Impact of Optical Coherence Tomography (OCT) for Periodontitis Diagnostics: Current Overview and Advances
Abstract
1. Introduction
2. Principles of Optical Coherence Tomography
- Fourier-domain OCT (FD-OCT): This technique captures the entire interference spectrum simultaneously using a spectrometer [24,37], and a Fourier transform is then applied to reconstruct the depth-resolved image [24,38]. FD-OCT provides significantly faster acquisition speeds compared to TD-OCT [24,37]. There are two main types of FD-OCT:
- ○
- Spectral-domain OCT (SD-OCT): Instead of a photo detector, SD-OCT employs a spectrometer to capture the image. The spectrometer records the entire optical spectrum of the backscattered light, utilizing all wavelengths to extract detailed information about the esamine tissue, and a Fourier transform is subsequently applied to generate the image [30]. SD-OCT enables cross-sectional imaging in the Fourier domain by measuring both the intensity of backscattered or reflected light and its time delay. Compared to TD-OCT, SD-OCT achieves higher imaging speed due to its non-mechanical scanning mechanism, offers superior axial resolution, and has also found applications in ophthalmology, cardiology, and dermatology [29].
- ○
- Swept-source OCT (SS-OCT): This technique employs a rapidly tunable laser that emits light at different wavelengths in quick succession, and the image is generated by analyzing the interference pattern as a function of wavelength variation [30]. Compared to SD-OCT, it has greater penetration depth, enhanced detection efficiency, extended imaging ranges, improved sensitivity with imaging depth, and dual-balanced detection capability [29].
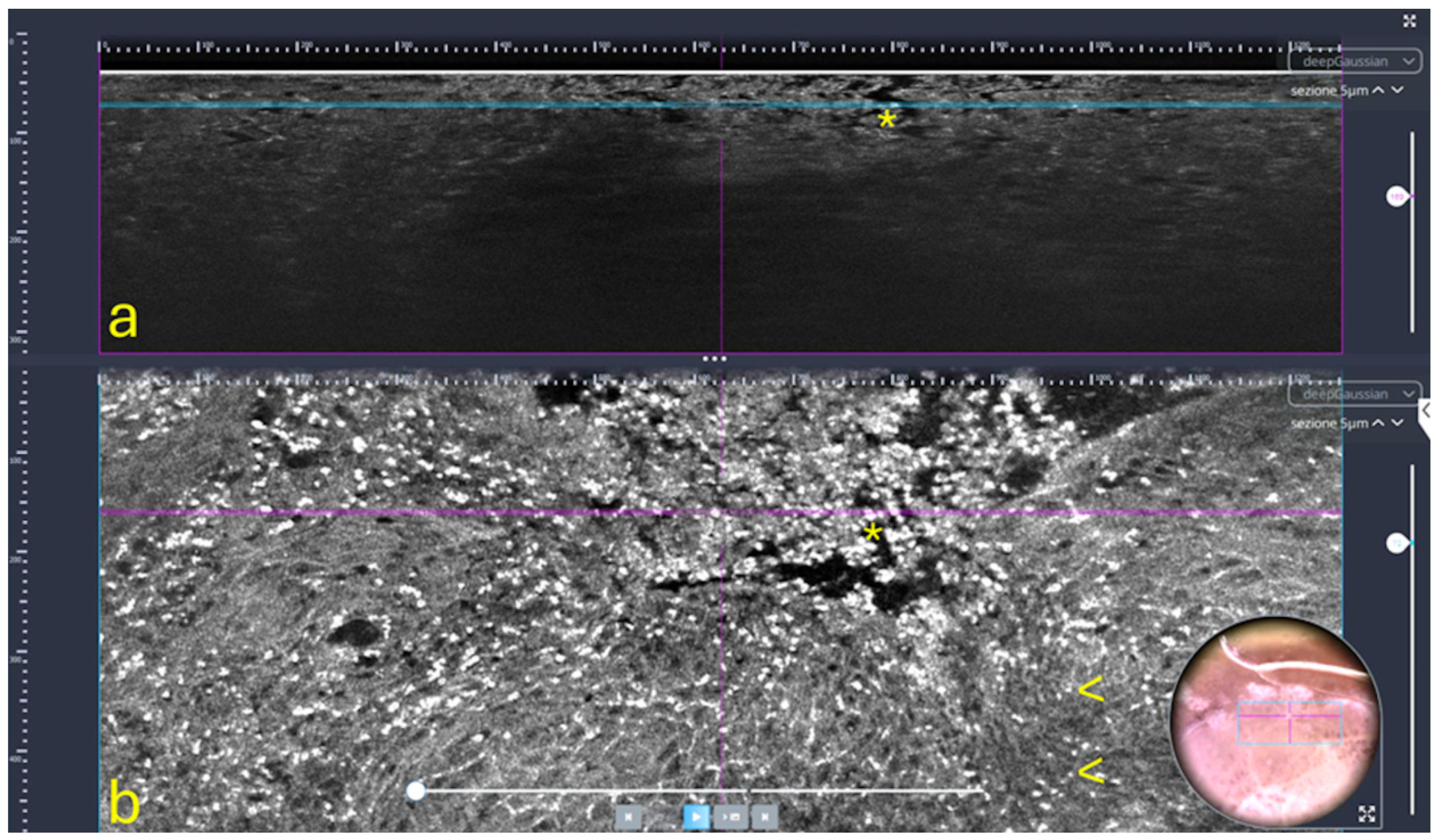
- Line-field confocal OCT (LC-OCT): This innovative technique is a recently developed non-invasive optical imaging technique designed for in vivo skin examination [39], which combines the principles of OCT and reflectance confocal microscopy (RCM), using line illumination and detection [40] (Figure 2). It provides high-resolution vertical images (B-scans), in real time from 8 to 10 frame/s, with an isotropic resolution of approximately 1 µm and a penetration depth of up to 500 µm [39,41,42]. LC-OCT is particularly well-suited for examining both healthy and pathological skin, enabling the visualization of cutaneous structures at the cellular level, including keratinocyte nuclei and the epidermal and dermal layers [39,41]. For this reason, it is used in the diagnosis, characterization, and therapeutic monitoring of various skin disorders, including benign and malignant skin tumors (such as melanoma, basal cell carcinoma, squamous cell carcinoma, and actinic keratosis), as well as inflammatory and infectious skin conditions [40]. AI and machine learning (ML) are emerging as essential tools for analyzing images obtained through LC-OCT and for detecting cutaneous anomalies. For instance, dedicated deep learning algorithms have been developed to assist in the analysis of these images, enabling the automatic segmentation of skin layers and keratinocyte nuclei. Moreover, AI, through convolutional neural networks, can assess the malignant potential of precancerous lesions, such as actinic keratoses (AK), by analyzing the undulation of the dermo-epidermal junction (DEJ) and quantifying cellular atypia [40].
3. OCT Applications in Periodontology
3.1. Assessment of Periodontal Tissue Structure
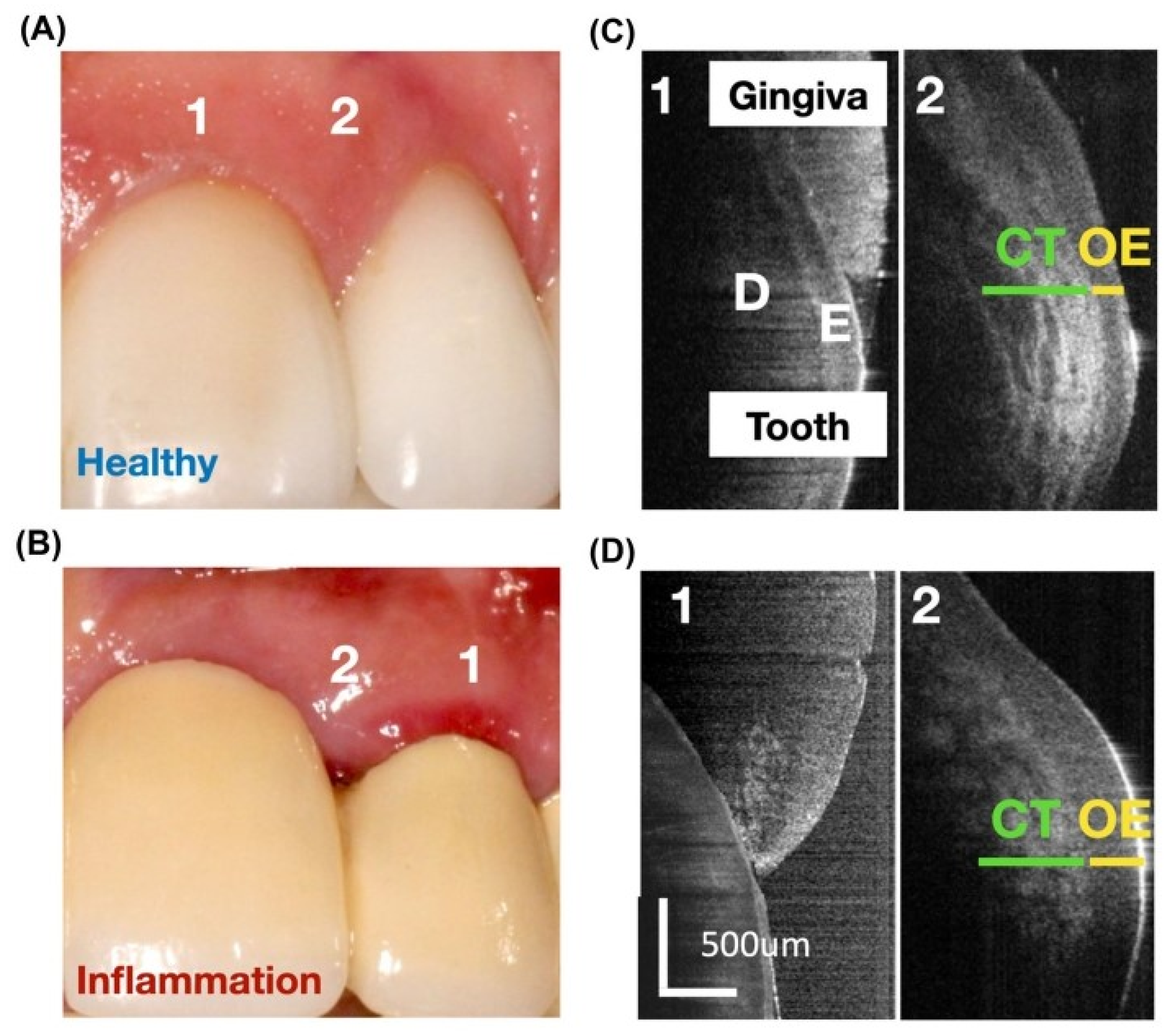
3.1.1. Gingiva
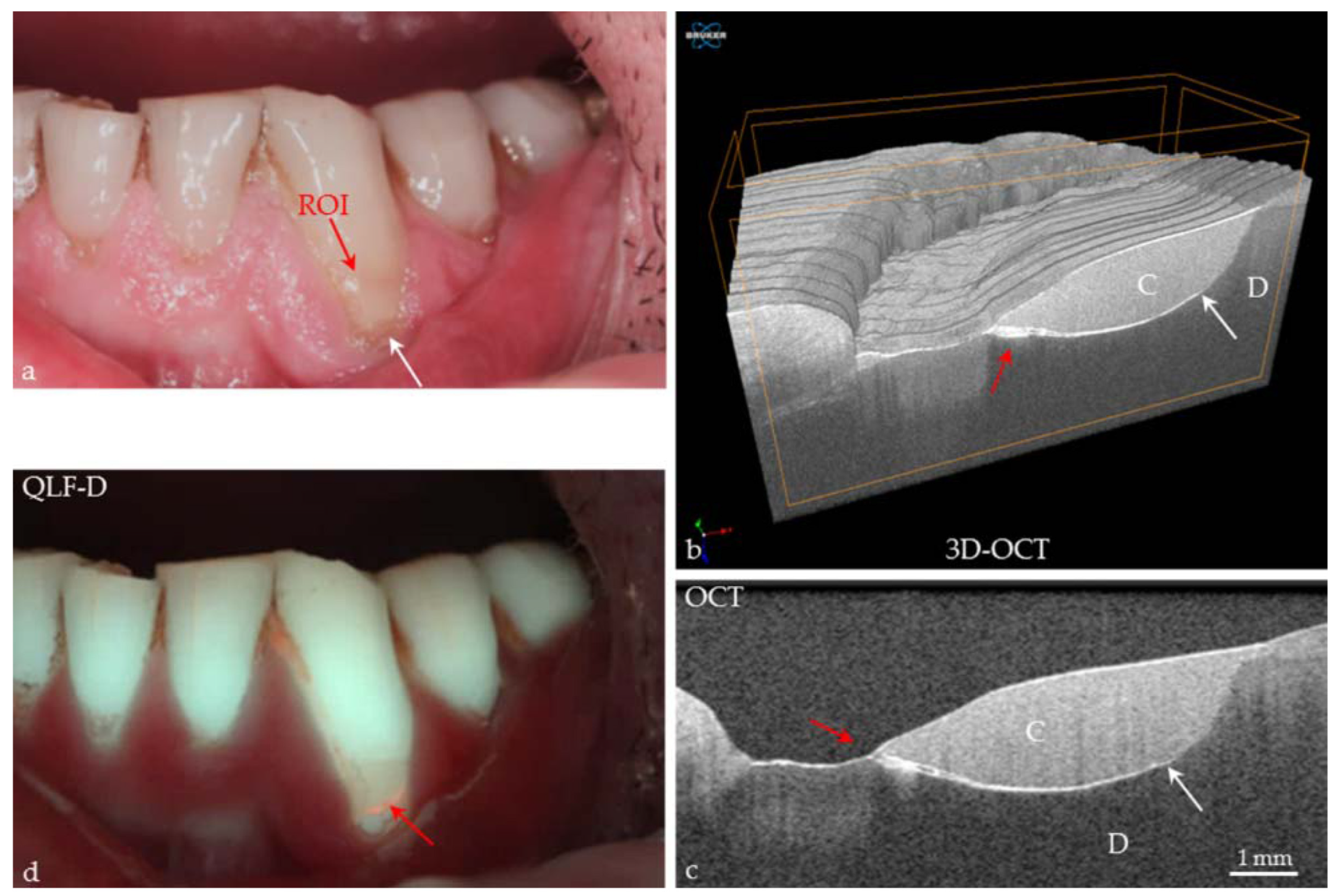
3.1.2. Alveolar Bone
3.1.3. Periodontal Ligament
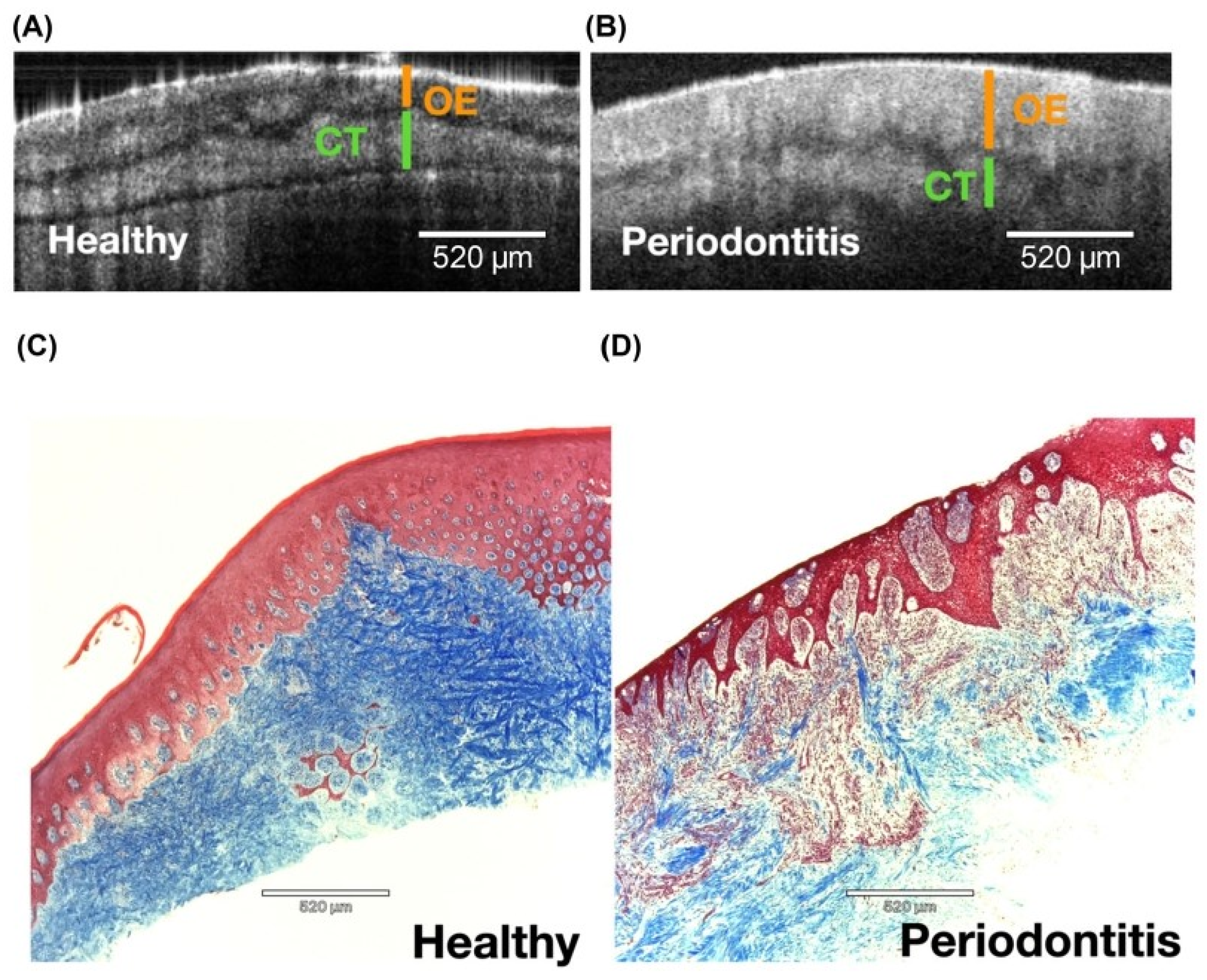
3.2. Detection of Periodontal Diseases
3.3. Evaluation of Periodontal Therapy Outcomes
3.3.1. Non-Surgical Periodontal Treatment
3.3.2. Regenerative Procedures
3.4. Monitoring of Peri-Implant Tissues
4. Advancements and Technological Innovations
4.1. High-Resolution 3D Imaging Capabilities
4.2. Integration of Artificial Intelligence and Machine Learning
4.3. Portable and Handheld OCT Devices
4.4. Potential for Real-Time Chair-Side Diagnostics
5. Challenges and Limitations
6. Future Perspectives
7. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OCT | Optical coherence tomography |
| 3D | Three-dimensional |
| CBCT | Cone-beam computed tomography |
| IU | Intraoral ultrasound |
| TD-OCT | Time-domain OCT |
| FD-OCT | Fourier-domain OCT |
| SD-OCT | Spectral-domain OCT |
| SS-OCT | Swept-source OCT |
| LC-OCT | Line-field confocal OCT |
| OCTA | OCT angiography |
| CBL | Crestal bone level |
| NAFLD | Non-alcoholic fatty liver disease |
| AI | Artificial intelligence |
| ML | Machine learning |
| CEJ | Cementoenamel junction |
| GT | Gingival thickness |
| MRI | Magnetic resonance imaging |
| IL-1β | Interleukin-1β |
| CNNs | Convolutional neural network |
| NLP | Natural language processing |
References
- Komori, T.; Kram, V.; Perry, S.; Pham, H.T.; Jani, P.; Kilts, T.M.; Watanabe, K.; Kim, D.G.; Martin, D.; Young, M.F. Type VI Collagen Deficiency Causes Enhanced Periodontal Tissue Destruction. J. Dent. Res. 2024, 103, 878–888. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Serra, S.; Boato, M.; Sculean, A. Relationship between periodontitis and systemic diseases: A bibliometric and visual study. Periodontol 2000 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Froum, S.; Wang, W.; Hafez, T.; Suzuki, T.; Yu, Y.; Cho, S.-C. Incision Design and Soft Tissue Management to Maintain or Establish an Interproximal Papilla Around Integrated Implants: A Case Series. Int. J. Periodontics Restor. Dent. 2018, 38, 61–69. [Google Scholar] [CrossRef]
- Jacobs, R.; Fontenele, R.C.; Lahoud, P.; Shujaat, S.; Bornstein, M.M. Radiographic Diagnosis of Periodontal Diseases—Current Evidence versus Innovations. Periodontology 2000 2024, 95, 51–69. [Google Scholar] [CrossRef]
- Corbet, E.; Ho, D.; Lai, S. Radiographs in Periodontal Disease Diagnosis and Management. Aust. Dent. J. 2009, 54, S27–S43. [Google Scholar] [CrossRef]
- Korostoff, J.; Aratsu, A.; Kasten, B.; Mupparapu, M. Radiologic Assessment of the Periodontal Patient. Dent. Clin. N. Am. 2016, 60, 91–104. [Google Scholar] [CrossRef]
- Wang, Z.; Song, J.H.; Kim, J.; Kwon, S.; Piao, X.; Oh, S.; Park, S.; Kim, S.; Ryu, J.; Kim, O.; et al. Metformin Reverses Periodontal Destruction Caused by Experimental Periodontitis by Inhibiting Interleukin-1β Activity. J. Periodontol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Huamán-Mendoza, A.A.; Pantigozo-Morán, F.L.; Da Silva, J.C.; Chuquimez-Ventura, C.V.; Holzhausen, M. Hotspots and Global Trends in Research of Host Immune Response in Periodontitis: A Bibliometric Analysis. J. Periodontol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Braun, X.; Ritter, L.; Jervøe-Storm, P.-M.; Frentzen, M. Diagnostic Accuracy of CBCT for Periodontal Lesions. Clin. Oral Investig. 2014, 18, 1229–1236. [Google Scholar] [CrossRef]
- Figueredo, C.A.; Lai, H.; Gibson, M.P.; Le, L.H.; Almeida, F.T.; Major, P.W. The Repeatability of Periodontal Imaging with Intraoral Ultrasound Scanning. Clin. Oral Investig. 2024, 28, 164. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; AlHadidi, A.; Vyas, R.; Bornstein, M.M.; Watanabe, H.; Tanaka, R. Nonionizing Diagnostic Imaging Modalities for Visualizing Health and Pathology of Periodontal and Peri-implant Tissues. Periodontology 2000 2024, 95, 87–101. [Google Scholar] [CrossRef]
- Figueredo, C.A.; Catunda, R.Q.; Gibson, M.P.; Major, P.W.; Almeida, F.T. Use of Ultrasound Imaging for Assessment of the Periodontium: A Systematic Review. J. Periodontal Res. 2024, 59, 3–17. [Google Scholar] [CrossRef]
- Gambino, A.; Martina, E.; Panzarella, V.; Ruggiero, T.; Haddad, G.E.; Broccoletti, R.; Arduino, P.G. Potential Use of Optical Coherence Tomography in Oral Potentially Malignant Disorders: In-Vivo Case Series Study. BMC Oral Health 2023, 23, 540. [Google Scholar] [CrossRef]
- Dai, X.; Tao, Y.; Zhou, J.; Zhou, Y.; Liang, S.; Ma, X. Global Burden and Trends of Severe Periodontitis among Women of Childbearing Age, 1990–2021. J. Periodontol. 2025. Online ahead of print. [Google Scholar] [CrossRef]
- Ali, S.; Gilani, S.B.S.; Shabbir, J.; Almulhim, K.S.; Bugshan, A.; Farooq, I. Optical Coherence Tomography’s Current Clinical Medical and Dental Applications: A Review. F1000Research 2021, 10, 310. [Google Scholar] [CrossRef]
- Song, D.; He, J.; Cheng, T.; Jin, L.; Li, S.; Chen, B.; Li, Y.; Liao, C. Cystathionine Γ-lyase Contributes to Exacerbation of Periodontal Destruction in Experimental Periodontitis under Hyperglycemia. J. Periodontol. 2025, 96, 255–267. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, G.; Zhang, J. Optical Coherence Tomography: Promising Imaging Technique for the Diagnosis of Oral Mucosal Diseases. Oral Dis. 2024, 30, 3638–3651. [Google Scholar] [CrossRef]
- Park, J.-H.; Son, K.-B.-D.; Son, Y.-T.; Kim, Y.-G.; Hwang, S.-M.; Hwang, J.-H.; Lee, J.-H.; Kim, H.-D.; Lee, K.-B.; Lee, J.-M. Comparative Evaluation of Laser System to Conventional Surgical Approaches in Periodontal Healing Using Optical Coherence Tomography. Appl. Sci. 2024, 14, 8854. [Google Scholar] [CrossRef]
- Putra, R.H.; Yoda, N.; Astuti, E.R.; Sasaki, K. Potential Imaging Capability of Optical Coherence Tomography as Dental Optical Probe: A Mini-Review. Appl. Sci. 2021, 11, 11025. [Google Scholar] [CrossRef]
- Lai, Y.-C.; Chiu, C.-H.; Cai, Z.-Q.; Lin, J.-Y.; Yao, C.-Y.; Lyu, D.-Y.; Lee, S.-Y.; Chen, K.-W.; Chen, I.-Y. OCT-Based Periodontal Inspection Framework. Sensors 2019, 19, 5496. [Google Scholar] [CrossRef]
- Krause, F.; Schmalz, G.; Park, K.J.; Schmidt, J.; Ziebolz, D.; Schneider, H.; Haak, R. Evaluation of Calculus Imaging on Root Surfaces by Spectral-Domain Optical Coherence Tomography. Photodiagnosis Photodyn. Ther. 2019, 25, 275–279. [Google Scholar] [CrossRef]
- Fernandes, L.O.; Mota, C.C.B.D.O.; Oliveira, H.O.; Neves, J.K.; Santiago, L.M.; Gomes, A.S.L. Optical Coherence Tomography Follow-up of Patients Treated from Periodontal Disease. J. Biophotonics 2019, 12, e201800209. [Google Scholar] [CrossRef]
- Fernandes, L.O.; Mota, C.C.B.O.; De Melo, L.S.A.; Da Costa Soares, M.U.S.; Da Silva Feitosa, D.; Gomes, A.S.L. In Vivo Assessment of Periodontal Structures and Measurement of Gingival Sulcus with Optical Coherence Tomography: A Pilot Study. J. Biophotonics 2017, 10, 862–869. [Google Scholar] [CrossRef]
- Park, J.-Y.; Chung, J.-H.; Lee, J.-S.; Kim, H.-J.; Choi, S.-H.; Jung, U.-W. Comparisons of the Diagnostic Accuracies of Optical Coherence Tomography, Micro-Computed Tomography, and Histology in Periodontal Disease: An Ex Vivo Study. J. Periodontal Implant. Sci. 2017, 47, 30. [Google Scholar] [CrossRef]
- Baek, J.H.; Na, J.; Lee, B.H.; Choi, E.; Son, W.S. Optical Approach to the Periodontal Ligament under Orthodontic Tooth Movement: A Preliminary Study with Optical Coherence Tomography. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 252–259. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kang, S.-R.; Park, H.-J.; Kim, J.-M.; Yi, W.-J.; Kim, T.-I. Improved Accuracy in Periodontal Pocket Depth Measurement Using Optical Coherence Tomography. J. Periodontal Implant. Sci. 2017, 47, 13. [Google Scholar] [CrossRef]
- Chang, W.-T.; Lyu, D.-Y.; Lai, Y.-L.; Yen, J.-Y.; Chen, Y.-C.; Lee, S.-Y. High-Precision and Non-Invasive Measurement of Crestal Bone Level by Optical Coherence Tomography. J. Dent. Sci. 2025, 20, 147–153. [Google Scholar] [CrossRef]
- Saggu, A.; Maguluri, G.; Grimble, J.; Park, J.; Hasturk, H.; Iftimia, N.; Sima, C. Raman Microspectroscopy/Micro-optical Coherence Tomography Approach for Chairside Diagnosis of Periodontal Diseases: A Pilot Study. J. Periodontol. 2022, 93, 1929–1939. [Google Scholar] [CrossRef]
- Varghese, M.; Varghese, S.; Preethi, S. Revolutionizing Medical Imaging: A Comprehensive Review of Optical Coherence Tomography (OCT). J. Opt. 2024, 1–18. [Google Scholar] [CrossRef]
- Janjua, O.S.; Jeelani, W.; Khan, M.I.; Qureshi, S.M.; Shaikh, M.S.; Zafar, M.S.; Khurshid, Z. Use of Optical Coherence Tomography in Dentistry. Int. J. Dent. 2023, 2023, 4179210. [Google Scholar] [CrossRef]
- Park, B.H.; Pierce, M.C.; Cense, B.; de Boer, J. Real-Time Multi-Functional Optical Coherence Tomography. Opt. Express 2003, 11, 782–793. [Google Scholar] [CrossRef]
- Wang, L.; Fu, R.; Xu, C.; Xu, M. Methods and Applications of Full-Filed Optical Coherence Tomography: A Review. J. Biomed. Opt. 2022, 27, 050901. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Chuang, C.-C.; Tsai, J.; Lin, K.-F.; Sun, C.-W. Dental Optical Coherence Tomography. Sensors 2013, 13, 8928–8949. [Google Scholar] [CrossRef]
- Gambino, A.; Cafaro, A.; Broccoletti, R.; Turotti, L.; Karimi, D.; Haddad, G.E.; Hopper, C.; Porter, S.R.; Chiusa, L.; Arduino, P.G. In Vivo Evaluation of Traumatic and Malignant Oral Ulcers with Optical Coherence Tomography: A Comparison between Histopathological and Ultrastructural Findings. Photodiagnosis Photodyn. Ther. 2022, 39, 103019. [Google Scholar] [CrossRef]
- Choma, M.; Sarunic, M.; Yang, C.; Izatt, J. Sensitivity Advantage of Swept Source and Fourier Domain Optical Coherence Tomography. Opt. Express 2003, 11, 2183. [Google Scholar] [CrossRef]
- Liu, B.; Brezinski, M.E. Theoretical and Practical Considerations on Detection Performance of Time Domain, Fourier Domain, and Swept Source Optical Coherence Tomography. J. Biomed. Opt. 2007, 12, 044007. [Google Scholar] [CrossRef]
- Bouma, B.E.; Yun, S.-H.; Vakoc, B.J.; Suter, M.J.; Tearney, G.J. Fourier-Domain Optical Coherence Tomography: Recent Advances toward Clinical Utility. Curr. Opin. Biotechnol. 2009, 20, 111–118. [Google Scholar] [CrossRef]
- Hillmann, D.; Bonin, T.; Lührs, C.; Franke, G.; Hagen-Eggert, M.; Koch, P.; Hüttmann, G. Common Approach for Compensation of Axial Motion Artifacts in Swept-Source OCT and Dispersion in Fourier-Domain OCT. Opt. Express 2012, 20, 6761. [Google Scholar] [CrossRef]
- Verzì, A.E.; Broggi, G.; Micali, G.; Sorci, F.; Caltabiano, R.; Lacarrubba, F. Line-field Confocal Optical Coherence Tomography of Psoriasis, Eczema and Lichen Planus: A Case Series with Histopathological Correlation. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1884–1889. [Google Scholar] [CrossRef]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.-L.; Dubois, A. Line-Field Confocal Optical Coherence Tomography (LC-OCT) for Skin Imaging in Dermatology. Life 2023, 13, 2268. [Google Scholar] [CrossRef]
- Monnier, J.; Tognetti, L.; Miyamoto, M.; Suppa, M.; Cinotti, E.; Fontaine, M.; Perez, J.; Orte Cano, C.; Yélamos, O.; Puig, S.; et al. In Vivo Characterization of Healthy Human Skin with a Novel, Non-invasive Imaging Technique: Line-field Confocal Optical Coherence Tomography. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2914–2921. [Google Scholar] [CrossRef]
- Dubois, A.; Levecq, O.; Azimani, H.; Siret, D.; Barut, A.; Suppa, M.; Del Marmol, V.; Malvehy, J.; Cinotti, E.; Rubegni, P.; et al. Line-Field Confocal Optical Coherence Tomography for High-Resolution Noninvasive Imaging of Skin Tumors. J. Biomed. Opt. 2018, 23, 106007. [Google Scholar] [CrossRef]
- Erdelyi, R.-A.; Duma, V.-F.; Sinescu, C.; Dobre, G.M.; Bradu, A.; Podoleanu, A. Dental Diagnosis and Treatment Assessments: Between X-Rays Radiography and Optical Coherence Tomography. Materials 2020, 13, 4825. [Google Scholar] [CrossRef]
- Kang, S.-R.; Kim, J.-M.; Kim, S.-H.; Park, H.-J.; Kim, T.-I.; Yi, W.-J. Tooth Cracks Detection and Gingival Sulcus Depth Measurement Using Optical Coherence Tomography. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; IEEE: Jeju, Republic of Korea, 2017; pp. 4403–4406. [Google Scholar]
- Di Stasio, D.; Lauritano, D.; Iquebal, H.; Romano, A.; Gentile, E.; Lucchese, A. Measurement of Oral Epithelial Thickness by Optical Coherence Tomography. Diagnostics 2019, 9, 90. [Google Scholar] [CrossRef]
- Kakizaki, S.; Aoki, A.; Tsubokawa, M.; Lin, T.; Mizutani, K.; Koshy, G.; Sadr, A.; Oda, S.; Sumi, Y.; Izumi, Y. Observation and Determination of Periodontal Tissue Profile Using Optical Coherence Tomography. J. Periodontal Res. 2018, 53, 188–199. [Google Scholar] [CrossRef]
- Mota, C.C.B.O.; Fernandes, L.O.; Cimões, R.; Gomes, A.S.L. Non-Invasive Periodontal Probing Through Fourier-Domain Optical Coherence Tomography. J. Periodontol. 2015, 86, 1087–1094. [Google Scholar] [CrossRef]
- Le, N.M.; Song, S.; Zhou, H.; Xu, J.; Li, Y.; Sung, C.; Sadr, A.; Chung, K.; Subhash, H.M.; Kilpatrick, L.; et al. A Noninvasive Imaging and Measurement Using Optical Coherence Tomography Angiography for the Assessment of Gingiva: An in Vivo Study. J. Biophotonics 2018, 11, e201800242. [Google Scholar] [CrossRef]
- Kim, S.-H.; Kim, J.; Yang, S.; Oh, S.-H.; Lee, S.-P.; Yang, H.J.; Kim, T.-I.; Yi, W.-J. Automatic and Quantitative Measurement of Alveolar Bone Level in OCT Images Using Deep Learning. Biomed. Opt. Express 2022, 13, 5468. [Google Scholar] [CrossRef]
- Colston, B.W., Jr.; Everett, M.J.; Da Silva, L.B.; Otis, L.L.; Nathel, H. Optical Coherence Tomography for Diagnosing Periodontal Disease. In Lasers in Dentistry III, Proceedings of the SPIE Photonics West 1997, San Jose, CA, USA, 8–14 February 1997; Wigdor, H.A., Featherstone, J.D.B., Rechmann, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 216–220. [Google Scholar]
- Isola, G.; Polizzi, A.; Santagati, M.; Alibrandi, A.; Iorio-Siciliano, V.; Ramaglia, L. Effect of Nonsurgical Mechanical Debridement with or Without Chlorhexidine Formulations in the Treatment of Peri-Implant Mucositis. A Randomized Placebo-Controlled Clinical Trial. Clin. Oral Implant. Res. 2025, 36, 566–577. [Google Scholar] [CrossRef]
- Kao, M.-C.; Lin, C.-L.; Kung, C.-Y.; Huang, Y.-F.; Kuo, W.-C. Miniature Endoscopic Optical Coherence Tomography for Calculus Detection. Appl. Opt. 2015, 54, 7419. [Google Scholar] [CrossRef]
- Tsubokawa, M.; Aoki, A.; Kakizaki, S.; Taniguchi, Y.; Ejiri, K.; Mizutani, K.; Koshy, G.; Akizuki, T.; Oda, S.; Sumi, Y.; et al. In Vitro and Clinical Evaluation of Optical Coherence Tomography for the Detection of Subgingival Calculus and Root Cementum. J. Oral Sci. 2018, 60, 418–427. [Google Scholar] [CrossRef]
- Hsieh, Y.-S.; Ho, Y.-C.; Lee, S.-Y.; Lu, C.-W.; Jiang, C.-P.; Chuang, C.-C.; Wang, C.-Y.; Sun, C.-W. Subgingival Calculus Imaging Based on Swept-Source Optical Coherence Tomography. J. Biomed. Opt. 2011, 16, 071409. [Google Scholar] [CrossRef]
- Șurlin, P.; Camen, A.; Stratul, S.I.; Roman, A.; Gheorghe, D.-N.; Herăscu, E.; Osiac, E.; Rogoveanu, I. Optical Coherence Tomography Assessment of Gingival Epithelium Inflammatory Status in Periodontal—Systemic Affected Patients. Ann. Anat.-Anat. Anz. 2018, 219, 51–56. [Google Scholar] [CrossRef]
- Surlin, P.; Didilescu, A.C.; Lazar, L.; Arsenie, C.C.; Camen, A.; Popescu, D.M.; Gheorghe, D.N.; Osiac, E.; Rogoveanu, I. Evaluation Through the Optical Coherence Tomography Analysis of the Influence of Non-Alcoholic Fatty Liver Disease on the Gingival Inflammation in Periodontal Patients. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 2935–2942. [Google Scholar] [CrossRef]
- Fernandes, L.O.; Graça, N.D.R.L.; Melo, L.S.A.; Silva, C.H.V.; Gomes, A.S.L. Monitoring the Gingival Regeneration after Aesthetic Surgery with Optical Coherence Tomography. In Lasers in Dentistry XXII, Proceedings of the SPIE Photonics West 2016, 13–18 February 2016, San Francisco, CA, USA; Rechmann, P., Fried, D., Eds.; SPIE: San Francisco, CA, USA, 2016; p. 96920Q. [Google Scholar]
- Graça, N.D.R.L.; Palmeira, A.R.D.B.L.S.; Fernandes, L.O.; Pedrosa, M.D.S.; Guimarães, R.P.; Dos Santos, S.C.; Gomes, A.S.L.; Da Silva, C.H.V. In Vivo Optical Coherence Tomographic Imaging to Monitor Gingival Recovery and the Adhesive Interface in Aesthetic Oral Rehabilitation: A Case Report. Imaging Sci. Dent. 2019, 49, 171. [Google Scholar] [CrossRef]
- Le, N.; Cheng, H.; Subhash, H.; Kilpatrick-Liverman, L.; Wang, R.K. Gingivitis Resolution Followed by Optical Coherence Tomography and Fluorescence Imaging: A Case Study. J. Biophotonics 2021, 14, e202100191. [Google Scholar] [CrossRef]
- Solomon, S.M.; Timpu, D.; Forna, D.A.; Stefanache, M.A.M.; Martu, S.; Stoleriu, S. AFM Comparative Study of Root Surface Morphology After Three Methods of Scaling. Mater. Plast. 2016, 53, 546–549. [Google Scholar]
- Sanda, M.; Shiota, M.; Imakita, C.; Sakuyama, A.; Kasugai, S.; Sumi, Y. The Effectiveness of Optical Coherence Tomography for Evaluating Peri-Implant Tissue: A Pilot Study. Imaging Sci. Dent. 2016, 46, 173. [Google Scholar] [CrossRef]
- Kim, S.; Kang, S.-R.; Park, H.-J.; Kim, B.; Kim, T.-I.; Yi, W.-J. Quantitative Measurement of Peri-Implant Bone Defects Using Optical Coherence Tomography. J. Periodontal Implant. Sci. 2018, 48, 84. [Google Scholar] [CrossRef]
- Elashiry, M.; Meghil, M.M.; Arce, R.M.; Cutler, C.W. From Manual Periodontal Probing to Digital 3-D Imaging to Endoscopic Capillaroscopy: Recent Advances in Periodontal Disease Diagnosis. J. Periodontal Res. 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Li, K.; Yang, J.; Liang, W.; Li, X.; Zhang, C.; Chen, L.; Wu, C.; Zhang, X.; Xu, Z.; Wang, Y.; et al. O-PRESS: Boosting OCT Axial Resolution with Prior Guidance, Recurrence, and Equivariant Self-Supervision. Med. Image Anal. 2025, 99, 103319. [Google Scholar] [CrossRef]
- Jerjes, W.; Stevenson, H.; Ramsay, D.; Hamdoon, Z. Enhancing Oral Cancer Detection: A Systematic Review of the Diagnostic Accuracy and Future Integration of Optical Coherence Tomography with Artificial Intelligence. J. Clin. Med. 2024, 13, 5822. [Google Scholar] [CrossRef]
- Won, J.; Huang, P.-C.; Spillman, D.R.; Chaney, E.J.; Adam, R.; Klukowska, M.; Barkalifa, R.; Boppart, S.A. Handheld Optical Coherence Tomography for Clinical Assessment of Dental Plaque and Gingiva. J. Biomed. Opt. 2020, 25, 116011. [Google Scholar] [CrossRef]
- Pitchika, V.; Büttner, M.; Schwendicke, F. Artificial Intelligence and Personalized Diagnostics in Periodontology: A Narrative Review. Periodontology 2000 2024, 95, 220–231. [Google Scholar] [CrossRef]
- Kapoor, R.; Whigham, B.T.; Al-Aswad, L.A. Artificial Intelligence and Optical Coherence Tomography Imaging. Asia-Pac. J. Ophthalmol. 2019, 8, 187–194. [Google Scholar] [CrossRef]
- Șalgău, C.A.; Morar, A.; Zgarta, A.D.; Ancuța, D.-L.; Rădulescu, A.; Mitrea, I.L.; Tănase, A.O. Applications of Machine Learning in Periodontology and Implantology: A Comprehensive Review. Ann. Biomed. Eng. 2024, 52, 2348–2371. [Google Scholar] [CrossRef]
- James, B.L.; Sunny, S.P.; Heidari, A.E.; Ramanjinappa, R.D.; Lam, T.; Tran, A.V.; Kankanala, S.; Sil, S.; Tiwari, V.; Patrick, S.; et al. Validation of a Point-of-Care Optical Coherence Tomography Device with Machine Learning Algorithm for Detection of Oral Potentially Malignant and Malignant Lesions. Cancers 2021, 13, 3583. [Google Scholar] [CrossRef]
- Jundaeng, J.; Chamchong, R.; Nithikathkul, C. Periodontitis Diagnosis: A Review of Current and Future Trends in Artificial Intelligence. Technol. Health Care 2025, 33, 473–484. [Google Scholar] [CrossRef]
- Bonny, T.; Al Nassan, W.; Obaideen, K.; Al Mallahi, M.N.; Mohammad, Y.; El-damanhoury, H.M. Contemporary Role and Applications of Artificial Intelligence in Dentistry. F1000Research 2023, 12, 1179. [Google Scholar] [CrossRef]
- Jung, W.; Kim, J.; Jeon, M.; Chaney, E.J.; Stewart, C.N.; Boppart, S.A. Handheld Optical Coherence Tomography Scanner for Primary Care Diagnostics. IEEE Trans. Biomed. Eng. 2011, 58, 741–744. [Google Scholar] [CrossRef]
- Monroy, G.L.; Won, J. Clinical Translation of Handheld Optical Coherence Tomography: Practical Considerations and Recent Advancements. J. Biomed. Opt. 2017, 22, 1–30. [Google Scholar] [CrossRef]
- Song, G.; Jelly, E.T.; Chu, K.K.; Kendall, W.Y.; Wax, A. A Review of Low-Cost and Portable Optical Coherence Tomography. Prog. Biomed. Eng. 2021, 3, 032002. [Google Scholar] [CrossRef]
- Li, D.; Ran, A.R.; Cheung, C.Y.; Prince, J.L. Deep Learning in Optical Coherence Tomography: Where Are the Gaps? Clin. Exp. Ophthalmol. 2023, 51, 853–863. [Google Scholar] [CrossRef]
- Bayer, A.; Akman, A. Artifacts and Anatomic Variations in Optical Coherence Tomography. Turk. J. Ophthalmol. 2020, 50, 99–106. [Google Scholar] [CrossRef]
- Sampson, D.M.; Dubis, A.M.; Chen, F.K.; Zawadzki, R.J.; Sampson, D.D. Towards Standardizing Retinal Optical Coherence Tomography Angiography: A Review. Light. Sci. Appl. 2022, 11, 63. [Google Scholar] [CrossRef]
- Reeß, L.G.; Salih, H.; Delikaya, M.; Paul, F.; Oertel, F.C. Barriers in Healthcare to the Use of Optical Coherence Tomography Angiography in Multiple Sclerosis. Neurol. Ther. 2025, 14, 45–56. [Google Scholar] [CrossRef]
- Untracht, G.R.; Chen, M.; Wijesinghe, P.; Mas, J.; Yura, H.T.; Marti, D.; Andersen, P.E.; Dholakia, K. Spatially Offset Optical Coherence Tomography: Leveraging Multiple Scattering for High-Contrast Imaging at Depth in Turbid Media. Sci. Adv. 2023, 9, eadh5435. [Google Scholar] [CrossRef]
- Badon, A.; Li, D.; Lerosey, G.; Boccara, A.C.; Fink, M.; Aubry, A. Smart Optical Coherence Tomography for Ultra-Deep Imaging through Highly Scattering Media. Sci. Adv. 2016, 2, e1600370. [Google Scholar] [CrossRef]
- Fujimoto, J.; Swanson, E. The Development, Commercialization, and Impact of Optical Coherence Tomography. Investig. Opthalmology Vis. Sci. 2016, 57, OCT1–OCT13. [Google Scholar] [CrossRef]
- Parihar, A.S.; Narang, S.; Tyagi, S.; Narang, A.; Dwivedi, S.; Katoch, V.; Laddha, R. Artificial Intelligence in Periodontics: A Comprehensive Review. J. Pharm. Bioallied Sci. 2024, 16, S1956–S1958. [Google Scholar] [CrossRef]
- Dimofte, R.A.; Popescu, D.M.; Dragomir, L.P.; Mărășescu, P.C.; Nicolae, F.M.; Gheorghe, D.N.; Foia, I.; Osiac, E.; Manolea, H.O.; Popescu, M.R.; et al. Evaluation of Gingival Epithelial Changes through Optical Coherence Tomography at Periodontal Patients Wearing Fixed Dental Prosthesis. Med. Surg. J. 2022, 126, 268–273. [Google Scholar] [CrossRef]
- Gambino, A.; Cabras, M.; Cafaro, A.; Broccoletti, R.; Carossa, S.; Hopper, C.; Conrotto, D.; Porter, S.R.; Arduino, P.G. Preliminary Evaluation of the Utility of Optical Coherence Tomography in Detecting Structural Changes during Photobiomodulation Treatment in Patients with Atrophic-Erosive Oral Lichen Planus. Photodiagnosis Photodyn. Ther. 2021, 34, 102255. [Google Scholar] [CrossRef]
- Soheili, F.; Delfan, N.; Masoudifar, N.; Ebrahimni, S.; Moshiri, B.; Glogauer, M.; Ghafar-Zadeh, E. Toward Digital Periodontal Health: Recent Advances and Future Perspectives. Bioengineering 2024, 11, 937. [Google Scholar] [CrossRef]
- Luchian, I.; Martu, I.; Ioanid, N.; Goriuc, A.; Vata, I.; Stefanache, A.M.; Hurjui, L.; Tatarciuc, M.; Matei, M.N.; Martu, S. Salivary IL-1β: A Biochemical Marker That Predicts Periodontal Disease in Orthodontic Treatment. Rev. Chim. 2016, 67, 2479–2483. [Google Scholar]
- Schneider, H.; Park, K.-J.; Häfer, M.; Rüger, C.; Schmalz, G.; Krause, F.; Schmidt, J.; Ziebolz, D.; Haak, R. Dental Applications of Optical Coherence Tomography (OCT) in Cariology. Appl. Sci. 2017, 7, 472. [Google Scholar] [CrossRef]
- Elbarbary, M.; Sgro, A.; Khazaei, S.; Goldberg, M.; Tenenbaum, H.C.; Azarpazhooh, A. The Applications of Ultrasound, and Ultrasonography in Dentistry: A Scoping Review of the Literature. Clin. Oral Investig. 2022, 26, 2299–2316. [Google Scholar] [CrossRef]
- Eugui, P. Advanced Methods for Tissue Scattering Characterization with OCT. Ph.D. Thesis, Medical University of Vienna, Vienna, Austria, 2020. [Google Scholar]
- Yeragi, E.; Nalawade, K.P.; Gotmare, S.; Yeragi, P.; Prabhu, V. Optical Coherence Tomography (Oct): 6th Generation Periodontal Probe??? A Review. IOSR J. Dent. Med. Sci. 2019, 18, 1–5. [Google Scholar]
- Ghaffari, M.; Zhu, Y.; Shrestha, A. A Review of Advancements of Artificial Intelligence in Dentistry. Dent. Rev. 2024, 4, 100081. [Google Scholar] [CrossRef]
- Alotaibi, G.; Awawdeh, M.; Farook, F.F.; Aljohani, M.; Aldhafiri, R.M.; Aldhoayan, M. Artificial Intelligence (AI) Diagnostic Tools: Utilizing a Convolutional Neural Network (CNN) to Assess Periodontal Bone Level Radiographically—A Retrospective Study. BMC Oral Health 2022, 22, 399. [Google Scholar] [CrossRef]
- Cholan, P.; Ramachandran, L.; Umesh, S.G.; Sucharitha, P.; Tadepalli, A. The Impetus of Artificial Intelligence on Periodontal Diagnosis: A Brief Synopsis. Cureus 2023, 15, e43583. [Google Scholar] [CrossRef]


| Study | Parameters Measured | Sample | Tool | Main Findings Between Modalities |
|---|---|---|---|---|
| Kakizaki et al. (2018) [46] | Thickness of gingiva, mucosa, and biologic width | 177 lower anterior teeth of 30 periodontally healthy patients | Dental swept-source OCT system (Prototype 2, Panasonic, Ehime, Japan); 1330 nm central wavelength laser source with 100 nm bandwidth at a scanning rate of 30 kHz | Demonstrated that OCT could visualize and measure the thickness of gingiva, mucosa, and biologic width |
| Fernandes et al. (2017) [23] | Gingival sulcus depth | All anterior teeth of 23 periodontally healthy patients, with a total of 445 buccal examination sites | Swept-source OCT system (model unspecified, Thorlabs, Newton, United States); 1325 nm central wavelength laser source with 100 nm bandwidth at a scanning rate of 16 kHz | 1. Mean buccal gingival sulcus depth measured by OCT < manual probing and automated probing with Florida Probe by 0.57 mm and 0.39 mm, respectively (p < 0.001) 2. Time needed to obtain OCT images >manual probing and automated probing by 17.84 min and 17.17 min, respectively (p < 0.001) |
| Study | Parameters Measured | Sample | Tool | Main Findings Between Modalities |
|---|---|---|---|---|
| Kim et al. (2018) [62] | Peri-implant bone defect | 15 implants were placed in 4 dead porcine mandibles; 75 bone defects were prepared | Swept-source OCT system (model unspecified, Oztec, Daegu, Korea); 1310 nm central wavelength laser source at a scanning rate of 50 kHz | Bone defect depth measured by OCT > caliper by 0.23 mm (p < 0.001) |
| Sanda et al. (2016) [61] | Peri-implant bone | Implants covered by pig’s oral mucosa, and implants embedded into dead pig’s jawbone | Dental swept-source OCT system (Prototype 2, Panasonic, Saijo, Ehime, Japan); 1330 nm central wavelength laser source with 100 nm bandwidth at a scanning rate of 30 kHz | 1. Implant surface could be clearly visualized if the mucosal thickness covering the implant was <1 mm 2. Clear images of the implant surface and peri-implant bone could not be obtained when the implants were embedded into the jawbone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigotti, P.; Polizzi, A.; Verzì, A.E.; Lacarrubba, F.; Micali, G.; Isola, G. Impact of Optical Coherence Tomography (OCT) for Periodontitis Diagnostics: Current Overview and Advances. Dent. J. 2025, 13, 305. https://doi.org/10.3390/dj13070305
Rigotti P, Polizzi A, Verzì AE, Lacarrubba F, Micali G, Isola G. Impact of Optical Coherence Tomography (OCT) for Periodontitis Diagnostics: Current Overview and Advances. Dentistry Journal. 2025; 13(7):305. https://doi.org/10.3390/dj13070305
Chicago/Turabian StyleRigotti, Pietro, Alessandro Polizzi, Anna Elisa Verzì, Francesco Lacarrubba, Giuseppe Micali, and Gaetano Isola. 2025. "Impact of Optical Coherence Tomography (OCT) for Periodontitis Diagnostics: Current Overview and Advances" Dentistry Journal 13, no. 7: 305. https://doi.org/10.3390/dj13070305
APA StyleRigotti, P., Polizzi, A., Verzì, A. E., Lacarrubba, F., Micali, G., & Isola, G. (2025). Impact of Optical Coherence Tomography (OCT) for Periodontitis Diagnostics: Current Overview and Advances. Dentistry Journal, 13(7), 305. https://doi.org/10.3390/dj13070305








