Biomechanical and Morphological Analyses of Enamel White Spot Lesions Treated by Different Therapeutic Approaches (In Vitro Comparative Study)
Abstract
1. Introduction
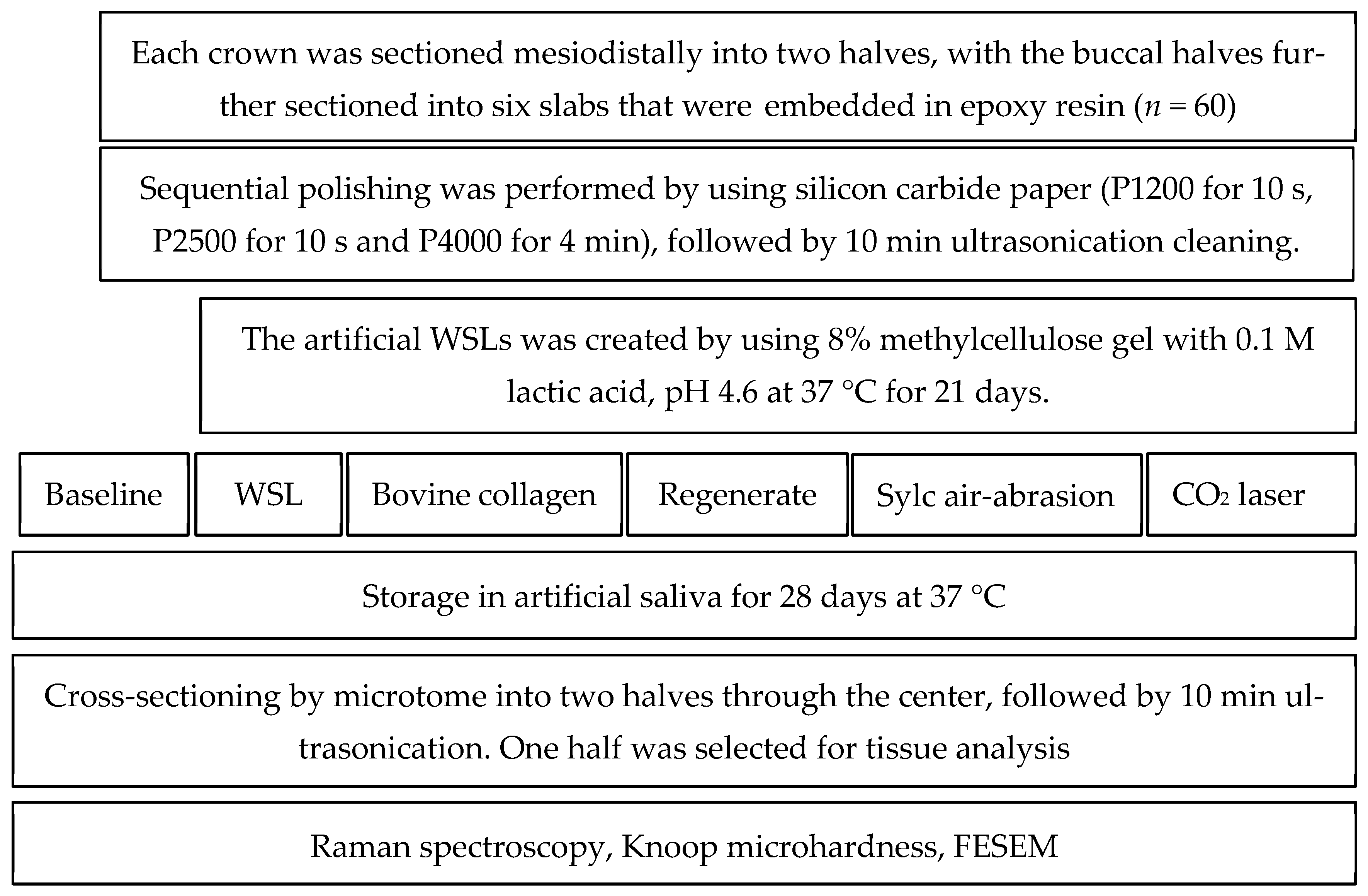
2. Materials and Methods
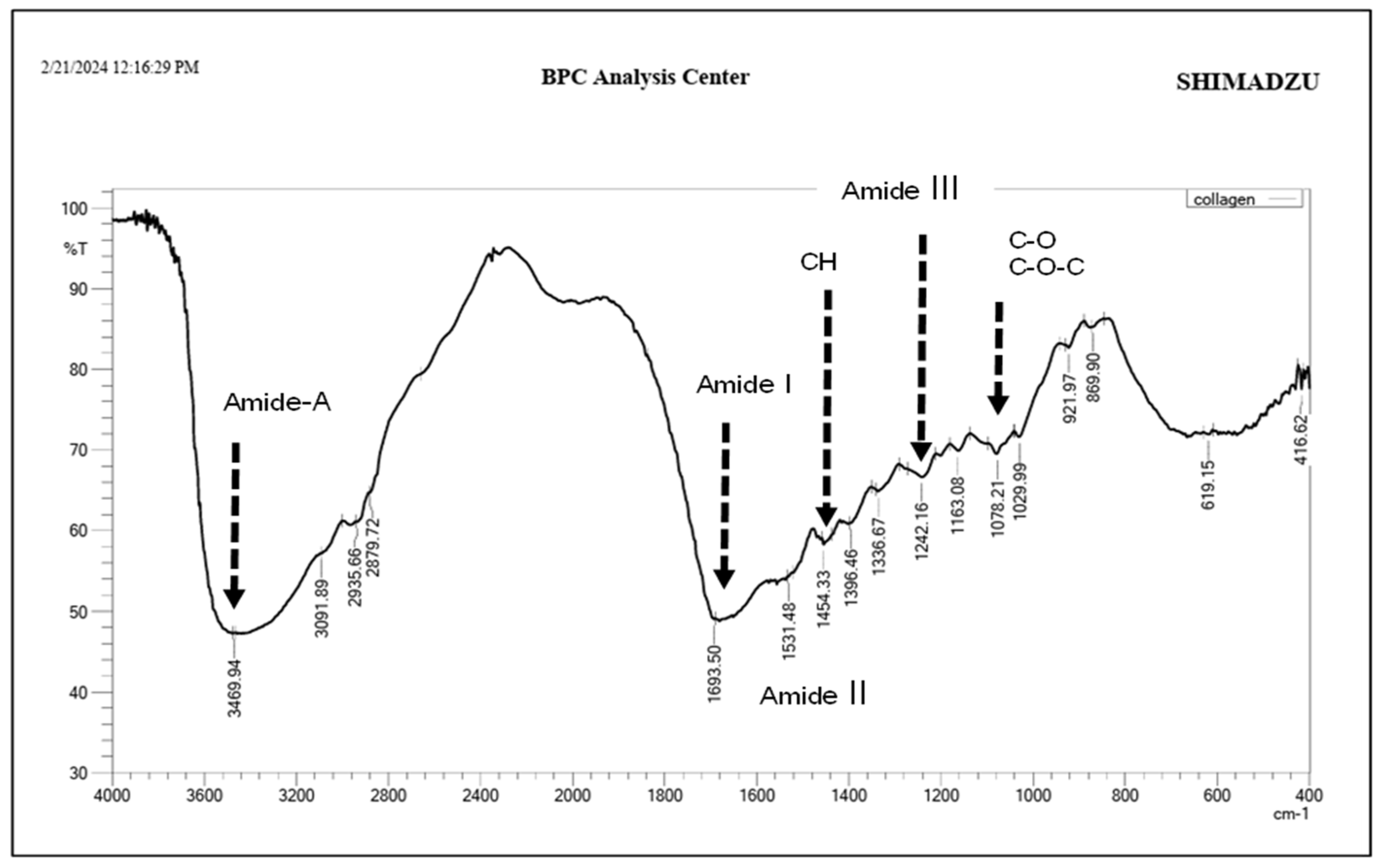
2.1. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis of Bovine Collagen
2.2. Sample Size
2.3. Specimen Preparation
2.4. Artificially Induced WSLs
2.5. Bovine Collagen Supplement Application
2.6. The Application of Regenerate Enamel Science System
2.7. Sylc Air Abrasion
2.8. Carbon Dioxide Laser
2.9. Chemical Characterization (Raman Spectroscopy)
2.10. Cross-Sectional Microhardness
2.11. Morphological Assessment by FESEM
2.12. Statistical Analyses
3. Results
3.1. FTIR Spectral Analysis
3.2. Chemical Analysis (Raman Results)
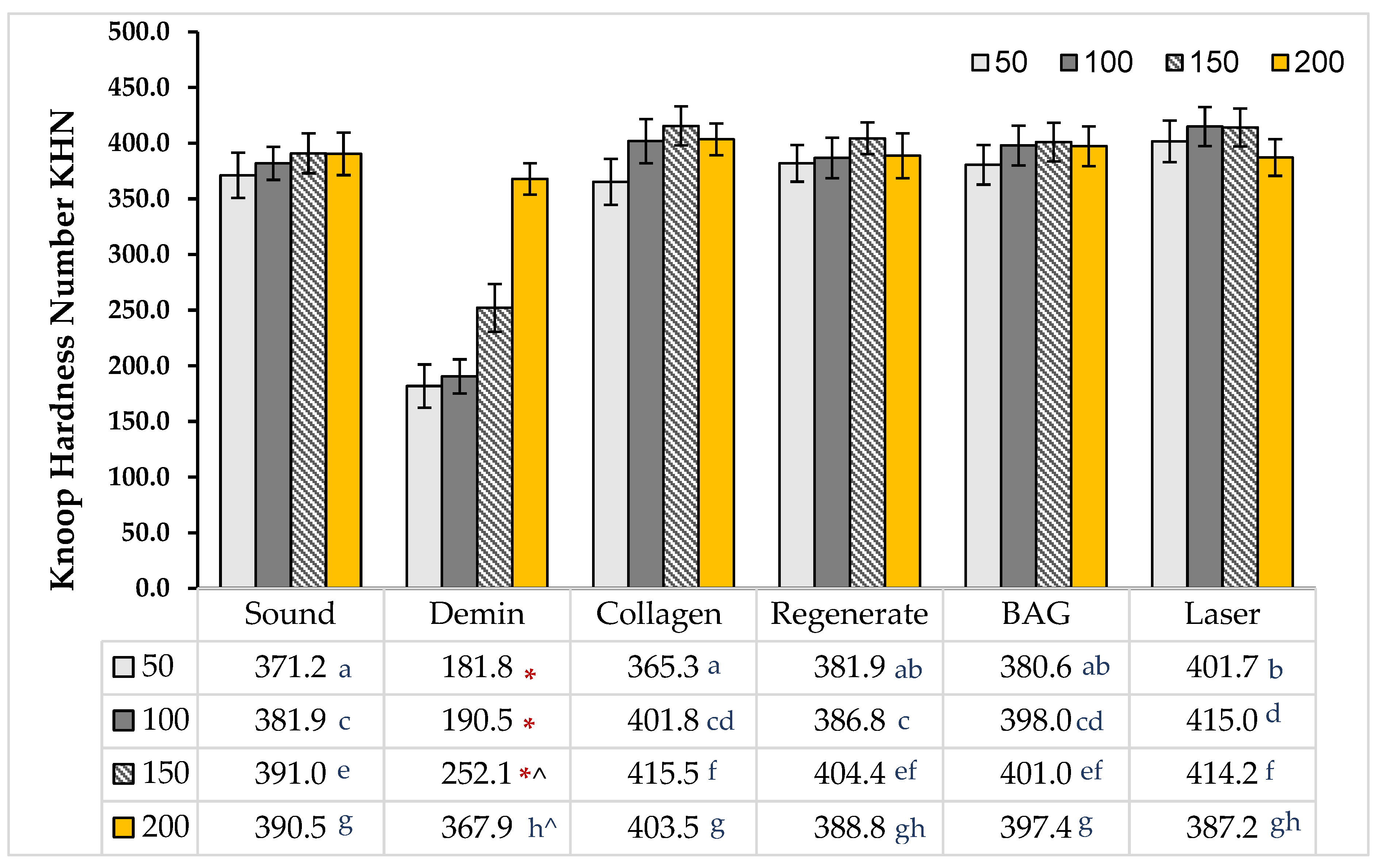
3.3. Knoop Microhardness
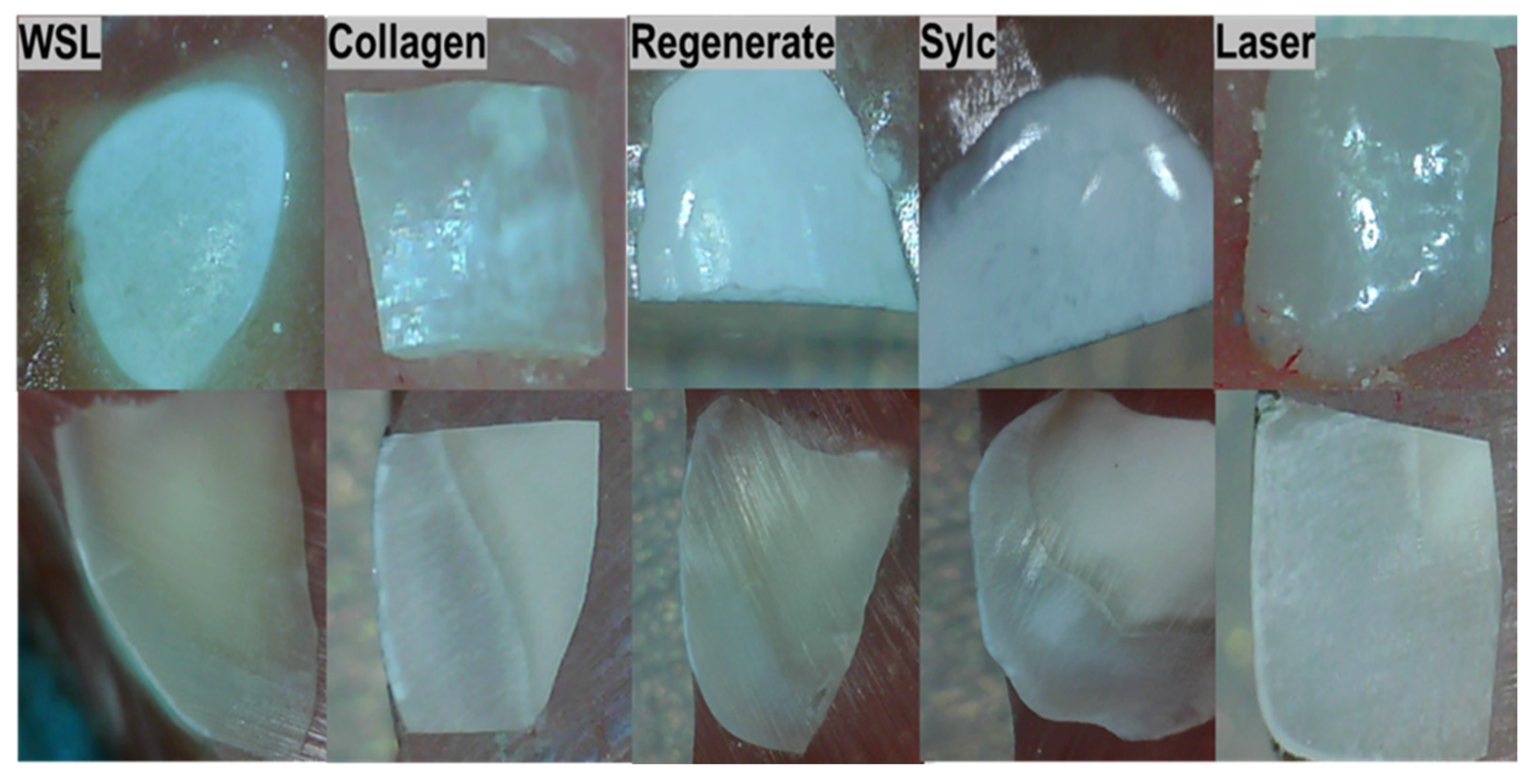
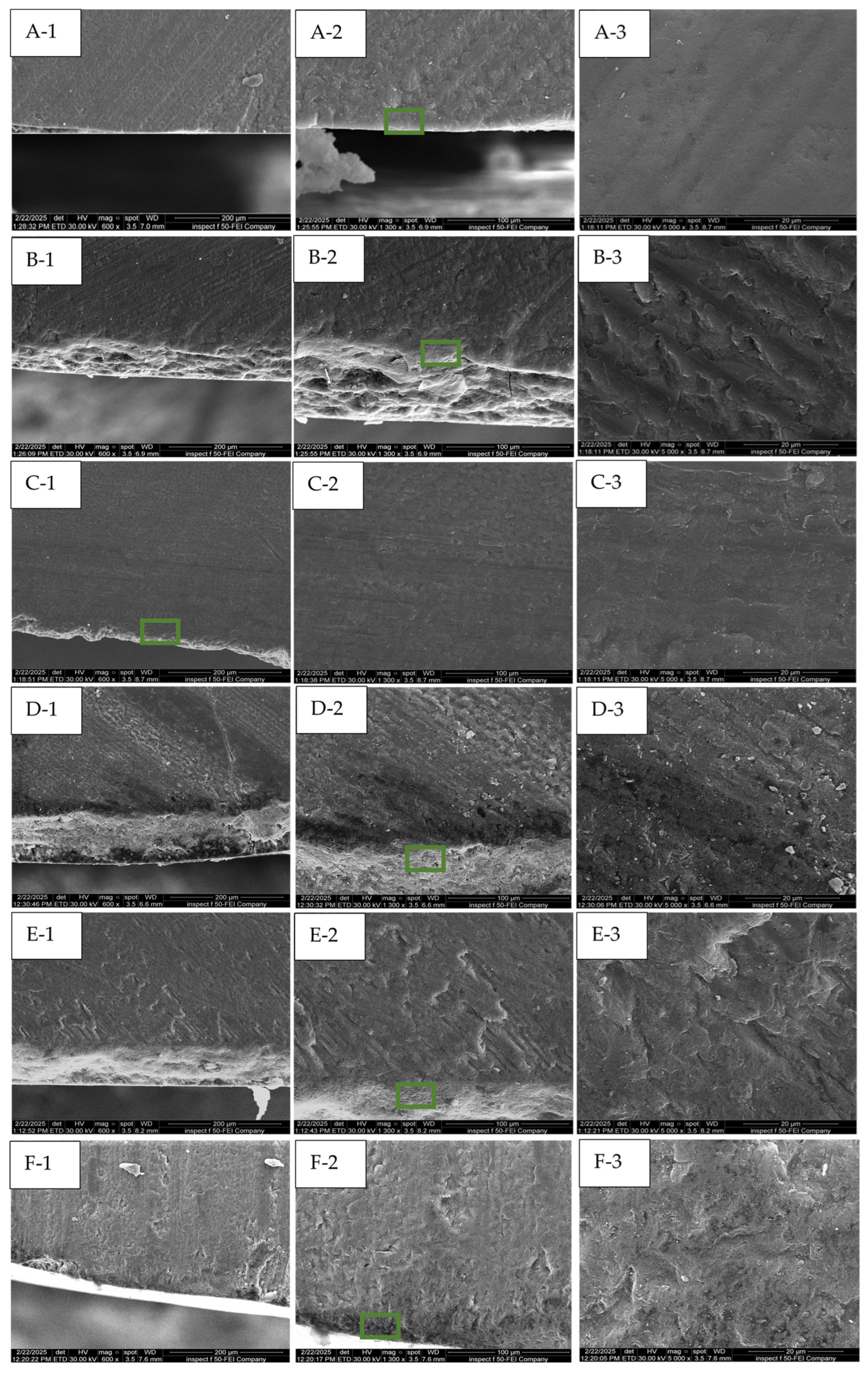
3.4. The Morphological Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paula, A.B.P.; Fernandes, A.R.; Coelho, A.S.; Marto, C.M.; Ferreira, M.M.; Caramelo, F.; Do Vale, F.; Carrilho, E. Therapies for white spot lesions—A systematic review. J. Evid. Based Dent. Pract. 2017, 17, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Deyhle, H.; White, S.N.; Bunk, O.; Beckmann, F.; Müller, B. Nanostructure of carious tooth enamel lesion. Acta Biomater. 2014, 10, 355–364. [Google Scholar] [CrossRef]
- Huang, G.J.; Roloff-Chiang, B.; Mills, B.E.; Shalchi, S.; Spiekerman, C.; Korpak, A.M.; Starrett, J.L.; Greenlee, G.M.; Drangsholt, R.J.; Matunas, J.C. Effectiveness of MI Paste Plus and PreviDent fluoride varnish for treatment of white spot lesions: A randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2013, 143, 31–41. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Cai, F.; Huq, N.L.; Burrow, M.F.; Reynolds, E.C. New approaches to enhanced remineralization of tooth enamel. J. Dent. Res. 2010, 89, 1187–1197. [Google Scholar] [CrossRef]
- Parker, A.S.; Patel, A.N.; Al Botros, R.; Snowden, M.E.; McKelvey, K.; Unwin, P.R.; Ashcroft, A.T.; Carvell, M.; Joiner, A.; Peruffo, M. Measurement of the efficacy of calcium silicate for the protection and repair of dental enamel. J. Dent. 2014, 42, S21–S29. [Google Scholar] [CrossRef] [PubMed]
- Shubbar, M.; Addie, A.; Al-Taee, L. The Effect of a Bioactive Oral System and CO2 Laser on Enamel Susceptibility to Acid Challenge. Diagnostics 2023, 13, 1087. [Google Scholar] [CrossRef]
- Bossù, M.; Saccucci, M.; Salucci, A.; Di Giorgio, G.; Bruni, E.; Uccelletti, D.; Sarto, M.S.; Familiari, G.; Relucenti, M.; Polimeni, A. Enamel remineralization and repair results of Biomimetic Hydroxyapatite toothpaste on deciduous teeth: An effective option to fluoride toothpaste. J. Nanobiotechnol. 2019, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.A.; Fleming, P.S.; Hill, R.G.; Patel, M.P. Enamel remineralization with novel bioactive glass air abrasion. J. Dent. Res. 2018, 97, 1438–1444. [Google Scholar] [CrossRef]
- Chabuk, M.M.; Al-Shamma, A.M. Surface roughness and microhardness of enamel white spot lesions treated with different treatment methods. Heliyon 2023, 9, e18283. [Google Scholar] [CrossRef]
- Banerjee, A.; Hameed, Z.; Chohan, M.A.; Patel, K.; Vaghela, J.; Sheikh, F.; Barker, N.; Shah, P.; Patel, D. Minimum Intervention Oral Care-incentivising preventive management of high needs/high caries-risk patients using phased courses of treatment. Br. Dent. J. 2024, 236, 379–382. [Google Scholar] [CrossRef]
- Pandya, M.; Diekwisch, T.G.H. Enamel biomimetics—Fiction or future of dentistry. Int. J. Oral Sci. 2019, 11, 8. [Google Scholar] [CrossRef]
- Alkilzy, M.; Qadri, G.; Splieth, C.H.; Santamaría, R.M. Biomimetic Enamel Regeneration Using Self-Assembling Peptide P11-4. Biomimetics 2023, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Kind, L.; Stevanovic, S.; Wuttig, S.; Wimberger, S.; Hofer, J.; Muller, B.; Pieles, U. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J. Dent. Res. 2017, 96, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, B.; Song, S.; Ma, M.; Si, S.; Wang, Y.; Xu, B.; Feng, K.; Wu, J.; Guo, Y. Bovine collagen peptides compounds promote the proliferation and differentiation of MC3T3-E1 pre-osteoblasts. PLoS ONE 2014, 9, e99920. [Google Scholar] [CrossRef] [PubMed]
- Souza-Gabriel, A.E.; Colucci, V.; Turssi, C.P.; Serra, M.C.; Corona, S.A. Microhardness and SEM after CO2 laser irradiation or fluoride treatment in human and bovine enamel. Microsc. Res. Tech. 2010, 73, 1030–1035. [Google Scholar] [CrossRef]
- Esteves-Oliveira, M.; Zezell, D.M.; Meister, J.; Franzen, R.; Stanzel, S.; Lampert, F.; Eduardo, C.P.; Apel, C. CO2 Laser (10.6 μm) parameters for caries prevention in dental enamel. Caries Res. 2009, 43, 261–268. [Google Scholar] [CrossRef]
- Esteves-Oliveira, M.; Pasaporti, C.; Heussen, N.; Eduardo, C.P.; Lampert, F.; Apel, C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J. Dent. 2011, 39, 414–421. [Google Scholar] [CrossRef]
- Eissa, N.M.; Elshourbagy, E.M.; Gomaa, N.E. Effect of sodium fluoride plus tricalcium phosphate with and without CO2 laser on remineralization of white spot lesions. Heliyon 2022, 8, e10752. [Google Scholar] [CrossRef]
- Kuramochi, E.; Iizuka, J.; Mukai, Y. Influences of bicarbonate on processes of enamel subsurface remineralization and demineralization: Assessment using micro-Raman spectroscopy and transverse microradiography. Eur. J. Oral Sci. 2016, 124, 554–558. [Google Scholar] [CrossRef]
- Almarsomy, D.H.; Al-Khayat, F.A.; Al-Taee, L.A. The preventive/therapeutic effect of CO2 laser and MI Paste Plus® on intact and demineralized enamel against Streptococcus mutans (In Vitro Study). Heliyon 2023, 9, e20310. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Lippert, F. Effect of Enamel Caries Lesion Baseline Severity on Fluoride Dose-Response. Int. J. Dent. 2017, 2017, 4321925. [Google Scholar] [CrossRef]
- Al-Taee, L.; Banerjee, A.; Deb, S. In-vitro adhesive and interfacial analysis of a phosphorylated resin polyalkenoate cement bonded to dental hard tissues. J. Dent. 2022, 118, 104050. [Google Scholar] [CrossRef]
- Amaechi, B.T.; Van Loveren, C. Fluorides and non-fluoride remineralization systems. Toothpastes Monogr. Oral Sci. 2013, 23, 15–26. [Google Scholar] [CrossRef]
- Hamdi, K.; Hamama, H.H.; Motawea, A.; Fawzy, A.; Mahmoud, S.H. Remineralization of early enamel lesions with a novel prepared tricalcium silicate paste. Sci. Rep. 2022, 12, 9926. [Google Scholar] [CrossRef] [PubMed]
- Belbachir, K.; Noreen, R.; Gouspillou, G.; Petibois, C. Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem. 2009, 395, 829–837. [Google Scholar] [CrossRef]
- Ko, A.C.T.; Choo-Smith, L.P.; Hewko, M.; Sowa, M.G.; Dong, C.C.S.; Cleghorn, B. Detection of early dental caries using polarized Raman spectroscopy. Opt. Express 2006, 14, 203–215. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Rehman, G.U.; Basavarajappa, S.; Al Khuraif, A.A.; Durgesh, B.H.; Khan, A.S.; Rehman, I.U. Applications of Raman spectroscopy in dentistry: Analysis of tooth structure. Appl. Spectrosc. Rev. 2015, 50, 332–350. [Google Scholar] [CrossRef]
- Magalhães, A.C.; Moron, B.M.; Comar, L.P.; Wiegand, A.; Buchalla, W.; Buzalaf, M.A.R. Comparison of cross-sectional hardness and transverse microradiography of artificial carious enamel lesions induced by different demineralising solutions and gels. Caries Res. 2009, 43, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Aljabo, A.; Strange, A.; Ibrahim, S.; Coathup, M.; Young, A.M.; Bozec, L.; Mudera, V. Demineralization—Remineralization dynamics in teeth and bone. Int. J. Nanomed. 2016, 11, 4743–4763. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Kayser, V.; Turton, D.A.; Aggeli, A.; Beevers, A.; Reid, G.D.; Beddard, G.S. Energy migration in novel pH-triggered self-assembled β-sheet ribbons. J. Am. Chem. Soc. 2004, 126, 336–343. [Google Scholar] [CrossRef]
- Yu, L.; Wei, M. Biomineralization of collagen-based materials for hard tissue repair. Int. J. Mol. Sci. 2021, 22, 944. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, J.; Firth, A.; Vernals, D.; Boden, N.; Robinson, C.; Shore, R.C.; Brookes, S.J.; Aggeli, A. Self-assembling peptide scaffolds promote enamel remineralization. J. Dent. Res. 2007, 86, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.; Zobrist, K.; Attin, T.; Wegehaupt, F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J. Appl. Oral Sci. 2016, 24, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Üstün, N.; Aktören, O. Analysis of efficacy of the self-assembling peptide-based remineralization agent on artificial enamel lesions. Microsc. Res. Tech. 2019, 82, 1065–1072. [Google Scholar] [CrossRef]
- Sun, Y.; Li, X.; Deng, Y.; Sun, J.N.; Tao, D.; Chen, H.; Hu, Q.; Liu, R.; Liu, W.; Feng, X.; et al. Mode of action studies on the formation of enamel minerals from a novel toothpaste containing calcium silicate and sodium phosphate salts. J. Dent. 2014, 42, S30–S38. [Google Scholar] [CrossRef]
- Soares, R.; De Ataide, I.D.N.; Fernandes, M.; Lambor, R. Assessment of enamel remineralisation after treatment with four different remineralising agents: A scanning electron microscopy (SEM) study. J. Clin. Diagn. Res. 2017, 11, ZC136–ZC141. [Google Scholar] [CrossRef]
- Rahee, S.S.; Jehad, R.H. Comparing the effectiveness of using three different re-mineralizing pastes on remineralisation of artificially induced white spot lesion. J. Baghdad Coll. Dent. 2023, 35, 35–45. [Google Scholar] [CrossRef]
- Liu, Y.; Hsu, C.Y.S. Laser-induced compositional changes on enamel: A FT-Raman study. J. Dent. 2007, 35, 226–230. [Google Scholar] [CrossRef]
- Rodríguez-Vilchis, L.E.; Contreras-Bulnes, R.; Olea-Mejìa, O.F.; Sánchez-Flores, I.; Centeno-Pedraza, C. Morphological and structural changes on human dental enamel after Er: YAG laser irradiation: AFM, SEM, and EDS evaluation. Photomed. Laser Surg. 2011, 29, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Garma, N.M.; Jasim, E.S. The effect of Er: YAG laser on enamel resistance to caries during orthodontic treatment: An in vitro study. J. Baghdad Coll. Dent. 2015, 27, 182–188. [Google Scholar] [CrossRef]


| PO4 ν1 | 50 µm | 100 µm | 150 µm | 200 µm | p Value |
|---|---|---|---|---|---|
| Baseline | 1222.7 ± 77.6 a | 1322.6 ± 94.9 c | 1347.4 ± 89.0 d | 1352.7 ± 103.9 e | 0.051 |
| WSLs | 777.7 ± 73.4 * | 880.8 ± 109.2 * | 1014.6 ± 103.5 * | 1290.5 ± 116.2 e | 0.000 |
| Collagen | 1226.5 ± 78.7 a | 1387.0 ± 94.3 c | 1360.2 ± 98.5 d | 1411.9 ± 98.0 e | 0.000 |
| Regenerate | 1340.1 ± 95.4 b | 1422.6 ± 92.6 c | 1372.4 ± 92.7 d | 1384.4 ± 78.0 e | 0.105 |
| Sylc | 1300.2 ± 86.4 ab | 1399.0 ± 97.7 c | 1350.0 ± 89.8 d | 1345.9 ± 80.4 e | 0.081 |
| Laser | 1268.5 ± 95.2 ab | 1341.3 ± 87.2 c | 1344.8 ± 93.6 d | 1359.3 ± 91.7 e | 0.053 |
| PO4 ν2 | |||||
| Baseline | 195.7 ± 27.7 f | 243.6 ± 25.1 | 283.1 ± 22.0 i | 272.4 ± 28.1 j | 0.000 |
| WSLs | 132.8 ± 27.5 * | 146.2 ± 23.0 * | 154.5 ± 24.2 * | 195.2 ± 24.8 * | 0.000 |
| Collagen | 192.6 ± 35.1 f | 288.8 ± 22.4 h | 309.8 ± 24.9 i | 292.2 ± 28.8 j | 0.000 |
| Regenerate | 327.1 ± 37.7 g | 364.8 ± 24.0 | 402.0 ± 24.2 | 306.0 ± 25.8 j | 0.000 |
| Sylc | 313.6 ± 28.2 g | 327.7± 28.7 h | 305.5 ± 26.4 i | 284.4 ± 26.2 j | 0.051 |
| Laser | 302.0± 37.8 g | 308.8 ± 37.4 h | 308.2 ± 27.0 i | 290.1 ± 25.6 j | 0.490 |
| PO4 ν4 | |||||
| Baseline | 164.3 ± 22.5 k | 168.1 ± 25.6 m | 230.5 ± 25.2 n | 260.5 ± 20.2 p | 0.000 |
| WSLs | 97.4 ± 20.9 * | 100.2 ± 22.8 * | 110.2 ± 22.8 * | 187.7 ± 26.9 * | 0.000 |
| Collagen | 161.4 ± 22.7 k | 182.8 ± 26.2 m | 252.6 ± 22.3 no | 286.1 ± 17.8 p | 0.000 |
| Regenerate | 266.8 ± 21.1 | 283.3 ± 28.3 | 310.9 ± 25.1 | 280.1 ± 18.9 p | 0.000 |
| Sylc | 217.4 ± 25.2 l | 249.3 ± 27.2 | 273.8 ± 23.9 o | 281.1 ± 30.0 p | 0.000 |
| Laser | 190.7 ± 22.9 l | 196.2 ± 22.5 m | 274.1 ± 18.6 o | 283.7 ± 27.5 p | 0.000 |
| Carbonate | |||||
| Baseline | 87.0 ± 20.1 r | 94.7 ± 25.2 tx | 96.2 ± 18.5 y | 99.8 ± 14.7 z | 0.552 |
| WSLs | 100.5 ± 23.8 r | 107.7 ± 18.1 t | 107.5 ± 21.1 y | 106.2 ± 23.0 z | 0.855 |
| Collagen | 88.6 ± 23.7 r | 86.8 ± 19.5 tx | 88.8 ± 20.6 y | 93.4 ± 20.1 z | 0.834 |
| Regenerate | 154.7 ± 18.3 | 166.7 ± 17.9 | 135.2 ± 22.4 | 104.5 ± 22.0 z | 0.000 |
| Sylc | 100.0 ± 24.2 r | 102.6 ± 18.4 t | 102.5 ± 16.7 y | 98.6 ± 19.2 z | 0.759 |
| Laser | 58.6 ± 19.9 | 73.3 ± 17.1 x | 84.7 ± 18.7 y | 99.3 ± 20.3 z | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Taee, L.A.H.; Al-Hyazaie, M.T.; Khalil, R.J.; Banerjee, A. Biomechanical and Morphological Analyses of Enamel White Spot Lesions Treated by Different Therapeutic Approaches (In Vitro Comparative Study). Dent. J. 2025, 13, 408. https://doi.org/10.3390/dj13090408
Al-Taee LAH, Al-Hyazaie MT, Khalil RJ, Banerjee A. Biomechanical and Morphological Analyses of Enamel White Spot Lesions Treated by Different Therapeutic Approaches (In Vitro Comparative Study). Dentistry Journal. 2025; 13(9):408. https://doi.org/10.3390/dj13090408
Chicago/Turabian StyleAl-Taee, Lamis Abdul Hammed, Mohammad Talal Al-Hyazaie, Rabeia J. Khalil, and Avijit Banerjee. 2025. "Biomechanical and Morphological Analyses of Enamel White Spot Lesions Treated by Different Therapeutic Approaches (In Vitro Comparative Study)" Dentistry Journal 13, no. 9: 408. https://doi.org/10.3390/dj13090408
APA StyleAl-Taee, L. A. H., Al-Hyazaie, M. T., Khalil, R. J., & Banerjee, A. (2025). Biomechanical and Morphological Analyses of Enamel White Spot Lesions Treated by Different Therapeutic Approaches (In Vitro Comparative Study). Dentistry Journal, 13(9), 408. https://doi.org/10.3390/dj13090408







