The Effect of Preventive Agents (Mouthwashes/Gels) on the Color Stability of Dental Resin-Based Composite Materials
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Specimen Preparation
4.3. Immersion of the Specimens
5. Conclusions
Conflicts of Interest
References
- Shikawa-Nagai, S.; Yoshida, A.; Da Silva, J.; Miller, L. Spectrophotometric Analysis of Tooth Color Reproduction on Anterior All-Ceramic Crowns: Part 1: Analysis and Interpretation of Tooth Color. J. Esthet. Restor. Dent. 2010, 22, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zafar, M.; Qasim, S.; Shahab, S.; Naseem, M.; AbuReqaiba, A. Advances in Nanotechnology for Restorative Dentistry. Materials 2015, 8, 717–731. [Google Scholar] [CrossRef]
- Yazici, A.; Celik, C.; Dayangaç, B.; Özgünaltay, G. The effect of curing units and staining solutions on the color stability of resin composites. Oper. Dent. 2007, 32, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Lim, H.; Lee, Y. Influence of nano-and micro-filler proportions on the optical property stability of experimental dental resin composites. Mater. Des. 2010, 31, 4719–4724. [Google Scholar] [CrossRef]
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials; Elsevier/Mosby: Philadelphia, PA, USA, 2012. [Google Scholar]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Khan, A.S.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, L.M.; Masouras, K.; Watts, D.C.; Pimenta, L.A.; Silikas, N. Effect of nanofillers’ size on surface properties after toothbrush abrasion. Am. J. Dent. 2009, 22, 60–64. [Google Scholar] [PubMed]
- Zamarripa, E.; Ancona, A.L.; D’Accorso, N.B.; Macchi, R.L.; Abate, P.F. Effect of energy density on color stability in dental resin composites under accelerated aging. Acta Odontol. Latinoam. 2008, 21, 11–15. [Google Scholar] [PubMed]
- Al-Samadani, K.H. Color stability of restorative materials in response to Arabic coffee, Turkish coffee and Nescafe. J. Contemp. Dent. Pract. 2013, 14, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Yuzugullu, B.; Erkut, S.; Yamanel, K. Effects of mouth rinses on color stability of resin composites. Eur. J. Dent. 2008, 2, 247–253. [Google Scholar] [PubMed]
- Mjör, I.A.; Moorhead, J.E.; Dahl, J.E. Reasons for replacement of restorations in permanent teeth in general dental practice. Int. Dent. J. 2000, 50, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, E.; Hansen, E.K. Surface discoloration of restorative resins in relation to surface softening and oral hygiene. Eur. J. Oral Sci. 1986, 94, 174–177. [Google Scholar] [CrossRef]
- Qasim, S.; Ramakrishnaiah, R.; Al-Kheraif, A.; Zafar, M.S. Influence of various bleaching regimes on surface roughness of resin composite and ceramic dental biomaterials. Technol. Health Care 2015, 24, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ullah, R.; Zafar, M.S. Oral and dental delivery of fluoride: A review. Fluoride 2015, 48, 195–204. [Google Scholar]
- Zafar, M.S.; Ahmed, N. Therapeutic roles of fluoride released from restorative dental materials. Fluoride 2015, 48, 184–194. [Google Scholar]
- Saffari, F.; Ardakani, M.D.; Zandi, H.; Heidarzadeh, H.; Moshafi, M.H. The Effects of Chlorhexidine and Persica Mouthwashes on Colonization of Streptococcus mutans on Fixed Orthodontics O-rings. J. Dent. 2015, 16, 54–57. [Google Scholar]
- Lewinstein, I.; Zenziper, E.; Block, J.; Kfir, A. Incorporation of chlorhexidine diacetate in provisional cements: Antimicrobial activity against Streptococcus mutans and the effect on tensile strength in vitro. Int. Endod. J. 2012, 45, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Zafar, M.S.; Farid, W.M.; Gazal, G. Assessment of Antimicrobial Efficacy of MTAD, Sodium Hypochlorite, EDTA and Chlorhexidine for Endodontic Applications: An In vitro Study. Middle East J. Sci. Res. 2014, 21, 353–357. [Google Scholar]
- Lamster, I.B. Antimicrobial mouthrinses and the management of periodontal diseases: Introduction to the supplement. J. Am. Dent. Assoc. 2006, 137, S5–S9. [Google Scholar] [CrossRef]
- Gagari, E.; Kabani, S. Adverse effects of mouthwash use: A review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 432–439. [Google Scholar] [CrossRef]
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Ten Bosch, J.; Coops, J. Tooth color and reflectance as related to light scattering and enamel hardness. J. Dent. Res. 1995, 74, 374–380. [Google Scholar] [CrossRef] [PubMed]
- ElEmbaby, A.E. The Effects of Mouth Rinses on the Color Stability of Resin-Based Restorative Materials. J. Esthet. Restor. Dent. 2014, 26, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Müjde, S.; Filiz, A.; Nilgün, Ö.; Neslihan, Ç.; Tolga, Y. Effect of Mouthrinses on Color Stability of Provisional Restorative Materials. Hacettepe Diş Hekimliği Fakültesi Dergisi 2008, 32, 2–11. [Google Scholar]
- Satou, N.; Khan, A.; Matsumae, I.; Satou, J.; Shintani, H. In vitro color change of composite-based resins. Dent. Mater. 1989, 5, 384–387. [Google Scholar] [CrossRef]
- Khokhar, Z.; Razzoog, M.; Yaman, P. Color stability of restorative resins. Quintessence Int. 1991, 22, 733–737. [Google Scholar]
- Ergücü, Z.; Türkün, L.; Aladag, A. Color stability of nanocomposites polished with one-step systems. Oper. Dent. 2008, 33, 413–420. [Google Scholar] [CrossRef] [PubMed]
- De Azevedo Miranda, D.; Bertoldo, C.E.; Aguiar, F.H.B.; Lima, D.A.N.L.; Lovadino, J.R. Effects of mouthwashes on Knoop hardness and surface roughness of dental composites after different immersion times. Braz. Oral Res. 2011, 25, 168–173. [Google Scholar] [CrossRef]
- Al-Samadani, K.H. Surface Hardness of Dental Composite Resin Restorations in Response to Preventive Agents. J. Contemp. Dent. Pract. 2016, 17, 978–984. [Google Scholar] [PubMed]
- Örtengren, U.; Anddersson, F.; Elgh, U.; Terselius, B.; Karlsson, S. Influence of PH and storage time on the sorption and solubility behaviour of three composite resin materials. J. Dent. 2001, 29, 35–41. [Google Scholar] [CrossRef]
- Shintani, H.; Satou, J.; Satou, N.; Hayashihara, H.; Inoue, T. Effects of various finishing methods on staining and accumulation of Streptococcus mutans HS-6 on composite resins. Dent. Mater. 1985, 1, 225–227. [Google Scholar] [CrossRef]
- Brook, A.; Smith, R.; Lath, D. The clinical measurement of tooth colour and stain. Int. Dent. J. 2007, 57, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Nasim, I.; Neelakantan, P.; Sujeer, R.; Subbarao, C. Color stability of microfilled, microhybrid and nanocomposite resins—An in vitro study. J. Dent. 2010, 38, e137–e142. [Google Scholar] [CrossRef] [PubMed]
- Davallo, A.; Tavangar, M.; Darabi, F.; Pourhabibi, Z.; Alamouti, N. The surface hardness value of a light cured hybrid composite resin after 12 h immersion in three alcohol-free mouthwashes. J. Dentomaxillofac. Radiol. Pathol. Surg. 2013, 2, 1–6. [Google Scholar]
- Um, C.M.; Ruyter, I. Staining of resin-based veneering materials with coffee and tea. Quintessence Int. 1991, 22, 377–386. [Google Scholar]
- Diab, M.; Zaazou, M.H.; Mubarak, E.H.; Fahmy, O.M.I. Effect of five commercial mouthrinses on the microhardness and color stability of two resin composite restorative materials. Aust. J. Basic Appl. Sci. 2007, 1, 667–674. [Google Scholar]
- Gürgan, S.; Önen, A.; Köprülü, H. In vitro effects of alcohol-containing and alcohol-free mouthrinses on microhardness of some restorative materials. J. Oral Rehabil. 1997, 24, 244–246. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Illumination. Colorimetry: Official Recommendations of the International Commission on Illumination Colorimetrie: Recommendations Officielles de la Commission Internationale de L'eclairage Farbmessung: Offizielle Empfehlungen der Internationalen Beleuchtungs-Kommission; Bureau Central de la CIE: Vienna, Austria, 1986. [Google Scholar]
- Ruyter, I.; Nilner, K.; Möller, B. Color stability of dental composite resin materials for crown and bridge veneers. Dent. Mater. 1987, 3, 246–251. [Google Scholar] [CrossRef]
- Vichi, A.; Ferrari, M.; Davidson, C.L. Color and opacity variations in three different resin-based composite products after water aging. Dent. Mater. 2004, 20, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Doray, P.G.; Li, D.; Powers, J.M. Color stability of provisional restorative materials after accelerated aging. J. Prosthodont. 2001, 10, 212–216. [Google Scholar] [CrossRef] [PubMed]
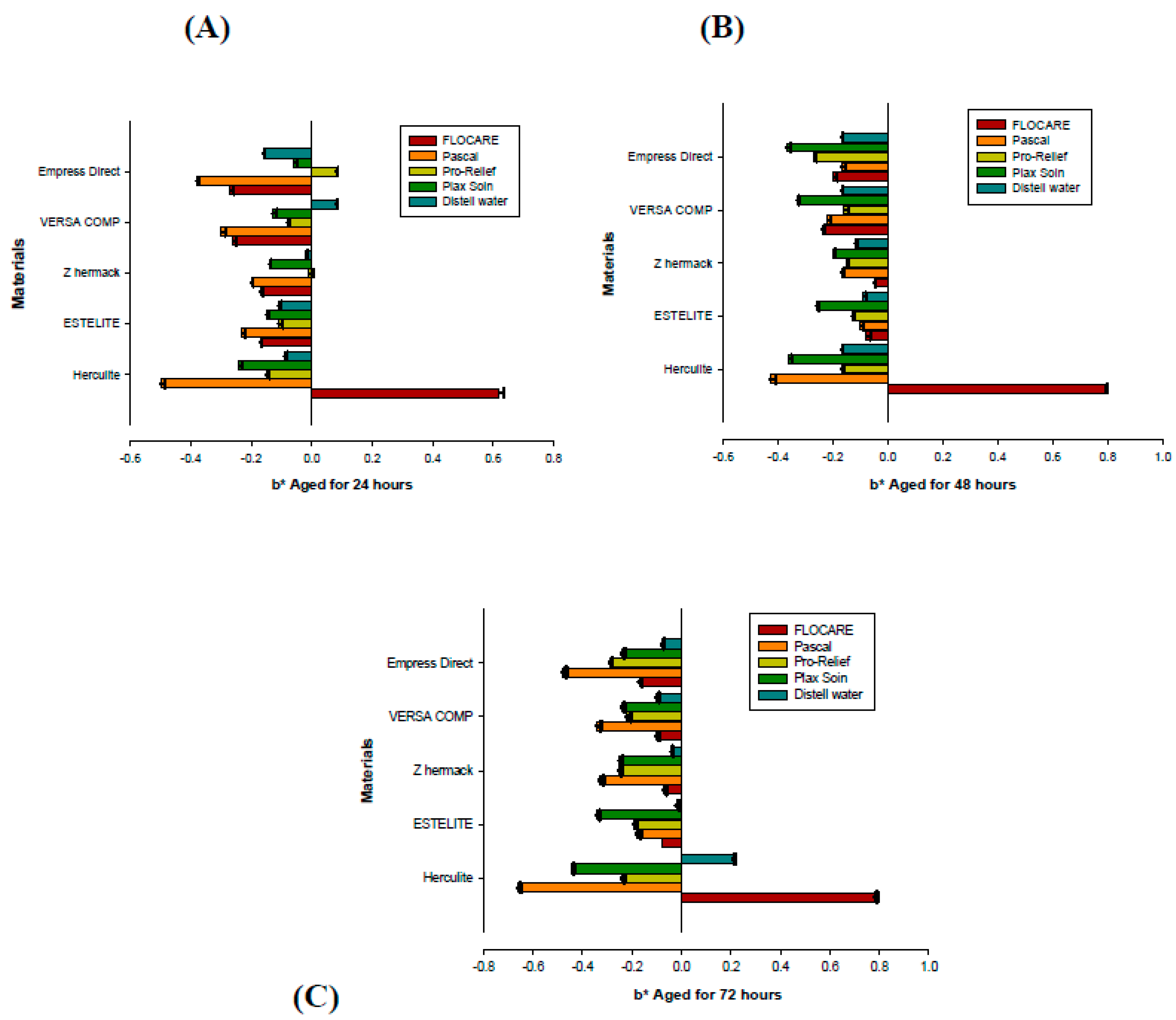
| Materials | 24 h | 48 h | 72 h | Significance |
|---|---|---|---|---|
| Flocare anti-caries gel (Group 1) Mean (St.Dev) | ||||
| Herculite TM Xrv Ultra | 0.69 (0.031) | 0.81 (0.047) | 0.84(0.038) | |
| Estelite Ʃ Quick | 0.58 (0.025) | 0.53 (0.030) | 0.64 (0.015) | |
| Z Hermack | 0.49 (0.010) | 0.45 (0.010) | 0.63 (0.025) | |
| Versa Comp Sultan | 0.73 (0.025) | 0.76 (0.036) | 0.76 (0.025) | |
| Empress® Direct IPS | 1.33 (0.025) | 1.27 (0.020) | 1.39 (0.015) | p = 0.000 |
| Pascal anti-caries gel (Group 2) | ||||
| Herculite TM Xrv Ultra | 0.98 (0.049) | 0.81 (0.031) | 1.06 (0.040) | |
| Estelite Ʃ Quick | 0.58 (0.021) | 0.64 (0.031) | 0.60 (0.015) | |
| Z Hermack | 0.34 (0.026) | 0.45 (0.015) | 0.70 (0.029) | |
| Versa Comp Sultan | 0.84 (0.021) | 0.87 (0.020) | 0.75 (0.025) | |
| Empress® Direct IPS | 1.04 (0.020) | 0.94 (0.031) | 1.12 (0.045) | p = 0.001 |
| Pro-Relief mouthwash (Group 3) | ||||
| Herculite TM Xrv Ultra | 0.70 (0.015) | 0.62 (0.025) | 0.99 (0.010) | |
| Estelite Ʃ Quick | 0.37(0.015) | 0.47 (0.015) | 0.83 (0.015) | |
| Z Hermack | 0.38 (0.015) | 0.56 (0.032) | 0.89 (0.015) | |
| Versa Comp Sultan | 0.21 (0.031) | 0.52 (0.025) | 0.83 (0.025) | |
| Empress® Direct IPS | 0.89 (0.021) | 1.33 (0.010) | 1.65 (0.026) | p = 0.042 |
| Plax Soin mouthwash (Group 4) | ||||
| Herculite TM Xrv Ultra | 0.25 (0.015) | 0.41 (0.021) | 0.54 (0.010) | |
| Estelite Ʃ Quick | 0.44 (0.010) | 0.50 (0.015) | 0.63 (0.021) | |
| Z Hermack | 0.38 (0.010) | 0.38 (0.006) | 0.68 (0.010) | |
| Versa Comp Sultan | 0.40 (0.015) | 1.01 (0.026) | 0.69 (0.010) | |
| Empress® Direct IPS | 1.05 (0.031) | 1.95 (0.021) | 1.36 (0.015) | p = 0.004 |
| Distilled Water (Group 5/control) | ||||
| Herculite TM Xrv Ultra | 0.32 (0.010) | 0.47 (0.015) | 0,65 (0.015) | |
| Estelite Ʃ Quick | 0.31 (0.010) | 0.44 (0.010) | 0.56 (0.021) | |
| Z Hermack | 0.24 (0.015) | 0.44 (0.010) | 0.54 (0.015) | |
| Versa Comp Sultan | 0.58 (0.015) | 0.58 (0.010) | 0.80 (0.015) | |
| Empress® Direct IPS | 0.76 (0.010) | 0.94 (0.015) | 1.07 (0.010) | p = 0.008 |
| Preventive Mouthwash | |||
| Name | Composition | Group | Manufacturer |
| Flocare Gel | Stannous Fluoride (0.4%) as active ingredient | 1 | Dentamerica® California, CA, USA |
| Pascal Gel | Topical Preventive Treatment Gel contains Acidulated phosphate fluoride | 2 | 2929 NE Northup EWay, Believue, WA 98004, USA |
| Pro-Relief Mouthwash | Arginine 0.8%, Sorbitol, Propylene Glycol, Tetrapotassium Pyrophosphate, Hydrogenated Castor Oil, PVM/MA Copolymer, Sodium Fluoride (225 ppm), Menthol, Sodium Saccharin, Citric Acid | 3 | Colgate Palmolive, Bangkok 10110, Thailand |
| Plax Soin Mouthwash | Cetylpyridinium Chloride 0.075% w/w, Sodium Fluoride 0.05% w/w, Water Glycerin, Propylene Glycol, Sorbitol, Sodium Saccharin, Menthol, Methylparaben | 4 | Colgate Palmolive, Bangkok 10110, Thailand |
| Composite Resin Materials | |||
| Materials | Type/Composition | Manufacturer | |
| Herculite TM Xrv Ultra | Nano-hybrid-composite, ethoxylated Bisphenol A-dimethacrylate, 2,2-ethylenedioxydiethyl dimethacrylate, 3-Methacryloxypropyltrimethoxysilane, bisphenol A-glycidyl methacrylate (BIS-GMA) | Kerr italia, S.rl Via passanti, 332 1-84018 Scafati (SA), Italy | |
| Estelite Ʃ Quick | Resin-based Restorative Material. Silica-zirconia fillers (82% by weight; 71% by volume), -methylethylidene)bis[4,1-phenyleneoxy(2-hydroxy-3,1-propanediyl)] bismethacrylate (10%–30%), ethylenedioxydiethyl dimethacrylate (5%–10%) | Tokuyama Dental Corporation 38-9, Taitou 1-chome, Tokyo, Japan | |
| Z Hermack | Universal Micro-hybrid Resin-Based composite. Highly dispersed silica fillers (0.04 µm), Dimethacrylate resin (EBDADMA), Triethyleneglycol dimethacrylate (TEGDMA), Photo initiators, Titanium oxide | Zhermack, 10045021 Badia Polesine (RO), Italy | |
| Versa Comp Sultan | Universal-Hybrid Composite. Barium Boron Fluoro Alumino Silicate, Glass, Amorphous silica, Urethane Modified Bis-GMA dimethacrylate, Polymerizable, Dimethacrylate, (1-methylethylidene)Bis[4,1-phenyleneoxy(2-hydroxy-3,1-propanediyl)] bismethacrylate | Sultan Health Care, 1301 Smile Way, York, PA 17404, USA | |
| Empress® Direct IPS | Direct Refill Enamel. Barium glass, ytterbium trifluoride, mixed oxide, silicon dioxide inorganic fillers (75–79 wt %; 52–59 vol % and size range of 40 nm and 3000 nm; mean particle size of 550 nm), dimethacrylates (20–21.5 wt %, opalescent shade 17 wt %) | Ivoclar vivadent, Bendererstrasse 29494 Schaan, Liechtenstein | |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Samadani, K.H. The Effect of Preventive Agents (Mouthwashes/Gels) on the Color Stability of Dental Resin-Based Composite Materials. Dent. J. 2017, 5, 18. https://doi.org/10.3390/dj5020018
Al-Samadani KH. The Effect of Preventive Agents (Mouthwashes/Gels) on the Color Stability of Dental Resin-Based Composite Materials. Dentistry Journal. 2017; 5(2):18. https://doi.org/10.3390/dj5020018
Chicago/Turabian StyleAl-Samadani, Khalid H. 2017. "The Effect of Preventive Agents (Mouthwashes/Gels) on the Color Stability of Dental Resin-Based Composite Materials" Dentistry Journal 5, no. 2: 18. https://doi.org/10.3390/dj5020018
APA StyleAl-Samadani, K. H. (2017). The Effect of Preventive Agents (Mouthwashes/Gels) on the Color Stability of Dental Resin-Based Composite Materials. Dentistry Journal, 5(2), 18. https://doi.org/10.3390/dj5020018




