A Thermal Imaging Camera as a Diagnostic Tool to Study the Effects of Occlusal Splints on the Elimination of Masticatory Muscle Tension
Abstract
1. Introduction
2. Material and Methods
- Group M received a Michigan splint;
- Group D received a double repositioning splint.
- Occlusal Splints
- Achieve centric relation;
- Provide freedom in the centric (0.5–1.0 mm flat surface);
- Establish canine guidance beginning ~1 mm from the centric;
- Eliminate incisal guidance from centric occlusion;
- Allow the ideal seating of the condyles in the articular fossae.
- Full biocompatibility, certified by the manufacturer, ensuring safety for intraoral use;
- Elasticity and durability, which allow for the repeated insertion and removal of the splint while maintaining mechanical strength under functional loading conditions.
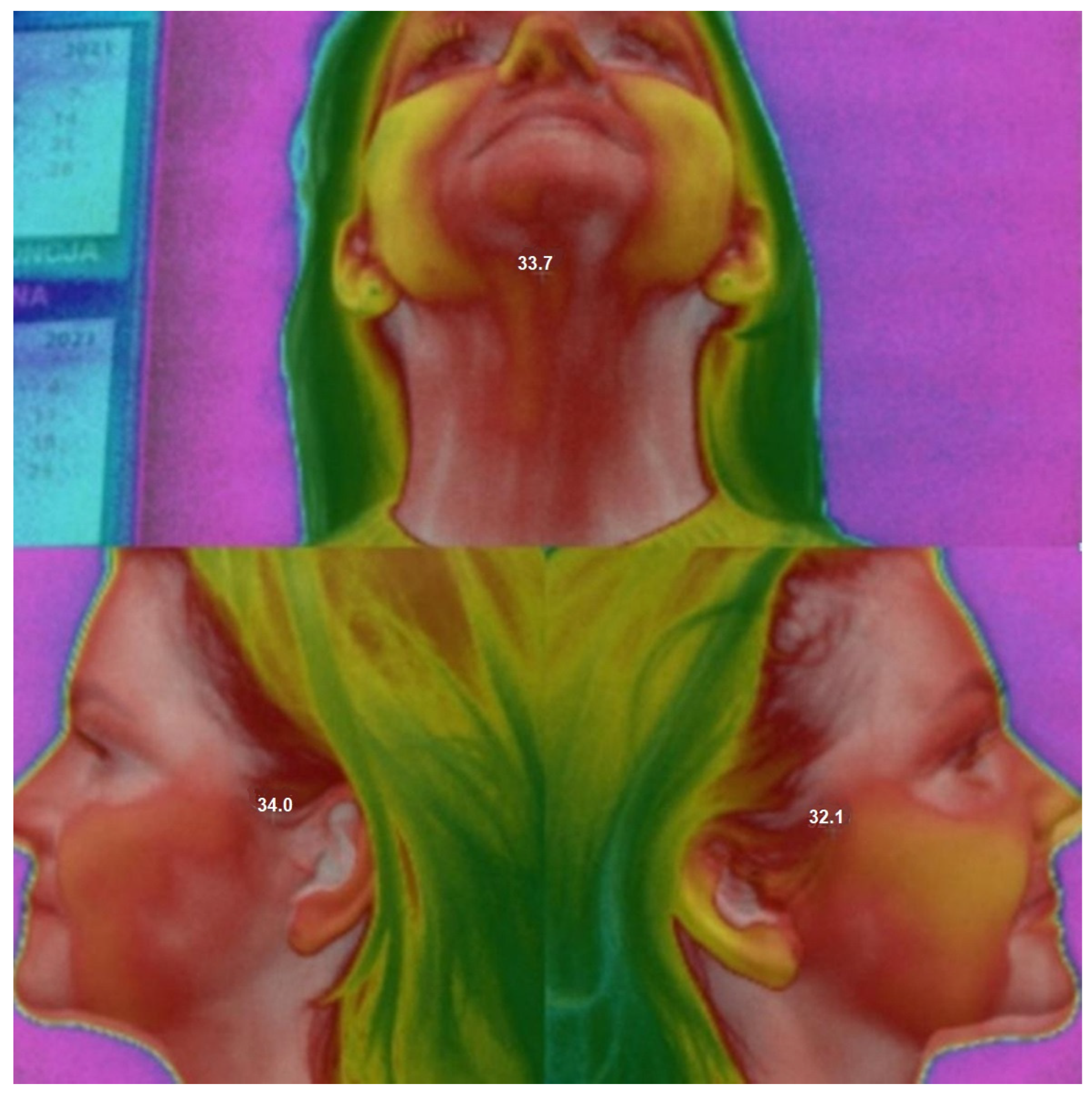
- Thermographic Measurements
- T1—Baseline (at rest, before splint application);
- T2—Immediately after splint placement;
- T3—30 min after splint placement.
- Active muscles generate more heat, which is transmitted via vascular and fascial structures.
- Thermal imaging can detect these changes with high accuracy.
- Overloaded or tense muscles are expected to exhibit higher surface temperatures compared to relaxed muscles.
2.1. Sample Size
- n—the number of patients in each group;
- α—the probability of a type I error (false positive);
- β—the probability of a type 2 error (false negative);
- d = ()/σ—Cohen’s effect size;
- —sample mean in both groups;
- σ—standard deviation.
2.2. Statistical Analysis
3. Results
- N—masseter muscle;
- OSP—suprahyoid muscles;
- OSL—sternocleidomastoid muscles.
- T1—baseline (before splint placement);
- T2—immediately after splint placement;
- T3—30 min after splint placement.
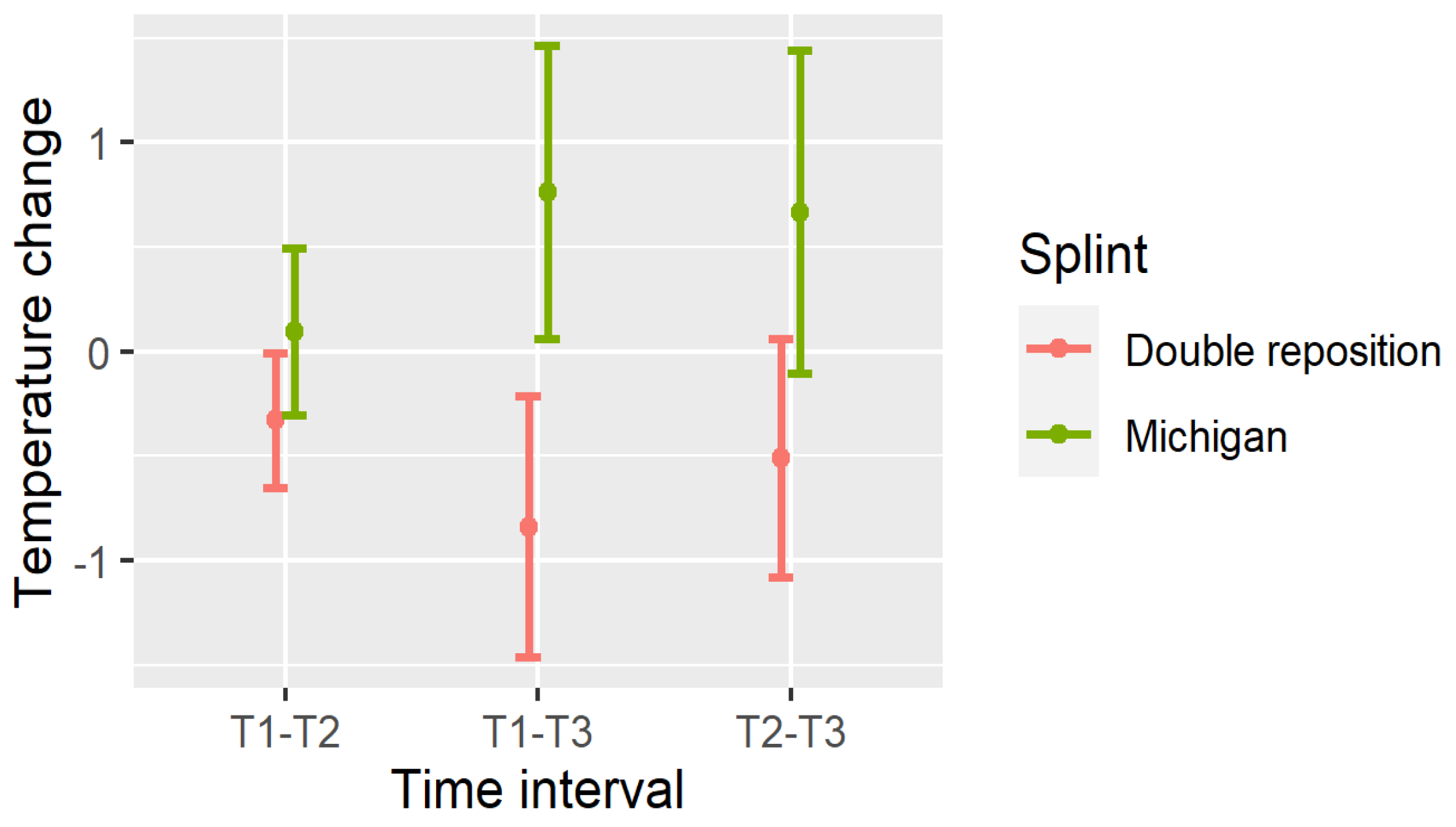
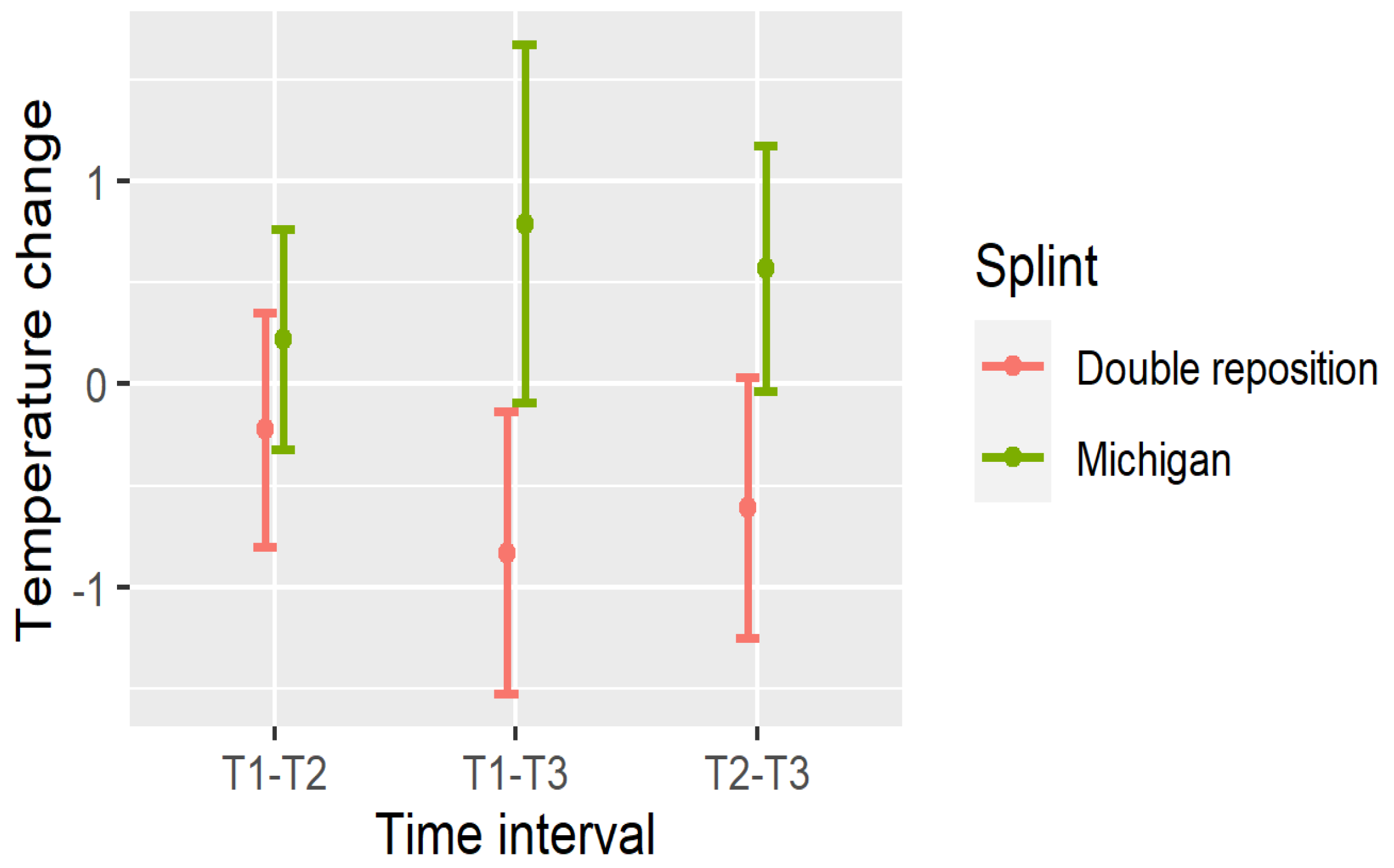
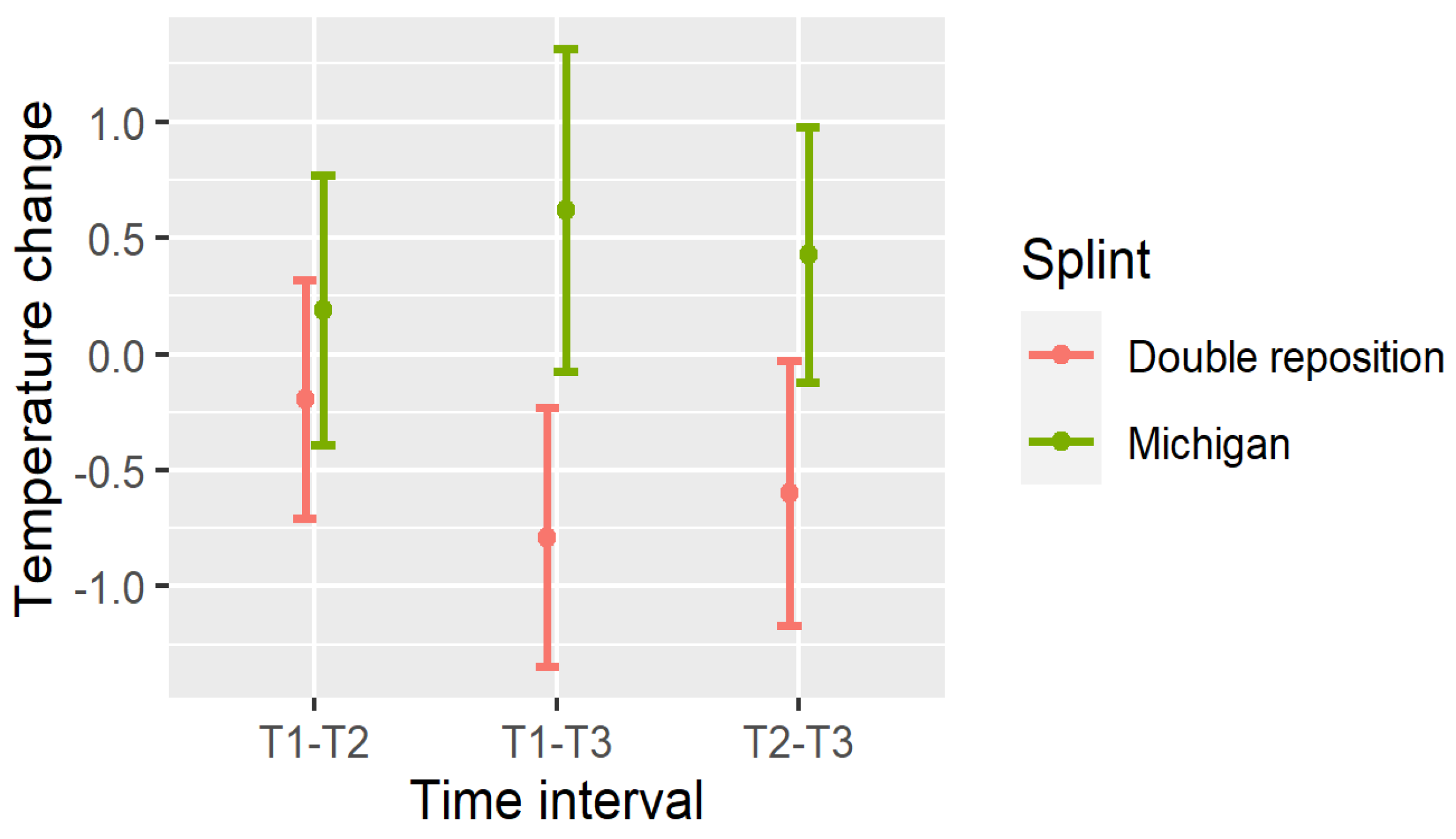
4. Changes in Temperature
- Dnij—change in temperature in the masseter muscle from time point Ti to Tj;
- DOSPij—change in the suprahyoid muscles;
- DOSLij—change in the sternocleidomastoid muscles.
- Masseter (DN): All intervals (T1–T2, T1–T3, T2–T3) showed significant reductions (p < 0.001);
- Suprahyoid (DOSP): Significant reductions were seen in T1–T3 and T2–T3, with a non-significant trend in T1–T2;
- Sternocleidomastoid (DOSL): There was a similar pattern, with the greatest drop observed in T1–T3 (p < 0.001).
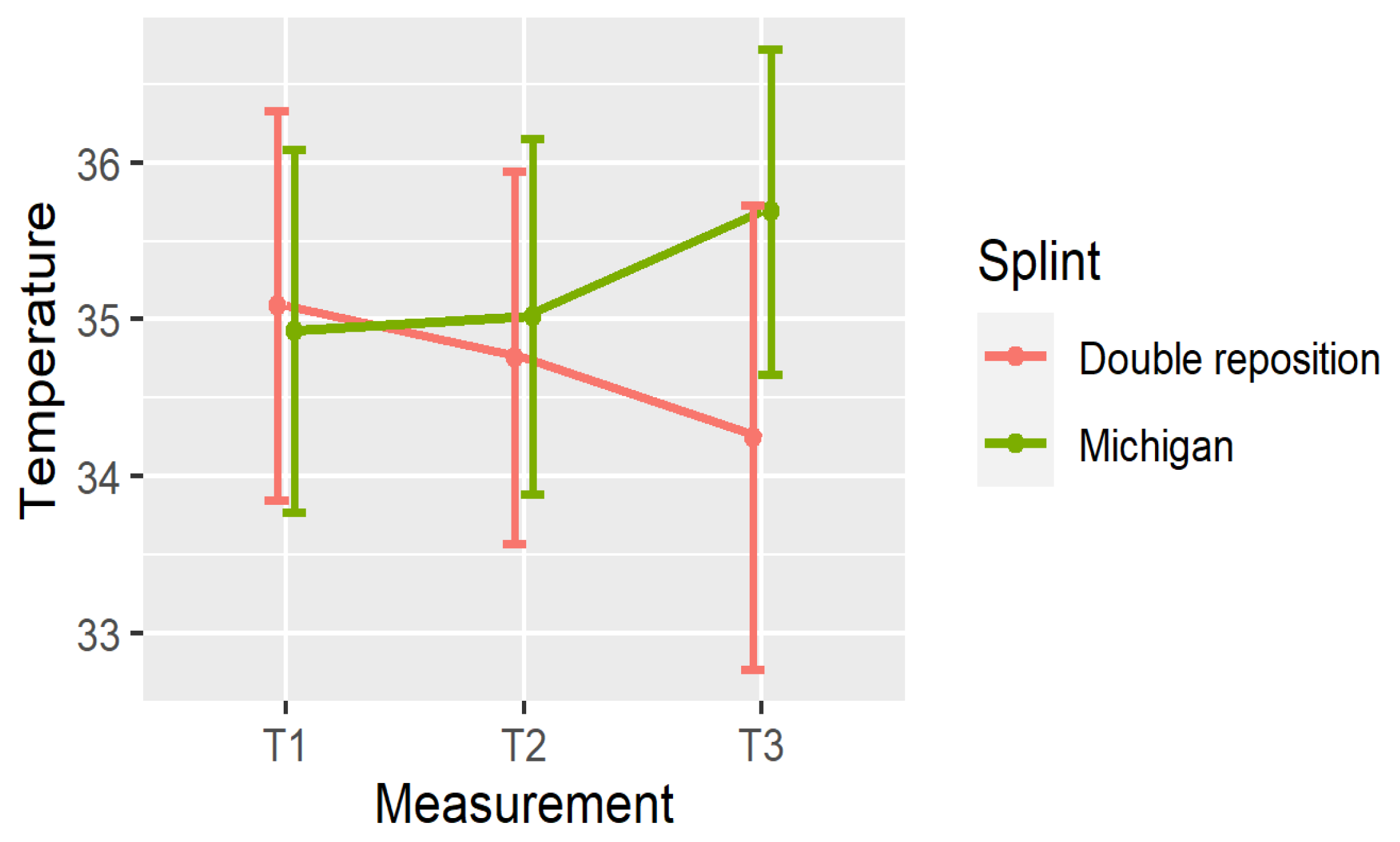
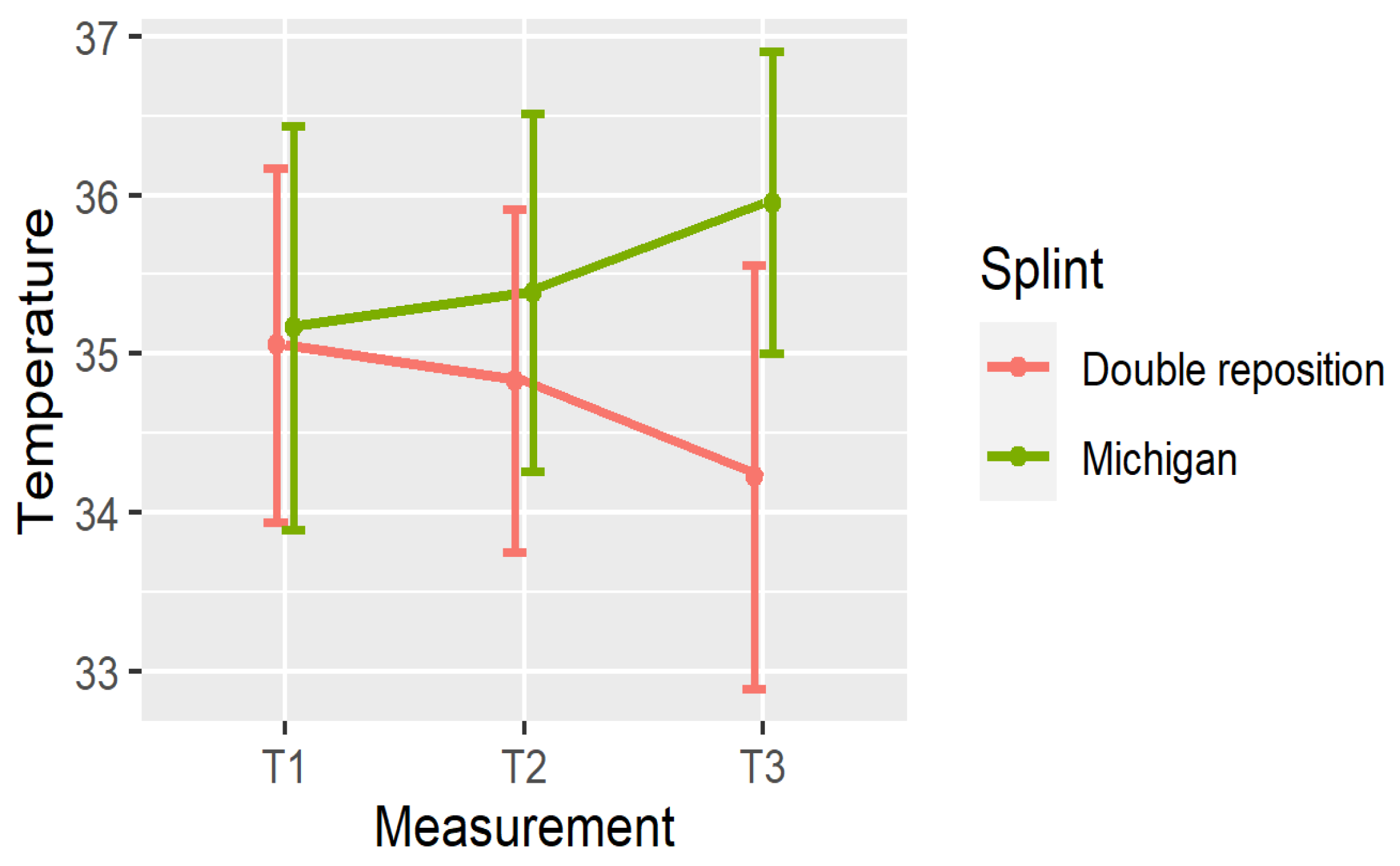
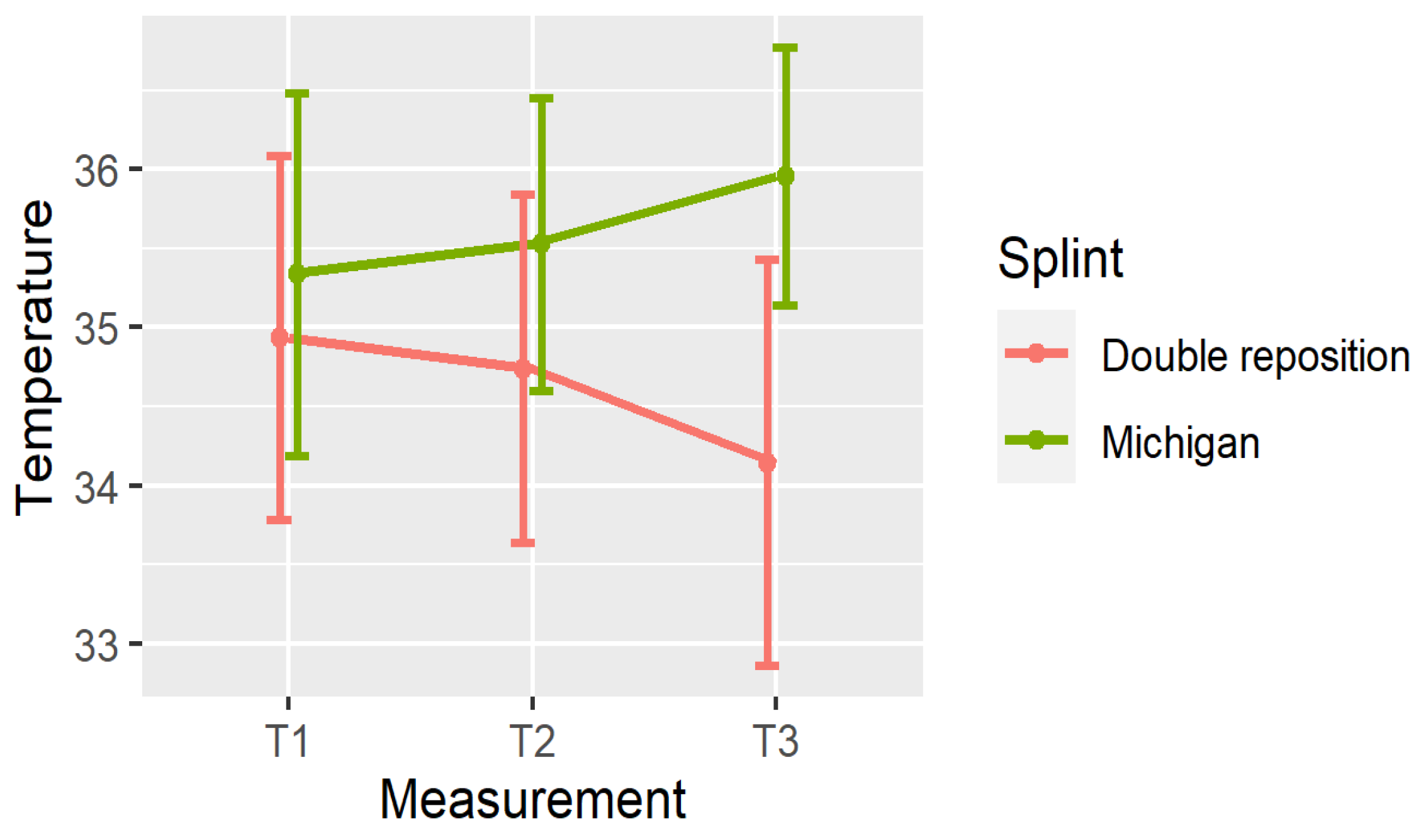
5. Comparison of the Splints
- In group D (double repositioning splint), temperature consistently decreased over time at all measured locations.
- In group M (Michigan splint), temperature consistently increased, particularly between T1 and T3.
6. Clinical Significance of Temperature Changes
7. Discussion
8. Conclusions
- Thermography using a thermal imaging camera is a precise, objective, and non-invasive diagnostic method that can be effectively applied to assess masticatory muscle tone in patients with temporomandibular disorders (TMDs).
- The type of occlusal splint significantly influences thermal patterns and, consequently, muscle activity.
- The double repositioning splint showed greater efficacy in reducing surface muscle temperature, suggesting its potential for short-term relief in patients with muscular or myofascial TMD.
- Further research should be conducted on larger and more diverse populations to confirm these findings and assess their generalizability across different age groups, sexes, and ethnic backgrounds.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCafferty, D.J.; Koprowski, R.; Herborn, K.; Tattersall, G.J.; Jerem, P.; Nord, A. Editorial: Advances in thermal imaging. J. Therm. Biol. 2021, 102, 103109. [Google Scholar] [CrossRef] [PubMed]
- Tattersall, G.J. Infrared thermography: A non-invasive window into thermal physiology. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef] [PubMed]
- Švantner, M.; Lang, V.; Skála, J.; Kohlschütter, T.; Honner, M.; Muzika, L.; Kosová, E. Statistical Study on Human Temperature Measurement by Infrared Thermography. Sensors 2022, 22, 8395. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.K.; Acharya, R. Remote-sensing infrared thermography. IEEE Eng. Med. Biol. 2009, 28, 76–83. [Google Scholar] [CrossRef]
- Hildebrandt, C.; Raschner, C.; Ammer, K. An Overview of Recent Application of Medical Infrared Thermography in Sports Medicine in Austria. Sensors 2010, 10, 4700–4715. [Google Scholar] [CrossRef]
- Liu, Q.; Li, M.; Wang, W.; Jin, S.; Piao, H.; Jiang, Y.; Li, N.; Yao, H. Infrared thermography in clinical practice: A literature review. Eur. J. Med. Res. 2025, 30, 33. [Google Scholar] [CrossRef] [PubMed]
- Antonaci, F.; Rossi, E.; Voiticovschi-Iosob, C.; Dalla Volta, G.; Marceglia, S. Frontal infrared thermography in healthy individuals and chronic migraine patients: Reliability of the method. Cephalalgia 2019, 39, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, G.; Capone, G.; Frize, M.; Zaffagnini, S.; Candrian, C.; Filardo, G. Cartilage. Infrared Thermography for the Evaluation of Inflammatory and Degenerative Joint Diseases: A Systematic Review. Cartilage 2021, 13 (Suppl. S2), 1790S–1801S. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.H.L.; Branco, R.L.L.; Siqueira, T.C.; de Souza, L.C.; Dalago, K.M.S.; Andrade, A. Clinical applicability of infrared thermography in rheumatic diseases: A systematic review. J. Therm. Biol. 2022, 104, 103172. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.; Souza Martins, L.; Freire, R.M.; . do Nascimento Silva, R.K.; Ferreira, J.J.A.; do Nascimento, J.A.; de Andrade, P.R. Accuracy of infrared thermography evaluation in burn wound healing: A systematic review and meta-analysis. J. Wound Care 2024, 33 (Suppl. S4a), cxviii–cxxix. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Yang, Q.; Han, D.; Chen, Z.; Cheng, Y. Application and prospects of infrared thermography in rheumatic diseases. Beijing Da Xue Xue Bao Yi Xue Ban = J. Peking University Health Sci. 2024, 56, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-S.; Cho, K.-J.; Park, S.-J.; Lee, B.N.; Hwang, Y.C.; Oh, W.M.; Hwang, I.N. Thermal analysis of bulk filled composite resin polymerization using various light curing modes according to the curing depth and approximation to the cavity wall. J. Appl. Oral Sci. 2013, 21, 293–299. [Google Scholar] [CrossRef]
- Mikulska, D.; Maleszka, R.; Parafiniuk, M. The usefulness of thermography as a diagnostic method in dermatology on the basis of clinical trials in 2001–2005. Ann. Acad. Medicae Stetin. 2006, 52, 91–97. [Google Scholar]
- Cavalheiro, A.L.; Costa, D.T.; Menezes, A.L.; Pereira, J.M.; Carvalho, E.M. Thermographic analysis and autonomic response in the hands of patients with leprosy. An. Bras. Dermatol. 2016, 91, 274–283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chudecka, M.; Lubkowska, A.; Leźnicka, K.; Krupecki, K. The Use of Thermal Imaging in the Evaluation of the Symmetry of Muscle Activity in Various Types of Exercises (Symmetrical and Asymmetrical). J. Hum. Kinet. 2015, 49, 141–147. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chudecka, M.; Lubkowska, A. The use of thermal imaging to evaluate body temperature changes of athletes during training and a study on the impact of physiological and morphological factors on skin temperature. Hum. Mov. 2012, 13, 33–39. [Google Scholar] [CrossRef]
- Chudecka, M.; Zaborski, D.; Lubkowska, A.; Grzesiak, W.; Modrzejewski, A.; Klimek, A. The Study of the Dynamics of Temperature Changes in the Selected Areas of Body Surface Induced by a 10-day Program of Systemic Cryostimulation with the Use of Thermovision. Aviat. Space Environ. Med. 2014, 85, 1170–1176. [Google Scholar] [CrossRef]
- Garstka, A.A.; Brzózka, M.; Bitenc-Jasiejko, A.; Ardan, R.; Gronwald, H.; Skomro, P.; Lietz-Kijak, D. Cause-effect relationships between painful TMD and postural and functional changes in the musculoskeletal system: A preliminary report. Pain. Res. Manag. 2022, 2022, 1429932. [Google Scholar] [CrossRef]
- Skorupska, E.; Rychlik, M.; Pawelec, W.; Bednarek, A.; Samborski, W. Trigger point-related sympathetic nerve activity in chronic sciatic leg pain: A case study. Acupunct. Med. 2014, 32, 418–422. [Google Scholar] [CrossRef]
- Kim, C. Consecutive digital infrared thermography in patients with temporomandibular joint arthralgia. Oral Biol. Res. 2019, 43, 32–39. [Google Scholar] [CrossRef]
- Pereira Americano, J.; Melo Pires, S.; Ambrósio Ferreira, L.; Lopes Devito, K. Can infrared thermography replace other methods of assessing orofacial pain intensity? Systematic review. BrJP 2024, 7, e20240049. [Google Scholar]
- Kitsoulis, P.; Marini, A.; Iliou, K.; Galani, V.; Zimpis, A.; Kanavaros, P.; Paraskevas, G. Signs and symptoms of temporomandibular joint disorders related to the degree of mouth opening and hearing loss. BMC Ear Nose Throat Disord. 2011, 11, 1–8. [Google Scholar] [CrossRef]
- Lietz-Kijak, D.; Kopacz, Ł.; Ardan, R.; Grzegocka, M.; Kijak, E. Assessment of the short- term effectiveness of kinesiotaping and trigger points release used in functional disorders of the masticatory muscles. Pain. Res. Med. 2018, 2018, 5464985. [Google Scholar] [CrossRef] [PubMed]
- Kopacz, Ł.; Ciosek, Ż.; Gronwald, H.; Skomro, P.; Ardan, R.; Lietz-Kijak, D. Comparative Analysis of the Influence of Selected Physical Factors on the Level of Pain in the Course of Temporomandibular Joint Disorders. Pain Res. Manag. 2020, 2020, 1036306. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Kijak, E.; Lietz-Kijak, D.; Śliwiński, Z.; Frączak, B. Muscle activity in the course of rehabilitation of masticatory motor system functional disorders. Adv. Hyg. Exp. Med. 2013, 67, 507–516. [Google Scholar] [CrossRef]
- Bodere, C.; Tea, S.H. Activity of masticatory muscles in subjects with different orofacial pain conditions. Pain 2005, 116, 33–41. [Google Scholar] [CrossRef]
- Peck, C.; Murray, G.; Gerzina, T. How does pain affect jaw muscle activity? The integrated pain adaptation model. Aust. Dent. J. 2008, 53, 201–207. [Google Scholar] [CrossRef]
- Fernandez-de-las-Penas, C.; Svensson, P. Myofascial temporomandibular disorder. Curr. Rheumatol. Rev. 2016, 12, 40–54. [Google Scholar]
- Fernandez-de-las-Penas, C.; Cuadrado, M.L.; Arendt-Nielsen, L.; Arendt-Nielsen, L.; Simons, D.G.; Pareja, J.A. Myofascial trigger points and sensitization: An updated pain model for tension-type headache. Cephalalgia 2007, 27, 383–393. [Google Scholar] [CrossRef]
- Ge, H.-Y.; Arendt-Nielsen, L. Latent myofascial trigger points. Curr. Pain Headache Rep. 2011, 15, 386–392. [Google Scholar] [CrossRef]
- Božović, D.; Ivkovic, N.; Račić, M.; Ristić, S. Salivary cortisol responses to acute stress in students with myofascial pain. Srp. Arh. Za Celok. Lek. 2018, 146, 20–25. [Google Scholar] [CrossRef]
- Jaeger, B. Myofascial trigger point pain. Alpha Omegan 2013, 106, 14–22. [Google Scholar] [PubMed]
- Fernandez-de-las-Penas, C.; Galan-del-Rio, F.; Fernandez-Carnero, J.; Pesquera, J.; Arendt-Nielsen, L.; Svensson, P. Bilateral widespread mechanical pain sensitivity in women with myofascial temporomandibular disorder: Evidence of impairment in central nociceptive processing. J. Pain 2009, 10, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Albagieh, H.; Alomran, I.; Binakresh, A.; Alhatarisha, N.; Almeteb, M.; Khalaf, Y.; Alqublan, A.; Alqahatany, M. Occlusal splints-types and effectiveness in temporomandibular disorder management. Saudi Dent J. 2023, 35, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Burns, R.H. TMD splint construction. J. Clin. Orthod. JCO 2022, 56, 384–389. [Google Scholar]
- Singh, B.P.; Singh, N.; Jayaraman, S.; Kirubakaran, R.; Joseph, S.; Muthu, M.S.; Jivnani, H.; Hua, F. Occlusal interventions for managing temporomandibular disorders. Cochrane Database Syst. Rev. 2024, 9, CD012850. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.N.; de Souza Ferreira, S.L.; Balata, P.M.M.; da Cunha, D.A.; Pernambuco, L.; da Silva, H.J. Thermography in complementary assessments of head and neck muscles: A scoping review. J. Oral Rehabil. 2022, 49, 1188–1196. [Google Scholar] [CrossRef]
- Manfredini, D.; Guarda-Nardini, L.; Wincour, E.; Piccotti, F.; Ahlberg, J.; Lobbezoo, F. Research diagnostic criteria for temporo- mandibular disorders. A systematic review of axis epidemiological findings. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, 453–462. [Google Scholar] [CrossRef]
- Costa, A.C.; Dibai Filho, A.V.; Packer, A.C.; Rodrigues-Bigaton, D. Intra and inter-rater reliability of infrared image analysis of masticatory and upper trapezius muscles in women with and without temporomandibular disorder. Braz. J. Phys. Ther. 2013, 17, 24–31. [Google Scholar] [CrossRef][Green Version]
- Rodrigues-Bigaton, D.; Dibai-Filho, A.V.; Packer, A.C.; Costa, A.C.; de Castro, E.M. Accuracy of two forms of infrared image analysis of the masticatory muscles in the diagnosis of myogenous temporomandibular disorder. J. Bodyw. Mov. Ther. 2014, 18, 49–55. [Google Scholar] [CrossRef]
- Haddad, D.S.; Brioschi, M.L.; Arita, E.S. Thermographic and clinical correlation of myofascial trigger points in the masticatory muscles. Dentomaxillofac. Radiol. 2012, 41, 621–629. [Google Scholar] [CrossRef]
- Magalhães, M.F.; Dibai-Filho, A.V.; de Oliveira Guirro, E.C.; Girasol, C.E.; de Oliveira, A.K.; Dias, F.R.C.; Guirro, R.R.D.J. Evolution of skin temperature after the application of compressive forces on tendon, muscle and myofascial trigger point. PLoS ONE 2015, 10, e0129034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, J.-Y.; Deng, D.-L.; He, B.-Y.; Tao, Y.; Niu, Y.-M.; Deng, M.-H. Efficacy of splint therapy for the management of temporomandibular disorders: A meta-analysis. Oncotarget 2016, 7, 84043–84053. [Google Scholar] [CrossRef] [PubMed]
- Clemente, M.; Mendes, J.; Moreira, A.; Vardasca, R.; Ferreira, A.; Amarante, J. Wind instrumentalists and temporomandibular disorder: From diagnosis to treatment. Dent. J. 2018, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, K.; Szyszka-Sommerfeld, L.; Trybek, G.; Piątkowska, D. Assessment of the sensitivity, specificity, and accuracy of thermography in identifying patients with TMD. Med. Sci. Monit. 2015, 21, 1485–1493. [Google Scholar]
- Barbosa, J.S.; Amorim, A.M.A.M.; Arruda, M.J.A.L.L.A.; Medeiros, G.B.S.; Freitas, A.P.L.F.; Vieira, L.E.M.; Melo, D.P.; Bemto, P.M. Infrared thermography assessment of patients with temporomandibular disorders. Dentomaxillofac. Radiol. 2020, 49, 20190392. [Google Scholar] [CrossRef]
- Clemente, M.; Coimbra, D.; Silva, A.; Aguiar Branco, C.; Pinho, J. Application of infrared thermal imaging in a violinist with temporomandibular disorder. Med. Probl. Perform. Artist. 2015, 30, 251–254. [Google Scholar] [CrossRef]
- Garstka, A.A.; Kozowska, L.; Kijak, K.; Brzózka, M.; Gronwald, H.; Skomro, P.; Lietz-Kijak, D. Accurate Diagnosis and Treatment of Painful Temporomandibular Disorders: A Literature Review Supplemented by Own Clinical Experience. Pain Res. Manag. 2023, 2023, 1002235. [Google Scholar] [CrossRef]
- Barão, V.A.R.; Gallo, A.K.G.; Zuim, P.R.J.; Garcia, A.R.; Assunção, W.G. Effect of occlusal splint treatment on the temperature of different muscles in patients with TMD. J. Prosthodont. Res. 2011, 55, 19–23. [Google Scholar] [CrossRef]
- de Melo, D.P.; Bento, P.M.; Peixoto, L.R.; Martins, S.K.L.D.; Martins, C.C. Is infrared thermography effective in the diagnosis of temporomandibular disorders? A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 185–192. [Google Scholar] [CrossRef]
- Dibai-Filho, A.V.; Costa, A.C.D.S.; Packer, A.C.; de Castro, E.M.; Rodrigues-Bigaton, D. Women with more severe degrees of temporomandibular disorder exhibit an increase in temperature over the temporomandibular joint. Saudi Dent. J. 2015, 27, 44–49. [Google Scholar] [CrossRef]
- Haddad, D.S.; Brioschi, M.L.; Vardasca, R.; Weber, M.; Crosato, E.M.; Arita, E.S. Thermographic characterization of masticatory muscle regions in volunteers with and without myogenous temporomandibular disorder: Preliminary results. Dentomaxillofac. Radiol. 2014, 43, 20130440. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Pain in the tissues surrounding the temporomandibular joint (TMJ) | Absence of pain in the TMJ region |
| Consent to avoid hot drinks and spicy foods | Lack of consent to follow dietary restrictions |
| Consent to avoid hot baths or microcirculation-stimulating activities | Lack of consent to avoid heat or circulatory stimulation |
| Age between 20 and 25 years | Age outside the 20–25 range |
| European origin (Polish ethnicity) | Non-European origin |
| Mean ± SD | [Min, Max] | 95% Conf. Interval | |
|---|---|---|---|
| group (D), double repositioning splint | |||
| N, T1 | 35.09 ± 1.24 | [31.8, 36.7] | (34.62, 35.55) |
| N, T2 | 34.76 ± 1.19 | [31.5, 36.4] | (34.31, 35.2) |
| N, T3 | 34.25 ± 1.48 | [30.1, 36.2] | (33.69, 34.8) |
| OSP, T1 | 35.05 ± 1.11 | [32, 36.6] | (34.64, 35.47) |
| OSP, T2 | 34.83 ± 1.08 | [31.9, 36.5] | (34.43, 35.23) |
| OSP, T3 | 34.22 ± 1.34 | [30.6, 36.4] | (33.72, 34.72) |
| OSL, T1 | 34.93 ± 1.15 | [32.1, 36.3] | (34.5, 35.36) |
| OSL, T2 | 34.74 ± 1.10 | [31.6, 36.2] | (34.33, 35.15) |
| OSL, T3 | 34.14 ± 1.28 | [31.1, 36.1] | (33.66, 34.62) |
| group (M), Michigan splint | |||
| N, T1 | 34.92 ± 1.16 | [32.1, 36.7] | (34.49, 35.36) |
| N, T2 | 35.02 ± 1.13 | [32.4, 36.9] | (34.6, 35.44) |
| N, T3 | 35.69 ± 1.04 | [33, 37.2] | (35.3, 36.07) |
| OSP, T1 | 35.16 ± 1.27 | [31.6, 36.9] | (34.69, 35.64) |
| OSP, T2 | 35.38 ± 1.13 | [33.1, 37.2] | (34.96, 35.8) |
| OSP, T3 | 35.95 ± 0.95 | [33.5, 37.8] | (35.6, 36.31) |
| OSL, T1 | 35.34 ± 1.15 | [32.6, 36.8] | (34.91, 35.77) |
| OSL, T2 | 35.53 ± 0.93 | [33.2, 36.9] | (35.18, 35.87) |
| OSL, T3 | 35.96 ± 0.81 | [34.3, 37.2] | (35.65, 36.26) |
| Mean | SD | p-Value | 95% CI | |
|---|---|---|---|---|
| group (D), double repositioning splint | ||||
| DN12 | −0.33 | 0.323 | <0.001 | (−0.451, −0.209) |
| DN13 | −0.84 | 0.625 | <0.001 | (−1.073, −0.607) |
| DN23 | −0.51 | 0.571 | <0.001 | (−0.723, −0.297) |
| DOSP12 | −0.223 | 0.578 | 0.129 | (−0.439, −0.008) |
| DOSP13 | −0.83 | 0.695 | <0.001 | (−1.089, −0.571) |
| DOSP23 | −0.607 | 0.642 | <0.001 | (−0.846, −0.367) |
| DOSL12 | −0.193 | 0.514 | 0.145 | (−0.385, −0.001) |
| DOSL13 | −0.79 | 0.557 | <0.001 | (−0.998, −0.582) |
| DOSL23 | −0.597 | 0.57 | <0.001 | (−0.810, −0.384) |
| group (M), Michigan splint | ||||
| DN12 | 0.095 | 0.398 | 0.603 | (−0.054, 0.244) |
| DN13 | 0.763 | 0.701 | <0.001 | (0.501, 1.024) |
| DN23 | 0.668 | 0.772 | <0.001 | (0.379, 0.956) |
| DOSP12 | 0.22 | 0.542 | 0.103 | (0.018, 0.422) |
| DOSP13 | 0.79 | 0.881 | <0.001 | (0.461, 1.119) |
| DOSP23 | 0.57 | 0.603 | <0.001 | (0.345, 0.795) |
| DOSL12 | 0.19 | 0.581 | 0.251 | (−0.027, 0.407) |
| DOSL13 | 0.62 | 0.694 | <0.001 | (0.361, 0.880) |
| DOSL23 | 0.43 | 0.549 | <0.001 | (0.225, 0.635) |
| Group (D), Double Repositioning Splint | Group (M), Michigan Splint | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | ||
| Temperature measurements | ||||||
| N, T1 | 35.09 | 1.24 | 34.92 | 1.158 | 0.604 | |
| N, T2 | 34.76 | 1.187 | 35.02 | 1.132 | 0.383 | |
| N, T3 | 34.25 | 1.483 | 35.69 | 1.036 | <0.001 | |
| OSP, T1 | 35.05 | 1.114 | 35.16 | 1.275 | 0.723 | |
| OSP, T2 | 34.83 | 1.079 | 35.38 | 1.128 | 0.057 | |
| OSP, T3 | 34.22 | 1.338 | 35.95 | 0.951 | <0.001 | |
| OSL, T1 | 34.93 | 1.149 | 35.34 | 1.147 | 0.179 | |
| OSL, T2 | 34.74 | 1.099 | 35.53 | 0.927 | 0.004 | |
| OSL, T3 | 34.14 | 1.284 | 35.96 | 0.815 | <0.001 | |
| Temperature change | ||||||
| DN12 | −0.33 | 0.323 | 0.095 | 0.398 | <0.001 | |
| DN13 | −0.84 | 0.625 | 0.76 | 0.701 | <0.001 | |
| DN23 | −0.51 | 0.571 | 0.67 | 0.772 | <0.001 | |
| DOSP12 | −0.223 | 0.578 | 0.22 | 0.542 | 0.009 | |
| DOSP13 | −0.83 | 0.695 | 0.79 | 0.881 | <0.001 | |
| DOSP23 | −0.607 | 0.642 | 0.57 | 0.603 | <0.001 | |
| DOSL12 | −0.193 | 0.514 | 0.19 | 0.581 | 0.027 | |
| DOSL13 | −0.79 | 0.557 | 0.62 | 0.694 | <0.001 | |
| DOSL23 | −0.597 | 0.570 | 0.43 | 0.549 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lietz-Kijak, D.; Garstka, A.A.; Szczucka, L.; Ardan, R.; Brzózka-Garstka, M.; Skomro, P.; D’Arcangelo, C. A Thermal Imaging Camera as a Diagnostic Tool to Study the Effects of Occlusal Splints on the Elimination of Masticatory Muscle Tension. Dent. J. 2025, 13, 313. https://doi.org/10.3390/dj13070313
Lietz-Kijak D, Garstka AA, Szczucka L, Ardan R, Brzózka-Garstka M, Skomro P, D’Arcangelo C. A Thermal Imaging Camera as a Diagnostic Tool to Study the Effects of Occlusal Splints on the Elimination of Masticatory Muscle Tension. Dentistry Journal. 2025; 13(7):313. https://doi.org/10.3390/dj13070313
Chicago/Turabian StyleLietz-Kijak, Danuta, Adam Andrzej Garstka, Lidia Szczucka, Roman Ardan, Monika Brzózka-Garstka, Piotr Skomro, and Camillo D’Arcangelo. 2025. "A Thermal Imaging Camera as a Diagnostic Tool to Study the Effects of Occlusal Splints on the Elimination of Masticatory Muscle Tension" Dentistry Journal 13, no. 7: 313. https://doi.org/10.3390/dj13070313
APA StyleLietz-Kijak, D., Garstka, A. A., Szczucka, L., Ardan, R., Brzózka-Garstka, M., Skomro, P., & D’Arcangelo, C. (2025). A Thermal Imaging Camera as a Diagnostic Tool to Study the Effects of Occlusal Splints on the Elimination of Masticatory Muscle Tension. Dentistry Journal, 13(7), 313. https://doi.org/10.3390/dj13070313







