Abstract
Objective: Burning mouth syndrome (BMS) is a chronic and often debilitating orofacial pain condition characterized by a burning sensation in the oral mucosa without clear abnormal lesions. While its etiology is considered multifactorial, the underlying pathophysiology remains unclear. This narrative review aims to synthesize existing functional magnetic resonance imaging (fMRI) studies to shed light on the central neural mechanisms contributing to BMS. Methods: A focused electronic search was conducted across the PubMed and J-STAGE databases for relevant articles published in English from January 2000 to May 2025. The review prioritized studies investigating brain structure and function using fMRI in individuals with BMS. Results: Our synthesis of the literature consistently demonstrated that the brains of individuals with BMS exhibit augmented connectivity within the medial pain system and a diminished gray matter volume in the medial prefrontal cortex (mPFC). These findings suggest a crucial role for altered brain circuitry, particularly a reduction in the output of the basal ganglia dopamine system, in the experience of BMS pain. Conclusions: The consistent fMRI findings strongly indicate that BMS involves significant functional and structural brain alterations. The observed changes in the mPFC and its connections to the basal ganglia dopamine system highlight this pathway as a potential target for both pharmacological and non-pharmacological neurological interventions for individuals with BMS.
1. Introduction
Burning mouth syndrome (BMS) is a challenging and often debilitating chronic orofacial pain syndrome, characterized by a persistent burning sensation in the oral mucosa without any clear physical lesions. Individuals with BMS endure constant or intermittent burning pain of unknown origin, which can last for months or even years, significantly diminishing their quality of life [1]. This condition occurs disproportionately in women, with an average age of onset typically in the perimenopausal or postmenopausal period. While the precise cause of BMS remains elusive, it is understood to be a complex interplay of factors, including neuropathic pain, hormonal changes, nutritional deficiencies, and psychological elements [2]. The International Classification of Orofacial Pain (ICOP) categorizes BMS as an idiopathic orofacial pain, notably lacking overt morphological alterations or evident nerve damage in the oral cavity [3].
Given the absence of detectable oral lesions, it has been increasingly speculated that the pain experienced in BMS may stem from disruptions within the brain’s pain-related circuits, including the pain matrix [4]. To gain deeper insight into these central mechanisms, this narrative review was conducted to comprehensively examine and synthesize the existing literature utilizing functional magnetic resonance imaging (fMRI) to explore brain structure and function in individuals with BMS. Our analysis aims to present a cohesive understanding of the neural alterations underlying this perplexing condition, aligning with the growing recognition of BMS as a centrally driven, nociplastic pain syndrome.
2. Materials and Methods
The aim of this study was to investigate what neural network changes identified by fMRI studies in adults diagnosed with BMS compared with healthy controls are associated with the pathological mechanisms of BMS (Table 1).

Table 1.
The PICO of this study.
To provide a comprehensive overview and synthesize key insights into the relationship between BMS and neural circuits, a narrative review was conducted. Rather than systematically mapping all available evidence, this approach focused on identifying and interpreting influential studies that shed light on brain structure and function in BMS patients, particularly those utilizing fMRI.
A targeted search for the pertinent literature was performed across the PubMed and J-STAGE databases, encompassing articles published in English from January 2000 to May 2025. The search strategy employed keywords such as burning mouth syndrome, neural circuits, functional magnetic resonance imaging, and mechanism, combined with Boolean operators to identify highly relevant studies. We prioritized original articles and conference proceedings that included fMRI data, such as resting-state fMRI, task-related fMRI, and diffusion tensor imaging (DTI), from patients diagnosed with BMS according to international criteria. Studies that compared BMS patients with control groups or examined differences within BMS patient populations were of particular interest. Exclusions included case reports, review articles, animal studies, and those employing brain imaging techniques other than fMRI. Studies focusing exclusively on secondary BMS or involving patients with fMRI contraindications were also not included. The focus was on gathering a rich set of information to construct a cohesive narrative, rather than on a formal assessment of individual study quality or risk of bias.
3. Results
3.1. Unraveling Brain Changes in Burning Mouth Syndrome
Our exploration of the literature aimed to understand the neural underpinnings of burning mouth syndrome (BMS) through fMRI studies. From an initial broad search, ten key papers emerged as particularly relevant, providing critical insights into how the brains of individuals with BMS differ from those of healthy controls. A PRISMA flow diagram is shown in Figure 1. These studies consistently revealed differences in neuronal connectivity and altered brain activity within regions crucial for pain perception and emotional processing in BMS patients. To better understand these structural and functional brain changes, we categorized the identified literature into two main approaches: those employing sensory stimulation during fMRI and those examining resting-state brain activity without external stimuli.
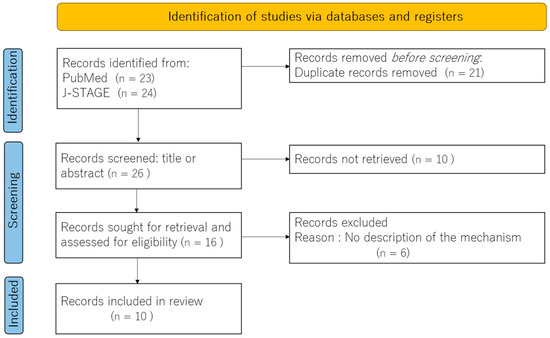
Figure 1.
A PRISMA flow diagram.
3.2. Brain Responses to Sensory Stimulation
Four of the included studies utilized fMRI while presenting specific sensory stimuli, offering a window into how BMS brains react to various inputs. These stimuli included noxious thermal stimulation to the lower lip [5,6], separate thermal stimulation to the right palm and right lower lip [7], and even tactile stimulation combined with the presentation of angry faces [8]. Across all these stimulated fMRI studies, individuals with BMS consistently showed heightened activation in neural regions comprising the “pain matrix” when compared to healthy controls.
Specifically, applying thermal stimuli to the trigeminal nerve area (which supplies sensation to the face) led to significantly greater activation in key areas like the medial prefrontal cortex (mPFC), the anterior cingulate cortex (ACC), the insular cortex (IC), and the hippocampus (Hc) in BMS patients [5,7]. For example, noxious thermal stimulation to the lower lip notably increased activation in the ACC and para-hippocampal gyrus [6]. Similarly, when gradually increasing painful thermal stimuli were applied to either the hand or lower lip, BMS brains demonstrated substantial sensitivity to these temperature changes, with the mPFC, IC, and ACC showing significantly elevated activity [7]. Even tactile stimuli, when an emotional state was induced, elicited a heightened sensitivity in individuals with BMS [8]. These findings are summarized in Table 2, providing a closer look at how BMS brains react under sensory challenge.

Table 2.
Results of the fMRI studies under specific stimuli.
3.3. Intrinsic Brain Activity and Structural Changes
The majority of the studies, six in total, investigated brain structure and function in BMS without external stimulation, allowing us to examine baseline brain activity and intrinsic networks when the patient is at rest. Although these studies used varied methodologies for analyzing fMRI data (making direct meta-analysis challenging), they yielded comparable and compelling results, consistently pointing to significant alterations in BMS brains.
A recurring finding across these non-stimulated fMRI studies was a reduction in gray matter volume in the mPFC and robust connectivity between the mPFC and the amygdala (AMY) in individuals with BMS. One study using resting-state fMRI specifically noted an increased gray matter volume in the Hc alongside a marked decrease in the mPFC in BMS patients compared to controls [9]. Another study, employing voxel-based morphometry, found a correlation between gray matter density in the pain matrix (including the ACC, cerebellar lobule, inferior temporal lobe, and mPFC) and pain intensity specifically in BMS individuals, a correlation absent in healthy controls and those with dysgeusia [10]. Gray matter density itself reflects the concentration of neural cells and their organization within a region.
Further insights into white matter structure and connectivity revealed that BMS brains exhibit significantly stronger connectivity within the medial pain system, as shown by probabilistic tractography. Interestingly, no significant differences were observed in the lateral pain system, which includes the somatosensory cortex [11]. The functional connectivity between the mPFC and the AMY in BMS patients was also found to be correlated with both the duration of the disease and the severity of symptoms [12]. A graph analysis of structural connectivity reinforced these findings, indicating stronger connectivity between regions of the medial pain system (such as the IC, AMY, and mPFC) when comparing BMS individuals to healthy controls [13]. Finally, evidence of astrocytic hypertrophy and microstructural alterations in the AMY of BMS patients points towards underlying functional and structural changes within the emotional processing system [14]. These six studies, focusing on non-stimulated fMRI, are summarized in Table 3, illustrating the complex baseline alterations in BMS brains.

Table 3.
Results of the fMRI studies without stimuli.
4. Discussion
4.1. Brain Alterations in Burning Mouth Syndrome
Our narrative review highlights that the perplexing experience of BMS likely stems from a complex interplay of factors, with emerging fMRI evidence strongly pointing to significant functional and structural alterations within the central nervous system. While peripheral small fiber neuropathy and hormonal shifts (particularly in postmenopausal women) are increasingly recognized as contributing elements, the central nervous system’s role in amplifying and perpetuating pain is paramount [15]. BMS can be understood as a form of central sensitization, where the brain becomes hypersensitive to sensory input, leading to amplified pain signals even without clear peripheral damage [16]. This maladaptive neuroplasticity, where the brain reorganizes in ways that reinforce pain perception, along with potential neurotransmitter imbalances, offers a compelling framework for understanding the chronic nature of BMS pain [17]. Functional and structural brain imaging, especially fMRI and DTI, provides invaluable insight into these neural mechanisms, revealing areas of altered activity and structural integrity. Our principal findings from the reviewed fMRI literature can be broadly categorized into functional impairment and structural abnormalities within the brains of individuals with BMS.
4.2. Functional Impairment: Reconfigured Pain and Emotional Networks
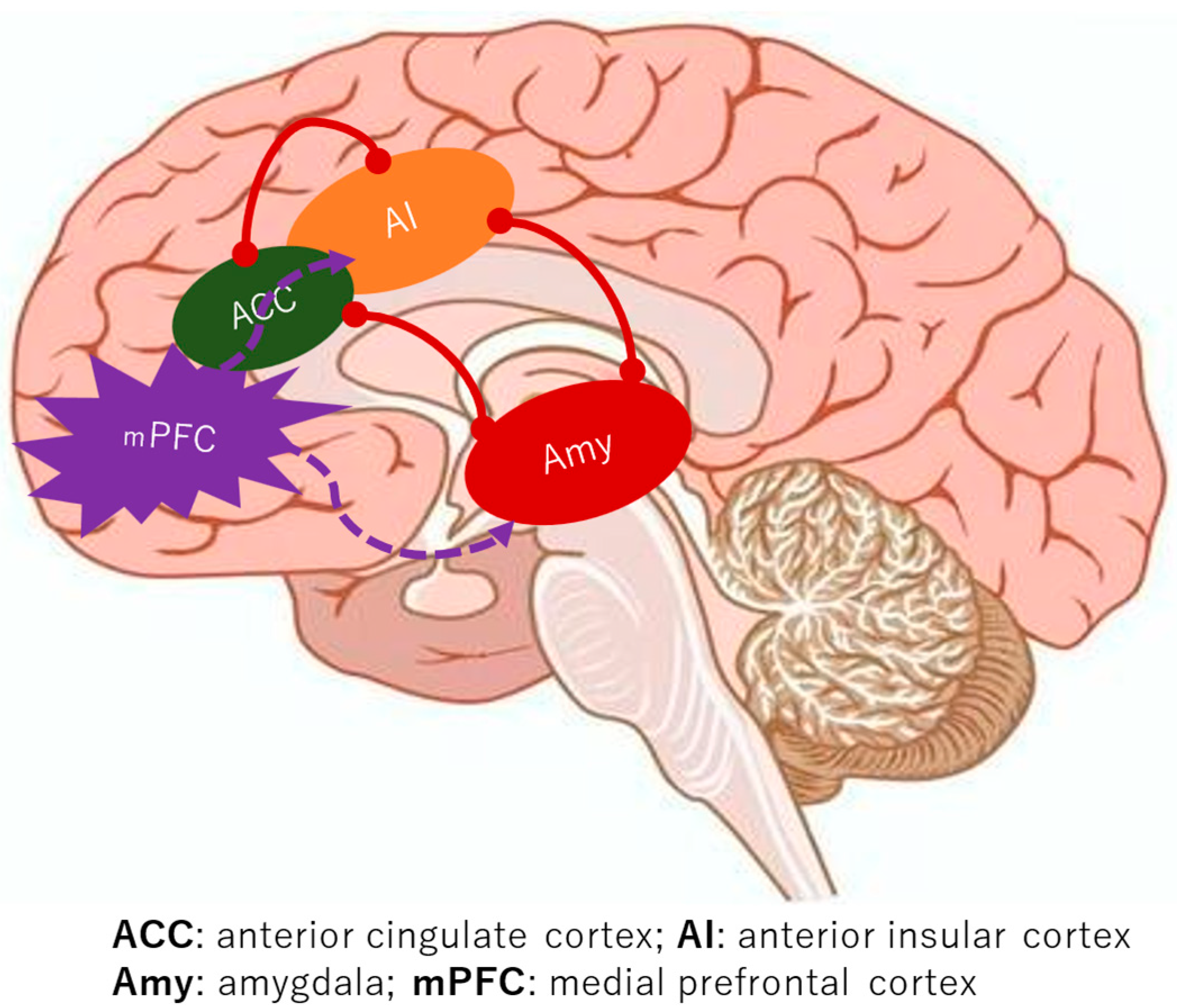
A consistent finding across the studies is the augmented functional connectivity within the medial pain system in BMS patients, encompassing key regions such as the mPFC, ACC, IC, Hc, and AMY [9,10,11,12,13,14]. Notably, the lateral pain system, including the somatosensory cortex, did not show similar discrepancies when compared to healthy controls [5,6,7]. The brain’s intricate functional networks, which govern everything from sensation to emotion, appear to be significantly reconfigured in BMS. A critical aspect of this re-wiring involves the salience network, a key functional brain network formed by the connectivity between the ACC and the IC [18]. This network is crucial for detecting and directing attention towards both external stimuli and internal bodily changes. In BMS, the robust functional connectivity of the salience network, particularly with emotional processing areas like the AMY, suggests a heightened sensitivity to internal signals (Figure 2). This heightened connectivity may lead to the perception of innocuous stimuli as significant changes, potentially triggering or intensifying the experience of pain. This characteristic of enhanced salience network activity and its strong connections to emotional circuits is also observed in other chronic pain conditions like fibromyalgia, hinting at a common neural signature for such disorders [19]. Furthermore, the strengthened connectivity between the salience network and memory-related regions, such as the Hc, offers a compelling explanation for the persistence of chronic pain even in the absence of ongoing noxious stimuli. It is plausible that past oral habits or painful dental procedures could have irritated trigeminal nerves, serving as an initial trigger [20]. The brain, particularly the IC, appears to store “molecular memories” of painful inflammation, and activation of the salience network might trigger the replay of these past painful experiences [21]. This suggests a mechanism where previous pain experiences alter neural connectivity, creating a robust circuit between the salience network, the AMY, and the Hc, thereby facilitating the regeneration of pain sensations.
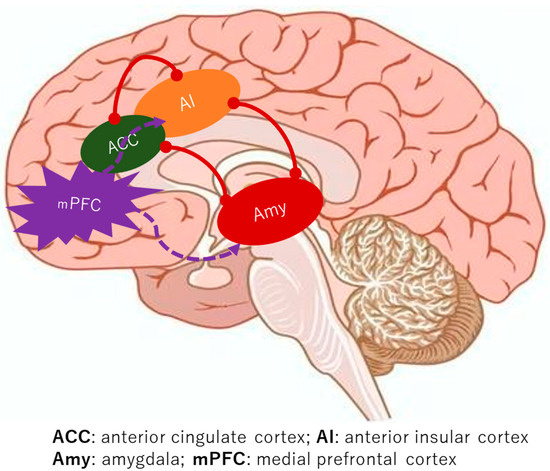
Figure 2.
The alterations in neural connectivity in individuals with BMS.
The brain’s intricate functional networks that regulate pain sensation and emotion undergo a profound reconfiguration in BMS. A fundamental element of this reconfiguration is the tight coupling of the mPFC and the emotional system.
4.3. Structural Abnormalities: Altered Brain Architecture
Beyond functional shifts, our review also highlights significant structural abnormalities in the brains of individuals with BMS. These include a reduction in gray matter volume in the mPFC, an increase in gray matter volume in the Hc, and an increase in astrocyte volumes in the AMY [12,14]. Gray matter volume, reflecting the overall size and cellular composition of a brain region, provides vital clues about the underlying pathology. The observed reduction in mPFC volume is particularly significant, given its pivotal role in pain perception and emotional regulation [22]. This decrease could contribute to heightened pain perception and a diminished capacity for pain tolerance in BMS patients. While the precise mechanism behind this volume reduction in chronic pain remains unclear, chronic stress associated with pain and imbalances in neurotransmitters like glutamate and GABA are plausible contributors. The mPFC’s close relationship with the basal ganglia, a region rich in dopamine-secreting neurons involved in pain inhibition, adds another layer of complexity [23]. A reduction in mPFC neurons and impaired function of dopaminergic neurons in the basal ganglia are interconnected and may reduce the brain’s natural analgesic effects, although the causal relationship is unclear [23]. This notion is supported by PET studies showing reduced dopamine D2 receptor activity and diminished endogenous dopamine levels in the striatum of BMS patients. Intriguingly, similar mPFC volume reductions and dopamine dysfunction have been observed in other chronic pain disorders of unknown etiology, suggesting a potential common neurobiological pathway [24]. The increased gray matter volume in the Hc in BMS patients presents a more nuanced finding, as the literature on hippocampal volume in chronic pain is often contradictory [25]. While some chronic pain conditions show hippocampal atrophy linked to memory and sleep issues, the increase observed in BMS (and in conditions like vestibulodynia) might reflect increased information processing, potentially driven by the overactive salience network [26]. Finally, structural alterations in the AMY, including astrocytic hypertrophy and microstructural changes, also appear central to BMS pathogenesis [9,14]. Animal models show that intense painful stimulation can alter AMY neurons, leading to ectopic pain without new stimuli [27]. This suggests that a history of oral procedures, frequently reported by BMS patients, might induce structural changes in the AMY, thereby lowering the pain threshold and contributing to BMS development.
In essence, both resting-state and stimulation-based fMRI provide compelling evidence that the intrinsic brain activity and neural responses to stimuli in individuals with BMS differ profoundly from those in healthy individuals. These studies collectively underscore the involvement of neural circuits, particularly those encompassing the ACC and mPFC, highlighting the significant emotional and cognitive components of BMS. This body of work reframes BMS not as a purely peripheral issue but as a nociplastic pain condition, characterized by an alteration in the pain-processing pathway within the central nervous system, even in the absence of overt tissue or nerve damage. The pain system itself undergoes hypersensitivity, leading to widespread and diffuse pain, often accompanied by fatigue, sleep disturbances, and mood changes, similar to conditions like fibromyalgia. Understanding BMS through this lens is crucial for developing more effective, centrally targeted therapeutic strategies.
5. Limitations
While this narrative review aimed to provide a comprehensive and interpretive synthesis of fMRI findings in burning mouth syndrome (BMS), it is important to acknowledge several limitations inherent to this methodological approach and the scope of our investigation.
Firstly, as a narrative review, this study does not adhere to the rigorous systematic protocols of a systematic review or meta-analysis. Consequently, there was no formal assessment of the methodological quality or risk of bias for individual studies. For data such as gray matter volume, due to different measurement methods and limited case numbers, it was not possible to enter these data into the Cochrane Review Manager software (RevMan 5.0) and synthesize them for review using Cochrane MetaView software. Our approach prioritized the comprehensive interpretation and synthesis of key concepts and findings over an exhaustive, protocol-driven aggregation of evidence. This means that while we aimed for a balanced overview, the depth of critical appraisal for each included paper was not as exhaustive as in systematic reviews, and the potential for selective reporting or subjective interpretation is acknowledged.
Secondly, this review serves as a pilot or proof-of-concept study to explore the feasibility and initial landscape of research in brain networks of individuals with BMS. As such, we intentionally limited our search to PubMed and J-STAGE to quickly identify key themes and gaps, with the intention of conducting a more exhaustive multi-database search in a subsequent, full-scale review. This limited scope means that relevant studies published in other databases (such as Web of Science, Embase, or the Cochrane Library), or in languages other than English, may have been inadvertently excluded. Future research employing more exhaustive and diverse search strategies across a wider array of databases would undoubtedly provide an even more comprehensive understanding of the topic.
Finally, while fMRI offers invaluable insights into the neural mechanisms of BMS by directly measuring brain activity and revealing involved regions and their connections in pain processing, it is crucial to recognize that fMRI measures brain activity, not pain itself. The observed brain activity changes must be interpreted in conjunction with other indices that directly evaluate pain, such as patient-reported outcomes or quantitative sensory testing. The complexity of pain perception means that while fMRI data strongly implicate altered brain function in BMS, further research integrating multiple assessment modalities is essential for a complete understanding of the patient’s lived experience of pain.
6. Conclusions
This narrative review synthesizes compelling fMRI evidence, demonstrating that burning mouth syndrome (BMS) is characterized by distinct structural and functional alterations within the brain’s pain and emotional processing networks. Our exploration of both stimulated and resting-state fMRI studies consistently reveals that the intrinsic brain activity and neural responses to stimuli in individuals with BMS diverge significantly from those of healthy controls. A central finding is the heightened connectivity within the salience network (comprising the ACC and IC) in BMS patients, and its strong connections to the AMY, Hc, and mPFC. This increased connectivity likely contributes to the persistent experience of pain, even in the absence of obvious external stimuli, by potentially amplifying the perception of internal bodily signals. Furthermore, we consistently observed a reduction in gray matter volume in the mPFC, which appears linked to diminished dopamine function in the basal ganglia. This structural change and its impact on the brain’s pain matrix suggest that the dopaminergic nervous system represents a promising avenue for future therapeutic interventions in BMS.
In essence, our narrative review underscores that BMS is not merely a peripheral discomfort but a complex condition rooted in demonstrable changes in brain structure and function. Understanding these central nervous system alterations is crucial for developing more effective, targeted treatments for individuals suffering from this challenging pain syndrome.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the Sunlight Brain Research Center, approval number SBRC-503-2.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ACC | Anterior cingulate cortex |
| AMY | Amygdala |
| BMS | Burning mouth syndrome |
| DTI | Diffusion tensor imaging |
| fMRI | Functional magnetic resonance imaging |
| Hc | Hippocampus |
| IC | Insular cortex |
| ICOP | International Classification of Orofacial Pain |
| mPFC | Medial prefrontal cortex |
| PET | Positron emission tomography |
References
- Aravindhan, R.; Vidyalakshmi, S.; Kumar, M.S.; Satheesh, C.; Balasubramanium, A.M.; Prasad, V.S. Burning mouth syndrome: A review on its diagnostic and therapeutic approach. J. Pharm. Bioallied Sci. 2014, 6 (Suppl. 1), S21–S25. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yoo, T.; Han, P.; Liu, Y.; Inman, J.C. A pragmatic evidence-based clinical management algorithm for burning mouth syndrome. J. Clin. Exp. Dent. 2018, 10, e321–e326. [Google Scholar] [CrossRef]
- International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [CrossRef]
- Nagamine, T. Pathogenesis of Orofacial Pain Based on Brain Circuits. Oral Dis. 2025. [Google Scholar] [CrossRef]
- Albuquerque, R.J.C.; de Leeuw, R.; Carlson, C.R.; Okeson, J.P.; Miller, C.S.; Andersen, A.H. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: An fMRI study. Pain 2006, 122, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, T.; Imamura, Y.; Kohashi, R.; Dezawa, K.; Nakaya, Y.; Sato, Y.; Watanabe, K.; Morimoto, Y.; Shizukuishi, T.; Abe, O.; et al. Spatial and Temporal Brain Responses to Noxious Heat Thermal Stimuli in Burning Mouth Syndrome. J. Dent. Res. 2016, 95, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Kohashi, R.; Shinozaki, T.; Sekine, N.; Watanabe, K.; Takanezawa, D.; Nishihara, C.; Ozasa, K.; Ikeda, M.; Noma, N.; Okada-Ogawa, A.; et al. Time-dependent responses in brain activity to ongoing hot stimulation in burning mouth syndrome. J. Oral. Sci. 2020, 62, 170–174. [Google Scholar] [CrossRef]
- Yoshino, A.; Okamoto, Y.; Doi, M.; Okada, G.; Takamura, M.; Ichikawa, N.; Yamawaki, S. Functional Alterations of Postcentral Gyrus Modulated by Angry Facial Expressions during Intraoral Tactile Stimuli in Patients with Burning Mouth Syndrome: A Functional Magnetic Resonance Imaging Study. Front. Psychiatry 2017, 8, 224. [Google Scholar] [CrossRef]
- Khan, S.A.; Keaser, M.L.; Meiller, T.F.; Seminowicz, D.A. Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 2014, 155, 1472–1480. [Google Scholar] [CrossRef]
- Sinding, C.; Gransjøen, A.M.; Schlumberger, G.; Grushka, M.; Frasnelli, J.; Singh, P.B. Grey matter changes of the pain matrix in patients with burning mouth syndrome. Eur. J. Neurosci. 2016, 43, 997–1005. [Google Scholar] [CrossRef]
- Wada, A.; Shizukuishi, T.; Kikuta, J.; Yamada, H.; Watanabe, Y.; Imamura, Y.; Shinozaki, T.; Dezawa, K.; Haradome, H.; Abe, O. Altered structural connectivity of pain-related brain network in burning mouth syndrome-investigation by graph analysis of probabilistic tractography. Neuroradiology 2017, 59, 525–532. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, X.; Chen, J.; Kong, L.; Qian, Z. Structural and Functional Connectivity Between the Amygdala and Orbital Frontal Cortex in Burning Mouth Syndrome: An fMRI Study. Front. Psychol. 2019, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, R.; Kamiya, K.; Inui, S.; Kato, S.; Suzuki, F.; Amemiya, S.; Shinozaki, T.; Takanezawa, D.; Kohashi, R.; Abe, O. Structural connectivity changes in the cerebral pain matrix in burning mouth syndrome: A multi-shell, multi-tissue-constrained spherical deconvolution model analysis. Neuroradiology 2021, 63, 2005–2012. [Google Scholar] [CrossRef]
- Kato, S.; Kurokawa, R.; Suzuki, F.; Amemiya, S.; Shinozaki, T.; Takanezawa, D.; Kohashi, R.; Abe, O. White and Gray Matter Abnormality in Burning Mouth Syndrome Evaluated with Diffusion Tensor Imaging and Neurite Orientation Dispersion and Density Imaging. Magn. Reson. Med. Sci. 2024, 23, 204–213. [Google Scholar] [CrossRef]
- Nagamine, T. Estrogen-Mediated Neural Mechanisms of Sex Differences in Burning Mouth Syndrome. Neurol. Int. 2025, 17, 61. [Google Scholar] [CrossRef]
- Monteserín-Matesanz, M.; Domínguez-Gordillo, A.A.; Esparza-Gómez, G.C.; Jiménez-Ortega, L.; Cerero-Lapiedra, R. Central sensitization in burning mouth syndrome: A practical approach using questionnaires. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2022, 133, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Jaffal, S.M. Neuroplasticity in chronic pain: Insights into diagnosis and treatment. Korean J. Pain 2025, 38, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Schimmelpfennig, J.; Topczewski, J.; Zajkowski, W.; Jankowiak-Siuda, K. The role of the salience network in cognitive and affective deficits. Front. Hum. Neurosci. 2023, 17, 1133367. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Garcia, A.; Leon-Llamas, J.L.; Villafaina, S.; Gusi, N. Fibromyalgia impact in the prefrontal cortex subfields: An assessment with MRI. Clin. Neurol. Neurosurg. 2022, 219, 107344. [Google Scholar] [CrossRef]
- Tınastepe, N.; Oral, K. Neuropathic pain after dental treatment. Agri 2013, 25, 1–6. [Google Scholar] [CrossRef]
- Koren, T.; Yifa, R.; Amer, M.; Krot, M.; Boshnak, N.; Ben-Shaanan, T.L.; Azulay-Debby, H.; Zalayat, I.; Avishai, E.; Hajjo, H.; et al. Insular cortex neurons encode and retrieve specific immune responses. Cell 2021, 184, 5902–5915.e17. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.Y.; Stohler, C.S.; Herr, D.R. Role of the Prefrontal Cortex in Pain Processing. Mol. Neurobiol. 2019, 56, 1137–1166. [Google Scholar] [CrossRef]
- Kang, D.; McAuley, J.H.; Kassem, M.S.; Gatt, J.M.; Gustin, S.M. What does the grey matter decrease in the medial prefrontal cortex reflect in people with chronic pain? Eur. J. Pain 2019, 23, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Hagelberg, N.; Forssell, H.; Rinne, J.O.; Scheinin, H.; Taiminen, T.; Aalto, S.; Luutonen, S.; Någren, K.; Jääskeläinen, S. Striatal dopamine D1 and D2 receptors in burning mouth syndrome. Pain 2003, 101, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Noorani, A.; Hung, P.S.; Zhang, J.Y.; Sohng, K.; Laperriere, N.; Moayedi, M.; Hodaie, M. Pain Relief Reverses Hippocampal Abnormalities in Trigeminal Neuralgia. J. Pain 2022, 23, 141–155. [Google Scholar] [CrossRef]
- Bhatt, R.R.; Gupta, A.; Rapkin, A.; Kilpatrick, L.A.; Hamadani, K.; Pazmany, E.; Van Oudenhove, L.; Stains, J.; Aerts, L.; Enzlin, P.; et al. Altered gray matter volume in sensorimotor and thalamic regions associated with pain in localized provoked vulvodynia: A voxel-based morphometry study. Pain 2019, 160, 1529–1540. [Google Scholar] [CrossRef]
- Sugimoto, M.; Takahashi, Y.; Sugimura, Y.K.; Tokunaga, R.; Yajima, M.; Kato, F. Active role of the central amygdala in widespread mechanical sensitization in rats with facial inflammatory pain. Pain 2021, 162, 2273–2286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).