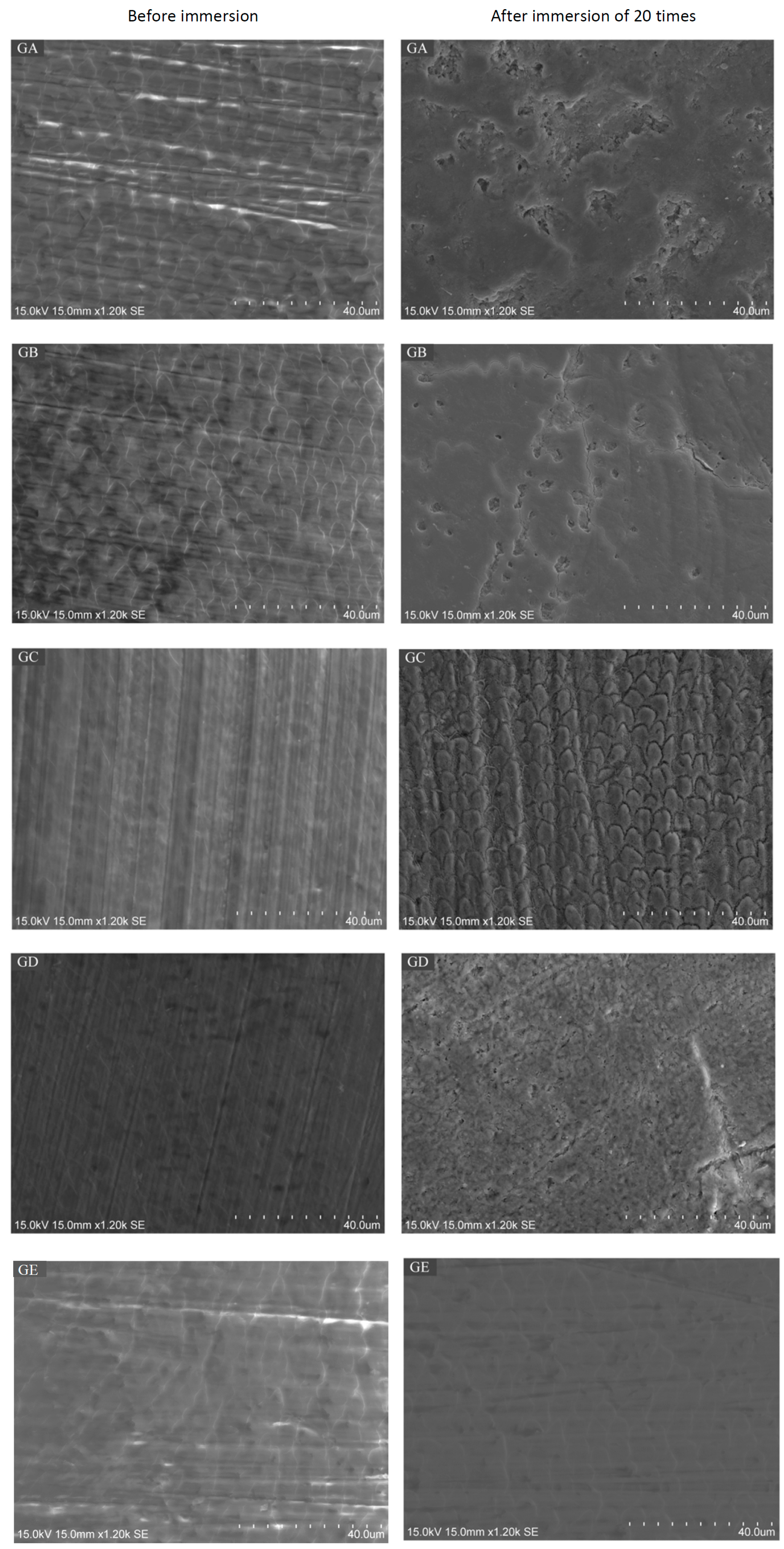
2.3. Scanning Electron Microscope (SEM) / Energy Dispersive X-Ray Spectrometry (EDS) Analysis
3. Discussion
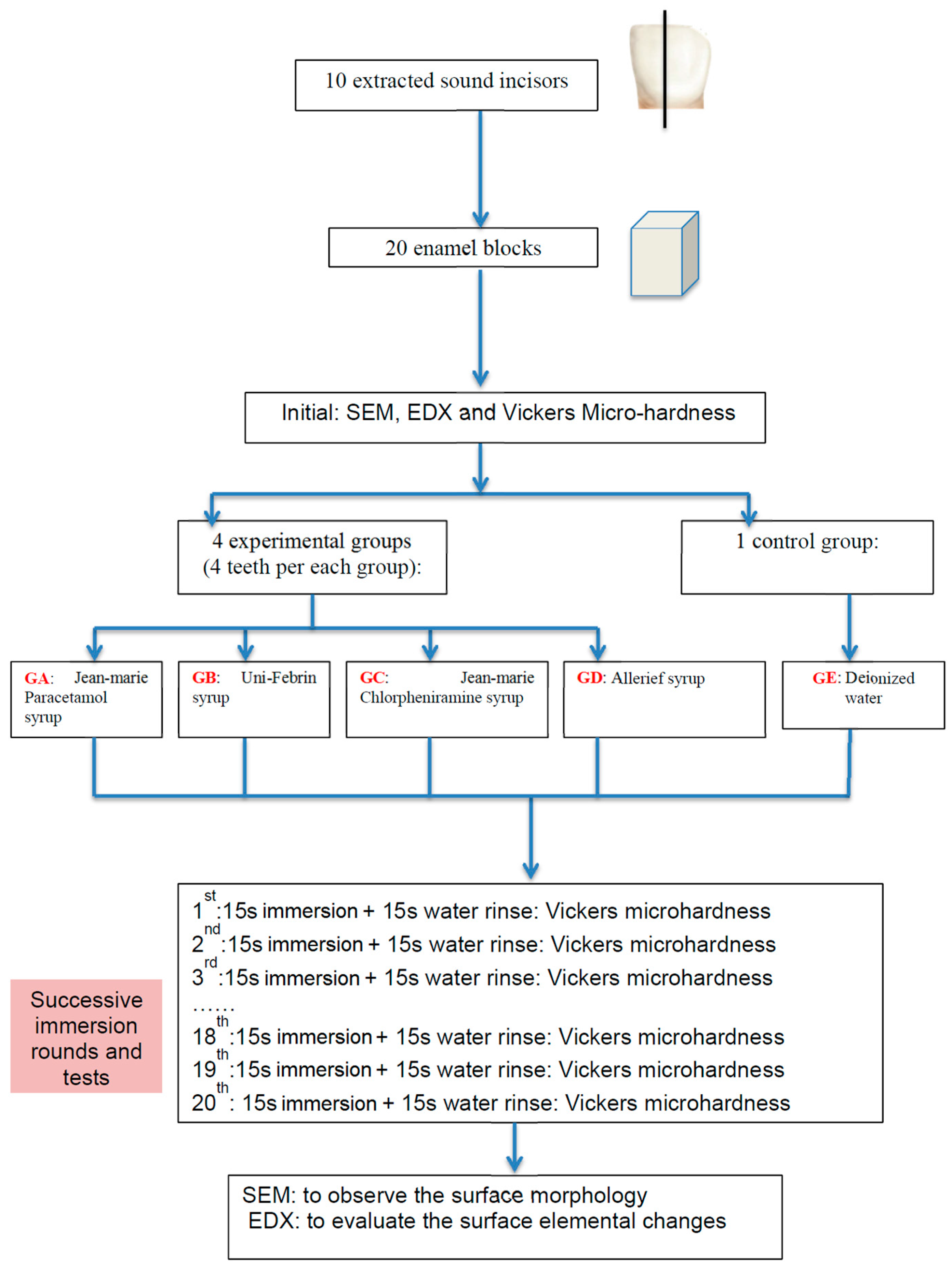
4. Materials and Methods
4.1. Teeth and Specimen Preparation
4.2. Oral Liquids
4.3. pH Measurement
4.4. Micro-Hardness Measurement
4.5. Scanning Electron Microscope Analysis
4.6. Energy Dispersive X-Ray Spectrometry Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bahal, P.; Djemal, S. Dental erosion from an excess of vitamin C. Case Rep. Dent. 2014, 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.; Dugmore, C. Pathological or physiological erosion—Is there a relationship to age? Clin. Oral Investig. 2008, 12, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Scheutzel, P. Etiology of dental erosion–intrinsic factors. Eur. J. Oral Sci. 1996, 104, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental erosion—An overview with emphasis on chemical and histopathological aspects. Caries Res. 2011, 45, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Dawes, C. What is the critical ph and why does a tooth dissolve in acid? Can. Dent. Assoc. 2003, 69, 722–724. [Google Scholar]
- Zhang, S.; Chau, A.M.H.; Lo, E.C.M.; Chu, C.-H. Dental caries and erosion status of 12-year-old Hong Kong children. BMC Public Health 2014, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Tahmassebi, J.F.; Duggal, M.S.; Malik-Kotru, G.; Curzon, M.E.J. Soft drinks and dental health: A review of the current literature. J. Dent. 2006, 34, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Mantonanaki, M.; Koletsi-Kounari, H.; Mamai-Homata, E.; Papaioannou, W. Dental erosion prevalence and associated risk indicators among preschool children in Athens, Greece. Clin. Oral Investig. 2013, 17, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Sturkenboom, M.; Verhamme, K.; Nicolosi, A.; Murray, M.; Neubert, A.; Caudri, D.; Picelli, G.; Sen, E.; Giaquinto, C.; Cantarutti, L.; et al. Drug use in children: Cohort study in three European countries. Br. Med. J. 2008, 337, 1338–1341. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Schaffner, M.; Jaeggi, T. Dental erosion-diagnosis and prevention in children and adults. Int. Dent. J. 2007, 57, 385–398. [Google Scholar] [CrossRef]
- Neves, B.G.; Farah, A.; Lucas, E.; de Sousa, V.P.; Maia, L.C. Are paediatric medicines risk factors for dental caries and dental erosion? Commun. Dent. Health 2010, 27, 46–51. [Google Scholar]
- Mennella, J.A.; Spector, A.C.; Reed, D.R.; Coldwell, S.E. The bad taste of medicines: Overview of basic research on bitter taste. Clin. Ther. 2013, 35, 1225–1246. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.; Baqir, W.; Nunn, J.H. Are sugars-free medicines more erosive than sugars-containing medicines? An in vitro study of paediatric medicines with prolonged oral clearance used regularly and long-term by children. Int. J. Paediatr. Dent. 2007, 17, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Liem, D.G.; Mennella, J.A. Heightened sour preferences during childhood. Chem. Senses 2003, 28, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Marquezan, M.; Pozzobon, R.; Oliveira, M. Medicines used by pediatric dentistry patients and its cariogenic potential. RPG Rev. Pos. Grad. 2007, 13, 334–339. [Google Scholar]
- Durward, C.; Thou, T. Dental caries and sugar-containing liquid medicines for children in new zealand. N. Z. Dent. J. 1997, 93, 124–129. [Google Scholar] [PubMed]
- Feigal, R.J.; Jensen, M.E.; Mensing, C.A. Dental caries potential of liquid medications. Pediatrics 1981, 68, 416–419. [Google Scholar] [PubMed]
- Babu, K.L.; Doddamani, G.M.; Naik, L.R.; Jagadeesh, K.N. Pediatric liquid medicaments—Are they cariogenic? An in vitro study. J. Int. Soc. Prev. Commun. Dent. 2014, 4, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Mukherjee, U.; Arora, V. Erosive potential of sugar free and sugar containing pediatric medicines given regularly and long term to children. Indian J. Pediatr. 2012, 79, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.L.G.; Rai, K.; Hedge, A.M. Pediatric liquid medicaments—Do they erode the teeth surface? An in vitro study: Part I. J. Clin. Pediatr. Dent. 2008, 32, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sahgal, J.; Sood, P.B.; Raju, O.S. A comparison of oral hygiene status and dental caries in children on long term liquid oral medications to those not administered with such medications. J. Indian Soc. Pedod. Prev. Dent. 2002, 20, 144–151. [Google Scholar] [PubMed]
- Cavalcanti, A.; de Oliveira, K.; Xavier, A.; Pinto, D.; Vieira, F. Evaluation of total soluble solids content (TSSC) and endogenous ph in antimicrobials of pediatric use. Indian J. Dent. Res. 2013, 24, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Strickley, R.G.; Iwata, Q.; Wu, S.; Dahl, T.C. Pediatric drugs-a review of commercially available oral formulations. J. Pharm. Sci. 2008, 97, 1731–1774. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, M.; Goodchild, J.H.; Epstein, J.B. Sugar content, cariogenicity, and dental concerns with commonly used medications. J. Am. Dent. Assoc. 2015, 146, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Featherstone, J.; Lussi, A. Understanding the chemistry of dental erosion. Dent. Eros. 2006, 20, 66–76. [Google Scholar]
- Shellis, R.P. A scanning electron-microscopic study of solubility variations in human enamel and dentine. Arch. Oral Biol. 1996, 41, 473–484. [Google Scholar] [CrossRef]
- Axelsson, P.D.D.S. Diagnosis and Risk Prediction of Dental Caries; Quintessence Publishing: Chicago, IL, USA, 2000. [Google Scholar]
- Pinto, S.C.S.; Batitucci, R.G.; Pinheiro, M.C.; Zandim, D.L.; Spin-Neto, R.; Sampaio, J.E.C. Effect of an acid diet allied to sonic toothbrushing on root dentin permeability: An in vitro study. Braz. Dent. J. 2010, 21, 390. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Pozzobon, R.T. Processed dairy beverages ph evaluation: Consequences of temperature variation. J. Clin. Pediatr. Dent. 2009, 33, 319. [Google Scholar] [CrossRef] [PubMed]
- Banan, L.K.; Hegde, A.M. Plaque and salivary ph changes after consumption of fresh fruit juices. J. Clin. Pediatr. Dent. 2005, 30, 9. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M.; Attin, T. Enzymes in the acquired enamel pellicle. Eur. J. Oral Sci. 2005, 113, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Lendenmann, U.; Grogan, J.; Oppenheim, F.G. Saliva and dental pellicle—A review. Adv. Dent. Res. 2000, 14, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Hannig, M.; Balz, M. Influence of in vivo formed salivary pellicle on enamel erosion. Caries Res. 1999, 33, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Edgar, W.M.; Dawes, C.; Mullane, D.M. Saliva and Oral Health; British Dental Association: London, UK, 2004. [Google Scholar]
- Meurman, J.H.; Ten Cate, J.M. Pathogenesis and modifying factors of dental erosion. Eur. J. Oral Sci. 1996, 104, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Voegel, J.C.; Belcourt, A.; Gillmeth, S. Dissolution of hydroxyapatites treated with salivary glycoproteins and fluoride. Caries Res. 1981, 243–249. [Google Scholar] [CrossRef]
- Attin, T.; Wegehaupt, F.J. Methods for Assessment of Dental Erosion; Karger: Basel, Switzerland, 2014. [Google Scholar]
- Gittings, S.; Turnbull, N.; Henry, B.; Roberts, C.J.; Gershkovich, P. Characterisation of human saliva as a platform for oral dissolution medium development. Eur. J. Pharm. Biopharm. 2015, 91, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K.T. Theory and measurement of the buffer value of bicarbonate in saliva. J. Theor. Biol. 1981, 90, 397–403. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Higham, S.M. Eroded enamel lesion remineralization by saliva as a possible factor in the site-specificity of human dental erosion. Arch. Oral Biol. 2001, 46, 697–703. [Google Scholar] [CrossRef]
- Gentile, E.; Di Stasio, D.; Santoro, R.; Contaldo, M.; Salerno, C.; Serpico, R.; Lucchese, A. In vivo microstructural analysis of enamel in permanent and deciduous teeth. Ultrastruct. Pathol. 2015, 39, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Low, I.M.; Duraman, N.; Davies, I.J. Microstructure-property relationships in human adult and baby canine teeth. Key Eng. Mater. 2006, 309, 23–26. [Google Scholar] [CrossRef]
- De Menezes Oliveira, M.A.H.; Torres, C.P.; Gomes-silva, J.M.; Chinelatti, M.A.; De Menezes, F.C.H.; Palma-dibb, R.G.; Borsatto, M.C. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc. Res. Tech. 2010, 73, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.J.; Tang, R.; Bonstein, T.; Bush, P.; Nancollas, G.H. Enamel demineralization in primary and permanent teeth. J. Dent. Res. 2006, 85, 359. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.K.; Sorvari, R.; Birkhed, D.; Meurman, J.H. Dental erosion in deciduous teeth—An in vivo and in vitro study. J. Dent. 2001, 29, 333–340. [Google Scholar] [CrossRef]
- Hunter, M.L.; West, N.X.; Hughes, J.A.; Newcombe, R.G.; Addy, M. Erosion of deciduous and permanent dental hard tissue in the oral environment. J. Dent. 2000, 28, 257–263. [Google Scholar] [CrossRef]
- Grenby, T.H.; Phillips, A.; Desai, T.; Mistry, M. Laboratory studies of the dental properties of soft drinks. Br. J. Nutr. 1989, 62, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A.; Jaeggi, T. Erosion—Diagnosis and risk factors. Clin. Oral Investig. 2008, 12, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.A.; West, N.X.; Parker, D.M.; van den Braak, M.H.; Addy, M. Effects of ph and concentration of citric, malic and lactic acids on enamel, in vitro. J. Dent. 2000, 28, 147–152. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raychaudhuri, U.; Chakraborty, R. Artificial sweeteners–a review. J. Food Sci. Technol. 2014, 51, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Pandit, N.K. Introduction to the Pharmaceutical Sciences An Integrated Approach; Soltis, R.P., Ed.; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2012. [Google Scholar]
- Hermsen, R.J.; Vrijhoef, M.M. Loss of enamel due to etching with phosphoric or maleic acid. Dent. Mater. 1993, 9, 332–336. [Google Scholar] [CrossRef]
- Fu, B.P.; Yuan, J.; Qian, W.X.; Shen, Q.Y.; Sun, X.M.; Hannig, M. Evidence of chemisorption of maleic acid to enamel and hydroxyapatite. Eur. J. Oral Sci. 2004, 112, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.E.; Bishara, S.E.; Damon, P.; Jakobsen, J.R. Evaluation of scotchbond multipurpose and maleic acid as alternative methods of bonding orthodontic brackets. Am. J. Orthod. Dentofac. Orthop. 1997, 111, 498–501. [Google Scholar] [CrossRef]
- Mahoney, E.K.; Rohanizadeh, R.; Ismail, F.S.M.; Kilpatrick, N.M.; Swain, M.V. Mechanical properties and microstructure of hypomineralised enamel of permanent teeth. Biomaterials 2004, 25, 5091–5100. [Google Scholar] [CrossRef] [PubMed]
- Fagrell, T.G.; Dietz, W.; Jälevik, B.; Norén, J.G. Chemical, mechanical and morphological properties of hypomineralized enamel of permanent first molars. Acta Odontol. Scand. 2010, 68, 215. [Google Scholar] [CrossRef] [PubMed]
- Kodaka, T.; Debari, K.; Kuroiwa, M. Mineral content of the innermost enamel in erupted human teeth. J. Electron Microsc. 1991, 40, 19–23. [Google Scholar]
- Sasaki, T.; Debrari, K.; Garant, P.R. Ameloblast modulation and changes in the Ca, P, and S content of developing enamel matrix revealed by SEM-EDX. J. Dent. Res. 1987, 66, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Jälevik, B.; Odelius, H.; Dietz, W.; Norén, J. Secondary ion mass spectrometry and X-ray microanalysis of hypomineralized enamel in human permanent first molars. Arch. Oral Biol. 2001, 46, 239–247. [Google Scholar] [CrossRef]
- Larsen, M.J. Chemical events during tooth dissolution. J. Dent. Res. 1990, 69, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Edgar, W.M. Sugar substitutes, chewing gumand dental caries-a review. Br. Dent. J. 1998, 184, 29–32. [Google Scholar] [CrossRef] [PubMed]
| Test Group | Main Component and Concentration | Brand | Manufacturer of Drugs | pH (± SD); (n = 3) |
|---|---|---|---|---|
| GA | Paracetamol (120 mg per 5 mL) | Jean-Marie Paracetamol syrup | Jean-Marie Pharmcal, Hong Kong | 4.97 ± 0.01 |
| GB | Uni-Febrin syrup | Universal Pharm, Hong Kong | 4.74 ± 0.01 | |
| GC | Chlorpheniramine (2 mg per 5 mL) | Jean-Marie Chlorpheniramine syrup | Jean-Marie Pharmcal, Hong Kong | 2.50 ± 0.01 |
| GD | Allerief syrup | Percuro Medica Ltd, UK | 5.77 ± 0.01 | |
| GE | Control group | Deionised water | 7.17 ± 0.06 |
| Dependent Variable: MHR | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Type III Sum of Squares | df | Mean Square | F | Sig. | Partial Eta Squared | Noncent. Parameter | Observed Power b |
| Corrected Model | 0.158 a | 5 | 0.032 | 119.792 | 0.000 | 0.858 | 598.961 | 1.000 |
| Intercept | 27.991 | 1 | 27.991 | 106,059.503 | 0.000 | 0.999 | 106,059.503 | 1.000 |
| Round | 0.093 | 1 | 0.093 | 354.279 | 0.000 | 0.782 | 354.279 | 1.000 |
| Drug Type | 0.064 | 4 | 0.016 | 60.926 | 0.000 | 0.711 | 243.706 | 1.000 |
| Error | 0.026 | 99 | 0.000 | |||||
| Total | 94.592 | 105 | ||||||
| Corrected Total | 0.184 | 104 | ||||||
| Dependent Variable: MHR | ||||||
|---|---|---|---|---|---|---|
| (I) Drug Type | (J) Drug Type | Mean Difference (I–J) | Std. Error | Sig. b | 95% Confidence Interval for Difference b | |
| Lower Bound | Upper Bound | |||||
| GA | GB | −0.033 * | 0.005 | 0.000 | −0.043 | −0.023 |
| GC | 0.004 | 0.005 | 0.422 | −0.006 | 0.014 | |
| GD | 0.007 | 0.005 | 0.180 | −0.003 | 0.017 | |
| GE | −0.056 * | 0.005 | 0.000 | −0.066 | −0.046 | |
| GB | GA | 0.033 * | 0.005 | 0.000 | 0.023 | 0.043 |
| GC | 0.037 * | 0.005 | 0.000 | 0.027 | 0.047 | |
| GD | 0.039 * | 0.005 | 0.000 | 0.030 | 0.049 | |
| GE | −0.023 * | 0.005 | 0.000 | −0.033 | −0.013 | |
| GC | GA | −0.004 | 0.005 | 0.422 | −0.014 | 0.006 |
| GB | −0.037 * | 0.005 | 0.000 | −0.047 | −0.027 | |
| GD | 0.003 | 0.005 | 0.604 | −0.007 | 0.013 | |
| GE | −0.060 * | 0.005 | 0.000 | −0.070 | −0.050 | |
| GD | GA | −0.007 | 0.005 | 0.180 | −0.017 | 0.003 |
| GB | −0.039 * | 0.005 | 0.000 | −0.049 | −0.030 | |
| GC | −0.003 | 0.005 | 0.604 | −0.013 | 0.007 | |
| GE | −0.063 * | 0.005 | 0.000 | −0.073 | −0.053 | |
| GE (Control) | GA | 0.056 * | 0.005 | 0.000 | 0.046 | 0.066 |
| GB | 0.023 * | 0.005 | 0.000 | 0.013 | 0.033 | |
| GC | 0.060 * | 0.005 | 0.000 | 0.050 | 0.070 | |
| GD | 0.063 * | 0.005 | 0.000 | 0.053 | 0.073 | |
| Based on estimated marginal means | ||||||
| Ca/P | Ca/C | Mg (wt %) | Na (wt %) | |||||
|---|---|---|---|---|---|---|---|---|
| Before Immersion | After 20 Rounds | Before Immersion | After 20 Rounds | Before Immersion | After 20 Rounds | Before Immersion | After 20 Rounds | |
| GA | 1.95 | 1.96 | 10.45 | 8.26 | 1.32 | 1.56 | 2.10 | 2.10 |
| GB | 1.99 | 1.92 | 10.65 | 8.71 | 1.26 | 1.48 | 2.02 | 2.00 |
| GC | 1.97 | 1.98 | 10.21 | 9.13 | 1.50 | 1.41 | 2.09 | 1.83 |
| GD | 1.94 | 1.94 | 9.79 | 8.63 | 1.46 | 1.55 | 2.10 | 1.95 |
| GE (control) | 1.80 | 1.76 | 7.85 | 6.76 | 1.62 | 1.52 | 2.46 | 2.25 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Tsoi, J.K.-H.; Wong, H.M.; Chu, C.H.; Matinlinna, J.P. Paediatric Over-the-Counter (OTC) Oral Liquids Can Soften and Erode Enamel. Dent. J. 2017, 5, 17. https://doi.org/10.3390/dj5020017
Zhao D, Tsoi JK-H, Wong HM, Chu CH, Matinlinna JP. Paediatric Over-the-Counter (OTC) Oral Liquids Can Soften and Erode Enamel. Dentistry Journal. 2017; 5(2):17. https://doi.org/10.3390/dj5020017
Chicago/Turabian StyleZhao, Dan, James Kit-Hon Tsoi, Hai Ming Wong, Chun Hung Chu, and Jukka P. Matinlinna. 2017. "Paediatric Over-the-Counter (OTC) Oral Liquids Can Soften and Erode Enamel" Dentistry Journal 5, no. 2: 17. https://doi.org/10.3390/dj5020017
APA StyleZhao, D., Tsoi, J. K.-H., Wong, H. M., Chu, C. H., & Matinlinna, J. P. (2017). Paediatric Over-the-Counter (OTC) Oral Liquids Can Soften and Erode Enamel. Dentistry Journal, 5(2), 17. https://doi.org/10.3390/dj5020017









