Analysis of Implant Osseointegration, Bone Repair, and Sinus Mucosa Integrity Using Bio-Oss® and Hyaluronic Acid-Polynucleotide Gel (Regenfast®) in Maxillary Sinus Augmentation in Rabbits
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Research Design
2.3. Animals Used
2.4. Sample Size Calculation
2.5. Randomization and Allocation Concealment
2.6. Implant Characteristics
2.7. Biomaterials Used
2.8. Anesthetic Protocol
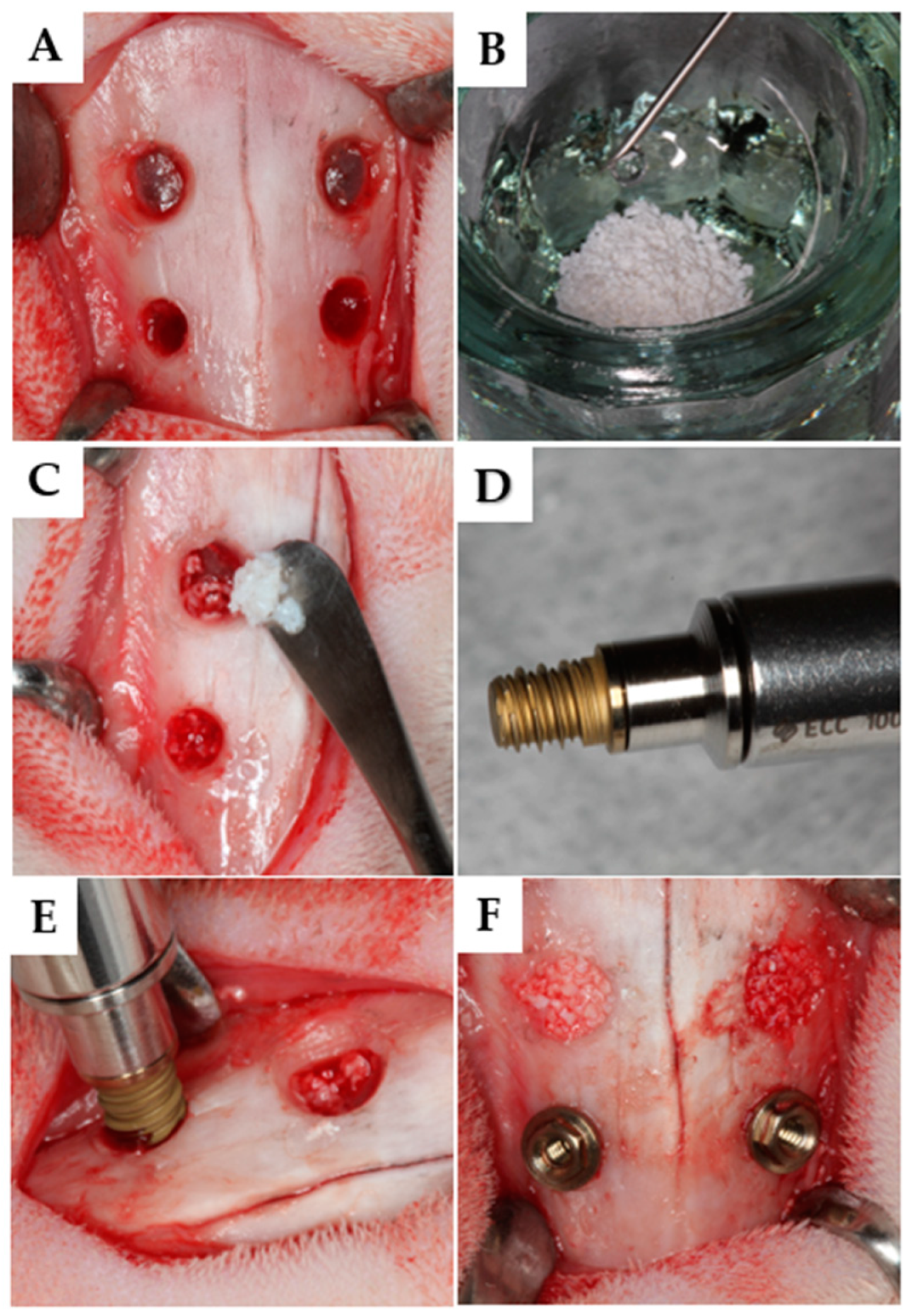
2.9. Surgical Procedures
2.10. Animal Care
2.11. Euthanasia
2.12. Tissue Processing
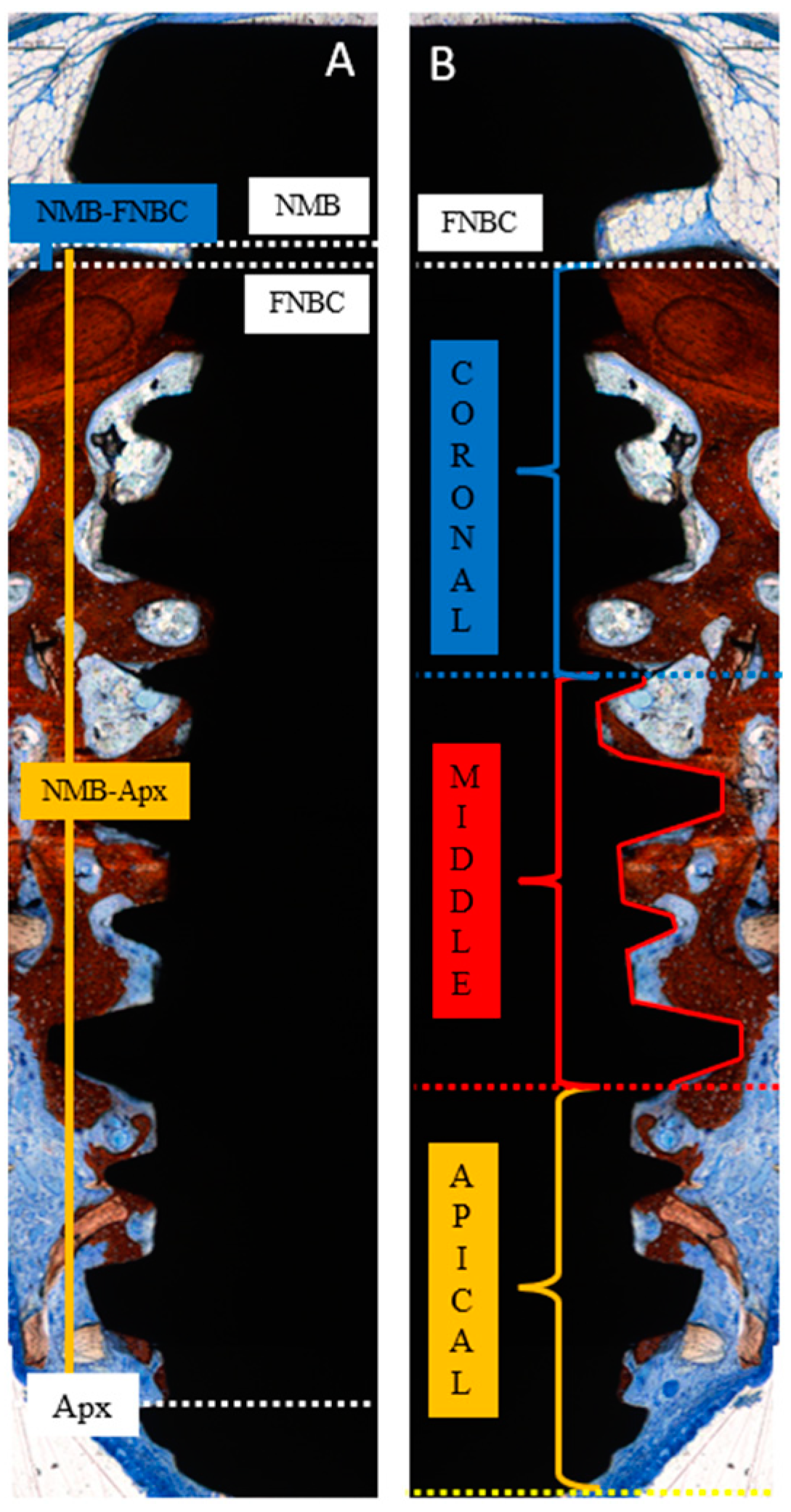
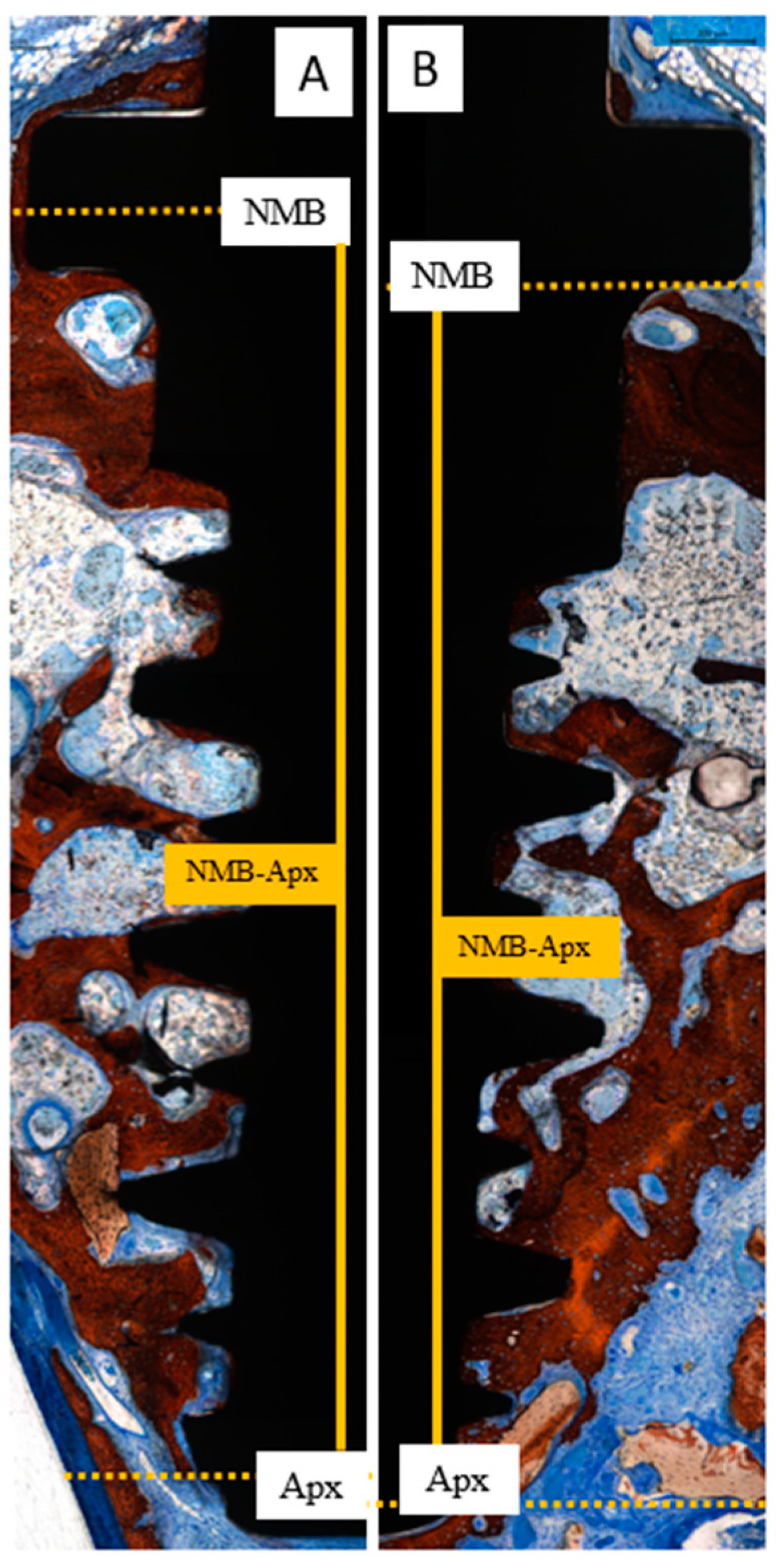
2.13. Histomorphometric Analysis
- NMB-FNBC: the distance from the native marginal bone (NMB) to the first newly formed bone contact (FNBC) at the most coronal point of osseointegration.
- NMB-Apx: the distance from the native marginal bone (NMB) to the most apical point of osseointegration (Apx).
2.14. Statistical Analysis
3. Results
3.1. Clinical Outcomes
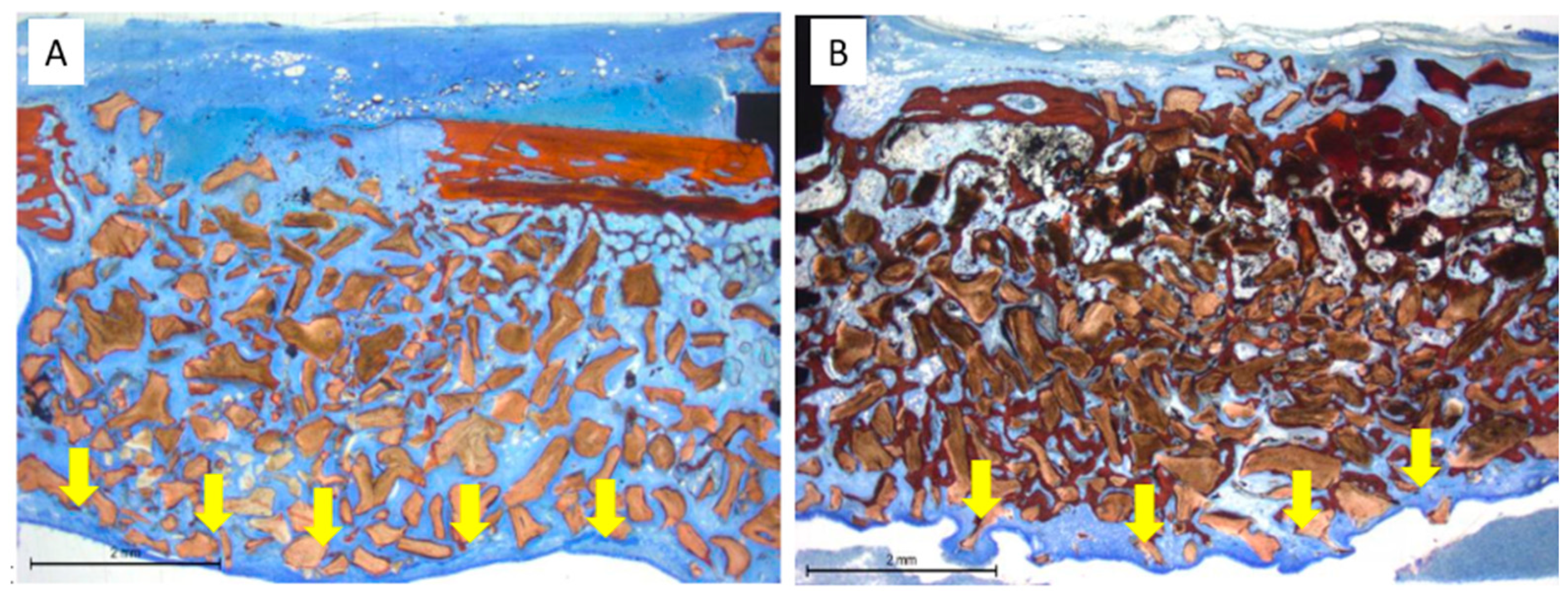
3.2. Histological Evaluation at the Only Grafted Sites
3.3. Histological Evaluation at the Implant Sites
3.4. Mucosa Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HyA: | Hyaluronic acid |
| PN: | Polynucleotides |
| NMB: | Native marginal bone |
| FNBC: | First newly formed bone contact |
| Apx | Apical extension of osseointegration |
References
- Del Fabbro, M.; Wallace, S.S.; Testori, T. Long-term implant survival in the grafted maxillary sinus: A systematic review. Int. J. Periodontics Restor. Dent. 2013, 33, 773–783. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 216–240. [Google Scholar] [CrossRef] [PubMed]
- Asai, S.; Shimizu, Y.; Ooya, K. Maxillary sinus augmentation model in rabbits: Effect of occluded nasal ostium on new bone formation. Clin. Oral Implant. Res. 2002, 13, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, W.F.; Eijsackers, F.A.; Bravenboer, N.; Ten Bruggenkate, C.M.; Remmelzwaal, S.; Schulten, E.A.J.M. Long-Term Bone Height Changes After Sinus Floor Elevation With Maxillary or Mandibular Bone Grafts: A Radiological Study. Clin. Implant. Dent. Relat. Res. 2025, 27, e70008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zijderveld, S.A.; Schulten, E.A.; Aartman, I.H.; ten Bruggenkate, C.M. Long-term changes in graft height after maxillary sinus floor elevation with different grafting materials: Radiographic evaluation with a minimum follow-up of 4.5 years. Clin. Oral Implant. Res. 2009, 20, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Cricchio, G.; Imburgia, M.; Sennerby, L.; Lundgren, S. Immediate loading of implants placed simultaneously with sinus membrane elevation in the posterior atrophic maxilla: A two-year follow-up study on 10 patients. Clin. Implant. Dent. Relat. Res. 2014, 16, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Johansson, A.S.; Cricchio, G.; Lundgren, S. Clinical outcome and factors determining new bone formation in lateral sinus membrane elevation with simultaneous implant placement without grafting material: A cross-sectional, 3–17 year follow-up study. Clin. Implant. Dent. Relat. Res. 2019, 21, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xi, Y.; Teng, F.; Wang, Z.; Yang, G. Comparison of Two Different Sizes of Deproteinized Bovine Bone Mineral Particles in Lateral Sinus Floor Elevation With Simultaneous Implant Placement: A Radiographic Study. Clin. Oral Implant. Res. 2025, 36, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Corbella, S.; Taschieri, S.; Weinstein, R.; Del Fabbro, M. Histomorphometric outcomes after lateral sinus floor elevation procedure: A systematic review of the literature and meta-analysis. Clin. Oral Implant. Res. 2016, 27, 1106–1122. [Google Scholar] [CrossRef]
- Jensen, T.; Schou, S.; Svendsen, P.A.; Forman, J.L.; Gundersen, H.J.G.; Terheyden, H.; Holmstrup, P. Volumetric changes of the graft after maxillary sinus floor augmentation with Bio-Oss and autogenous bone in different ratios: A radiographic study in minipigs. Clin. Oral Implant. Res. 2012, 23, 902–910. [Google Scholar] [CrossRef]
- Eken, S.; Guler Ayyıldız, B.; Altay, B.; Arı, N.S.; Özatik, O. Clinical, Radiological, and Histomorphometric Comparison of the Use of Deproteinized Bovine Bone Mineral and Titanium-Prepared Platelet-Rich Fibrin in Maxillary Sinus Augmentation: A Split-Mouth Randomized Controlled Clinical Study. J. Oral Maxillofac. Surg. 2025, 83, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft in animals: A systematic review. Int. J. Oral Maxillofac. Surg. 2012, 41, 114–120. [Google Scholar] [CrossRef]
- Krennmair, G.; Schwarze, U.Y.; Weinländer, M.; Forstner, T.; Malek, M.; Krennmair, S. Maxillary Sinus Augmentation with Anorganic Bovine Bone Mineral of Different Particle Sizes: A Split-Mouth Study with Histomorphometric, Radiographic, and Clinical Analyses. Int. J. Oral Maxillofac. Implant. 2024, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Kungvarnchaikul, I.; Subbalekha, K.; Sindhavajiva, P.R.; Suwanwela, J. Deproteinized bovine bone and freeze-dried bone allograft in sinus floor augmentation: A randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2023, 25, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Wiesheu, S.; Schlegel, K.A.; Adler, W.; Kesting, M.R.; Matta, R.E.; Möst, T. Three-dimensional assessment after maxillary sinus grafting with a bovine, a porcine, and a synthetic bone substitute material. A randomized controlled clinical trial. Int. J. Comput. Dent. 2024, 27, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Baba, S.; Botticelli, D.; Masuda, K.; Xavier, S.P. Comparison of histomorphometry and microCT after sinus augmentation using xenografts of different particle sizes in rabbits. Oral Maxillofac. Surg. 2020, 24, 57–64. [Google Scholar] [CrossRef]
- Xu, H.; Shimizu, Y.; Asai, S.; Ooya, K. Grafting of deproteinized bone particles inhibits bone resorption after maxillary sinus floor elevation. Clin. Oral Implant. Res. 2004, 15, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Busenlechner, D.; Huber, C.D.; Vasak, C.; Dobsak, A.; Gruber, R.; Watzek, G. Sinus augmentation analysis revised: The gradient of graft consolidation. Clin. Oral Implant. Res. 2009, 20, 1078–1083. [Google Scholar] [CrossRef]
- Godoy, E.P.; Alccayhuaman, K.A.A.; Botticelli, D.; Amaroli, A.; Balan, V.F.; Silva, E.R.; Xavier, S.P. Osteoconductivity of Bovine Xenograft Granules of Different Sizes in Sinus Lift: A Histomorphometric Study in Rabbits. Dent. J. 2021, 9, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galindo-Moreno, P.; Hernández-Cortés, P.; Aneiros-Fernández, J.; Camara, M.; Mesa, F.; Wallace, S.; O’Valle, F. Morphological evidences of Bio-Oss® colonization by CD44-positive cells. Clin. Oral Implant. Res. 2014, 25, 366–371. [Google Scholar] [CrossRef]
- Iaquinta, M.R.; De Pace, R.; Benkhalqui, A.; D’Agostino, A.; Trevisiol, L.; Finotti, A.; Breveglieri, G.; Tognon, M.; Martini, F.; Mazzoni, E. Secretome Release During In Vitro Bone Marrow-Derived Mesenchymal Stem Cell Differentiation Induced by Bio-Oss® Collagen Material. Int. J. Mol. Sci. 2025, 26, 3807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dehkordi, A.N.; Babaheydari, F.M.; Chehelgerdi, M.; Dehkordi, S.R. Skin tissue engineering: Wound healing based on stem-cell- based therapeutic strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.T.; Govoni, P.; Belletti, S.; Squadrito, F.; Guizzardi, S.; Galli, C. Polynucleotide biogel enhances tissue repair, matrix deposition and organization. J. Biol. Regul. Homeost. Agents 2021, 35, 355–362. [Google Scholar] [CrossRef]
- Vanelli, R.; Costa, P.; Rossi, S.M.P.; Benazzo, F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: A randomized, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 901–907. [Google Scholar] [CrossRef]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Massirone, A.; Palmieri, I.P.; Papagni, M.; Priori, M.; Trocchi, G. Consensus report on the use of PN-HPTTM (polynucleotides highly purified technology) in aesthetic medicine. J. Cosmet. Dermatol. 2021, 20, 922–928. [Google Scholar] [CrossRef]
- Spicer, A.P.; Tien, J.Y. Hyaluronan and morphogenesis. Birth Defects Res. Part C Embryo Today Rev. 2004, 72, 89–108. [Google Scholar] [CrossRef] [PubMed]
- Treger, D.; Zhang, L.; Jia, X.; Hui, J.H.; Gantumur, M.A.; Hui, M.; Liu, L. A clinical study of the local injection of a freshly manufactured 35 kDa hyaluronan fragment for treating chronic wounds. Int. Wound J. 2024, 21, e14906. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, L.; Ma, X.; Jia, X.; Hui, J.H.; Hui, M. Application of 35 kDa Hyaluronic Acid Fragment in Managing Persistent Localized Pain in Rheumatoid Arthritis: A Case Report. Clin. Case Rep. 2025, 13, e70361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Frenkel, J.S. The role of hyaluronan in wound healing. Int. Wound J. 2014, 11, 159–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donegan, G.C.; Hunt, J.A.; Rhodes, N. Investigating the importance of flow when utilizing hyaluronan scaffolds for tissue engineering. J. Tissue Eng. Regen. Med. 2010, 4, 83–95. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, X.; Qin, L.; Zhai, M.; Yuan, J.; Chen, J.; Li, D. Effect of hyaluronic acid in bone formation and its applications in dentistry. J. Biomed. Mater. Res. Part A 2016, 104, 1560–1569. [Google Scholar] [CrossRef] [PubMed]
- Oksala, O.; Salo, T.; Tammi, R.; Hakkinen, L.; Jalkanen, M.; Inki, P.; Larjava, H. Expression of proteoglycans and hyaluronan during wound healing. J. Histochem. Cytochem. 1995, 43, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Fujioka-Kobayashi, M.; Müller, H.D.; Mueller, A.; Lussi, A.; Sculean, A.; Schmidlin, P.R.; Miron, R.J. In vitro effects of hyaluronic acid on human periodontal ligament cells. BMC Oral Health 2017, 17, 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bertl, K.; Bruckmann, C.; Isberg, P.E.; Klinge, B.; Gotfredsen, K.; Stavropoulos, A. Hyaluronan in non-surgical and surgical periodontal therapy: A systematic review. J. Clin. Periodontol. 2015, 42, 236–246. [Google Scholar] [CrossRef]
- Maniwa, N.; Xavier, S.P.; Scombatti de Souza, S.L.; Silva, E.R.; Botticelli, D.; Morinaga, K.; Baba, S. Sequential Bone Repair in Rabbit Sinus Lifts Using Bio-Oss and Hyaluronic Acid-Polynucleotide Gel (Regenfast). J. Funct. Biomater. 2024, 15, 361. [Google Scholar] [CrossRef]
- Martins, S.H.L.; Cadore, U.B.; Novaes ABJr Messora, M.R.; Ghiraldini, B.; Bezerra, F.J.B.; Botticelli, D.; de Souza, S.L.S. Evaluation of Bone Response to a Nano HÁ Implant Surface on Sinus Lifting Procedures: Study in Rabbits. J. Funct. Biomater. 2022, 13, 122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dupont, W.D.; Plummer, W.D., Jr. Power and sample size calculations. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; Arvidsson, A.; Andersson, M.; Kjellin, P.; Albrektsson, T.; Wennerberg, A. Nano hydroxyapatite structures influence early bone formation. J. Biomed. Mater. Res. Part A 2008, 87, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Meirelles, L.; Currie, F.; Jacobsson, M.; Albrektsson, T.; Wennerberg, A. The effect of chemical and nanotopographical modifications on the early stages of osseointegration. Int. J. Oral Maxillofac. Implant. 2008, 23, 641–647. [Google Scholar] [PubMed]
- Jimbo, R.; Coelho, P.G.; Bryington, M.; Baldassarri, M.; Tovar, N.; Currie, F.; Hayashi, M.; Janal, M.N.; Andersson, M.; Ono, D.; et al. Nano hydroxyapatite-coated implants improve bone nanomechanical properties. J. Dent. Res. 2012, 91, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, M.; Henriques, J.; Martins, G.; Guerra, F.; Judas, F.; Figueiredo, H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes—comparison with human bone. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.R.; Cha, J.K.; Lim, H.C.; Lee, J.S.; Choi, S.H.; Jung, U.W. De novo bone formation underneath the sinus membrane supported by a bone patch: A pilot experiment in rabbit sinus model. Clin. Oral Implant. Res. 2017, 28, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Silva, E.R.; Apaza Alccayhuaman, K.A.; Botticelli, D.; Xavier, S.P. Histologic and Micro-CT Analyses at Implants Placed Immediately After Maxillary Sinus Elevation Using Large or Small Xenograft Granules: An Experimental Study in Rabbits. Int. J. Oral Maxillofac. Implant. 2020, 35, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.G.; Wennström, J.L.; Lindhe, J. Modeling of the buccal and lingual bone walls of fresh extraction sites following implant installation. Clin. Oral Implant. Res. 2006, 17, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, K.; Becker, W.; Persson, R.; Baker, D.A.; Rohrer, M.D.; O’Neal, R.B. Evaluation of titanium implants placed into simulated extraction sockets: A study in dogs. Int. J. Oral Maxillofac. Implant. 1999, 14, 351–360. [Google Scholar] [PubMed]
- Miki, M.; Botticelli, D.; Silva, E.R.; Xavier, S.P.; Baba, S. Incidence of Sinus Mucosa Perforations During Healing After Sinus Elevation Using Deproteinized Bovine Bone Mineral as Grafting Material: A Histologic Evaluation in a Rabbit Model. Int. J. Oral Maxillofac. Implant. 2021, 36, 660–668. [Google Scholar] [CrossRef] [PubMed]






| Control | Test (Regenfast®) | |||||
|---|---|---|---|---|---|---|
| New Bone | Xenograft | Soft Tissue | New Bone | Xenograft | Soft Tissue | |
| Total | 7.3 ± 2.4 ‡ | 41.6 ± 8.1 | 51.1 ± 8.6 | 7.7 ± 2.4 ‡ | 37.9 ± 9.7 | 54.3 ± 11.3 |
| Sub-Mucosa | 8.5 ± 4.6 ‡ | 40.0 ± 12.3 | 51.5 ± 12.0 | 8.2 ± 3.2 ‡ | 40.1 ± 11.4 | 51.7 ± 11.2 |
| Anterior | 7.2 ± 4.5 ‡ | 37.6 ± 13.7 | 55.3 ± 15.2 | 5.5 ± 4.7 ‡ | 33.1 ± 9.3 | 61.4 ± 11.0 |
| Central | 5.8 ± 4.1 ‡ | 45.6 ± 19.1 | 48.6 ± 16.2 | 6.5 ± 4.4 ‡ | 41.5 ± 10.3 | 51.9 ± 12.1 |
| Posterior | 9.0 ± 5.5 ‡ | 39.7 ± 7.8 * | 51.3 ± 11.1 | 12.8 ± 6.4 ‡ | 32.7 ± 10.4 * | 54.5 ± 13.1 |
| Sub-Window | 5.0 ± 4.3 ‡ | 46.4 ± 13.7 | 48.6 ± 10.2 | 5.0 ± 5.6 ‡ | 40.1 ± 13.1 | 54.9 ± 15.4 |
| Control | Test (Regenfast®) | |||||
|---|---|---|---|---|---|---|
| New Bone | Xenograft | Soft Tissue | New Bone | Xenograft | Soft Tissue | |
| Total | 26.8 ± 10.0 *‡ | 37.1 ± 7.3 ‡ | 36.2 ± 8.8 | 37.2 ± 6.7 *‡ | 29.7 ± 6.3 ‡ | 33.2 ± 3.3 |
| Sub-mucosa | 27.6 ± 15.0 ‡ | 36.5 ± 8.1 ‡ | 36.0 ± 8.4 | 38.1 ± 12.1 ‡ | 29.9 ± 7.6 ‡ | 31.9 ± 5.9 |
| Anterior | 30.1 ± 16.3 ‡ | 31.9 ± 14.3 | 37.9 ± 15.1 | 42.9 ± 10.9 ‡ | 25.9 ± 9.2 ‡ | 31.2 ± 4.1 |
| Central | 22.9 ± 11.8 ‡ | 39.1 ± 8.4 | 37.9 ± 9.6 | 31.3 ± 14.6 ‡ | 39.9 ± 15.1 ‡ | 28.8 ± 2.9 |
| Posterior | 29.9 ± 12.2 ‡ | 37.8 ± 9.3 ‡ | 32.3 ± 16.8 | 40.8 ± 14.3 ‡ | 25.9 ± 16.3 ‡ | 33.3 ± 3.1 |
| Sub-Window | 22.7 ± 15.8 ‡ | 40.5 ± 9.5 | 36.8 ± 14.8 | 31.8 ± 15.8 ‡ | 26.4 ± 9.6 | 41.8 ± 12.4 |
| Two Weeks | Ten Weeks | |||
|---|---|---|---|---|
| NMB-Apx | NMB-B | NMB-Apx | NMB-B | |
| Control | 3.68 ± 1.10 * | 0.49 ± 0.65 ‡ | 3.73 ± 0.27 | 0.04 ± 0.05 ‡ |
| Test (Regenfast®) | 2.62 ± 1.14 * | 0.20 ± 0.12 | 3.84 ± 0.25 | 0.36 ± 0.51 |
| p-value | 0.031 | 0.300 | 0.604 | 0.625 |
| Total | Apical | Middle | Coronal | ||
|---|---|---|---|---|---|
| Two weeks | Control | 16.1 ± 8.2 ‡ | 5.5 ± 3.4 ‡ | 18.2 ± 15.4 ‡ | 24.4 ± 16.5 ‡ |
| Test (Regenfast®) | 17.9 ± 8.8 ‡ | 4.0 ± 4.5 ‡ | 15.0 ± 18.2 ‡ | 34.7 ± 7.6 | |
| p-value | 0.350 | 0.544 | 0.602 | 0.064 | |
| Ten weeks | Control | 42.3 ± 7.8 ‡ | 29.0 ± 16.0 ‡ | 41.3 ± 10.8 ‡ | 56.7 ± 11.2 ‡ |
| Test (Regenfast®) | 40.2 ± 9.8 ‡ | 27.2 ± 12.8 ‡ | 48.0 ± 19.1 ‡ | 45.4 ± 23.9 | |
| p-value | 0.687 | 0.833 | 0.352 | 0.295 |
| Pristine µm | Thinned Mucosae | Perforations | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Mean µm | Min µm | <10 µm | No | Dimension µm | Sinus | |||
| 2 weeks | Control | 117 ± 41 | 31 | 16 ± 11 | 4 | 7 | 2 | 311 ± 76 | 2 |
| Test | 115 ± 50 | 27 | 21 ± 11 | 4 | 1 | 1 | 2005 | 1 | |
| 10 weeks | Control | 97 ± 33 | 64 | 22 ± 4 | 3 | 12 | 14 | 337 ± 486 | 6 |
| Test | 122 ± 38 | 62 | 23 ± 5 | 1 | 15 | 5 | 1522 ± 2095 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omori, H.; Botticelli, D.; Silva, E.R.; Xavier, S.P.; Souza, S.L.S.d.; Kusano, K.; Baba, S. Analysis of Implant Osseointegration, Bone Repair, and Sinus Mucosa Integrity Using Bio-Oss® and Hyaluronic Acid-Polynucleotide Gel (Regenfast®) in Maxillary Sinus Augmentation in Rabbits. Dent. J. 2025, 13, 293. https://doi.org/10.3390/dj13070293
Omori H, Botticelli D, Silva ER, Xavier SP, Souza SLSd, Kusano K, Baba S. Analysis of Implant Osseointegration, Bone Repair, and Sinus Mucosa Integrity Using Bio-Oss® and Hyaluronic Acid-Polynucleotide Gel (Regenfast®) in Maxillary Sinus Augmentation in Rabbits. Dentistry Journal. 2025; 13(7):293. https://doi.org/10.3390/dj13070293
Chicago/Turabian StyleOmori, Hiroyuki, Daniele Botticelli, Erick Ricardo Silva, Samuel Porfirio Xavier, Sérgio Luis Scombatti de Souza, Kaoru Kusano, and Shunsuke Baba. 2025. "Analysis of Implant Osseointegration, Bone Repair, and Sinus Mucosa Integrity Using Bio-Oss® and Hyaluronic Acid-Polynucleotide Gel (Regenfast®) in Maxillary Sinus Augmentation in Rabbits" Dentistry Journal 13, no. 7: 293. https://doi.org/10.3390/dj13070293
APA StyleOmori, H., Botticelli, D., Silva, E. R., Xavier, S. P., Souza, S. L. S. d., Kusano, K., & Baba, S. (2025). Analysis of Implant Osseointegration, Bone Repair, and Sinus Mucosa Integrity Using Bio-Oss® and Hyaluronic Acid-Polynucleotide Gel (Regenfast®) in Maxillary Sinus Augmentation in Rabbits. Dentistry Journal, 13(7), 293. https://doi.org/10.3390/dj13070293










