The Effects of Scan Body Geometry on the Precision and the Trueness of Implant Impressions Using Intraoral Scanners: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Design and Protocol
2.2. Eligibility Criteria
2.2.1. Study Types
2.2.2. Participants
2.2.3. Interventions and Comparisons
2.2.4. Outcomes
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Extraction
2.5. Risk of Bias Assessment
2.6. Data Synthesis
2.7. Ethical Considerations
3. Results
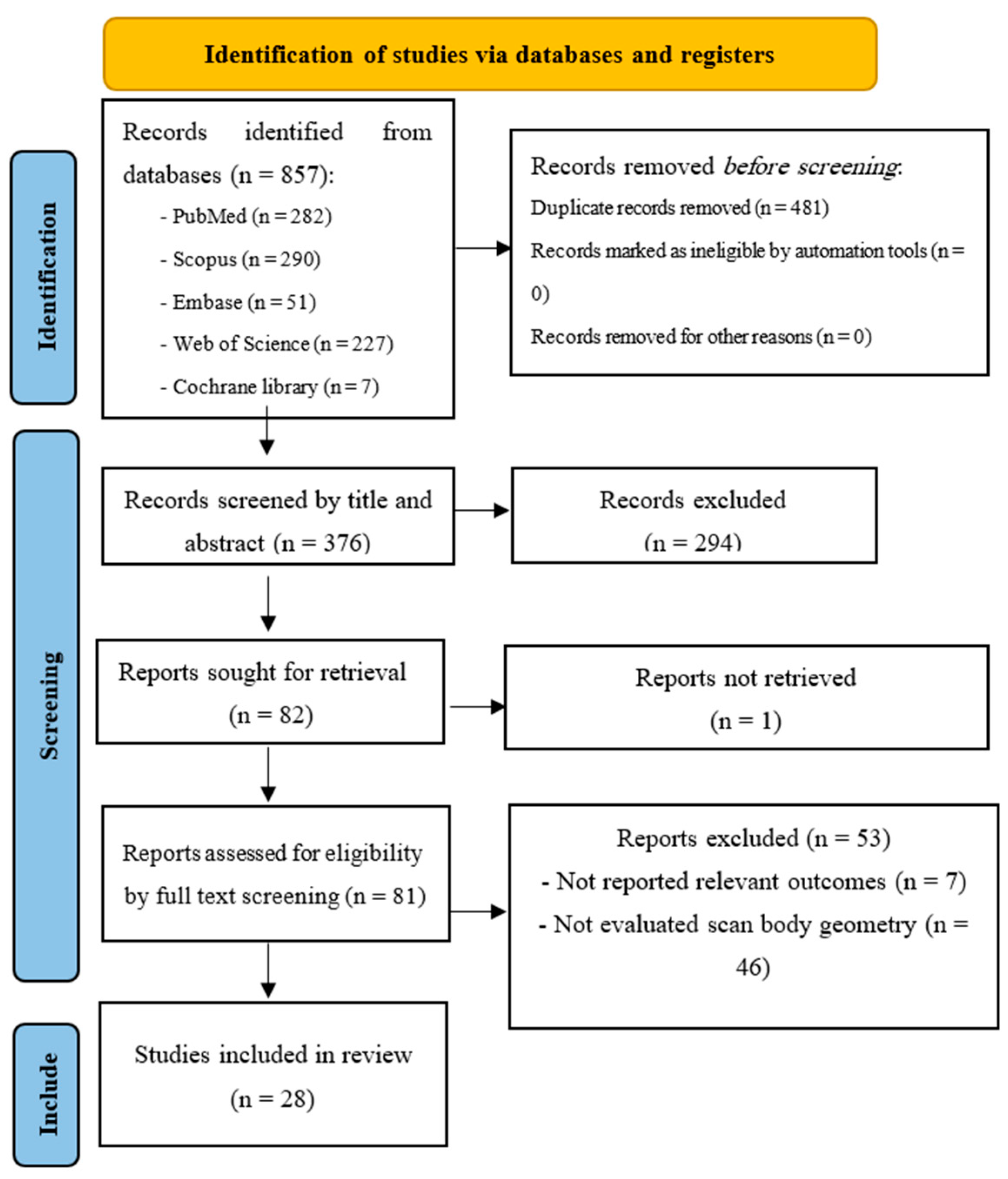
3.1. Study Selection
3.2. Study Characteristics
3.3. Scan Body Geometry
3.4. Quality Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IOSs | Intraoral scanners |
| PICO | Population, intervention, comparison, outcome |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| MeSH | Medical Subject Headings |
| CAD/CAM | Computer-aided design/computer-aided manufacturing |
| QUIN | Quality Assessment Tool For In Vitro Studies |
| PEEK | Polyether ether ketone |
| RMS | Root mean square |
References
- Alikhasi, M.; Alsharbaty, M.H.M.; Moharrami, M. Digital Implant Impression Technique Accuracy: A Systematic Review. Implant Dent. 2017, 26, 929–935. [Google Scholar] [CrossRef]
- Lee, H.; So, J.S.; Hochstedler, J.L.; Ercoli, C. The accuracy of implant impressions: A systematic review. J. Prosthet. Dent. 2008, 100, 285–291. [Google Scholar] [CrossRef]
- Marques, S.; Ribeiro, P.; Falcão, C.; Lemos, B.F.; Ríos-Carrasco, B.; Ríos-Santos, J.V.; Herrero-Climent, M. Digital Impressions in Implant Dentistry: A Literature Review. Int. J. Environ. Res. Public Health 2021, 18, 1020. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Vazouras, K.; Chen, Y.-w.; Kotina, E.; Natto, Z.; Kang, K.; Chochlidakis, K. Digital vs Conventional Implant Impressions: A Systematic Review and Meta-Analysis. J. Prosthodont. 2020, 29, 660–678. [Google Scholar] [CrossRef]
- Rutkunas, V.; Gedrimiene, A.; Adaskevicius, R.; Al-Haj Husain, N.; Özcan, M. Comparison of the Clinical Accuracy of Digital and Conventional Dental Implant Impressions. Eur. J. Prosthodont. Restor. Dent. 2020, 28, 173–181. [Google Scholar] [CrossRef]
- Schmidt, A.; Schlenz, M.A.; Liu, H.; Kämpe, H.S.; Wöstmann, B. The Influence of Hard- and Software Improvement of Intraoral Scanners on the Implant Transfer Accuracy from 2012 to 2021: An In Vitro Study. Appl. Sci. 2021, 11, 7166. [Google Scholar] [CrossRef]
- Gehrke, P.; Rashidpour, M.; Sader, R.; Weigl, P. A systematic review of factors impacting intraoral scanning accuracy in implant dentistry with emphasis on scan bodies. Int. J. Implant Dent. 2024, 10, 20. [Google Scholar] [CrossRef]
- Tan, J.Z.H.; Tan, M.Y.; See Toh, Y.L.; Wong, K.Y.; Tan, K.B.C. Three-dimensional positional accuracy of intraoral and laboratory implant scan bodies. J. Prosthet. Dent. 2022, 128, 735–744. [Google Scholar] [CrossRef]
- Mizumoto, R.M.; Yilmaz, B. Intraoral scan bodies in implant dentistry: A systematic review. J. Prosthet. Dent. 2018, 120, 343–352. [Google Scholar] [CrossRef]
- Eggmann, F.; Blatz, M.B. Recent Advances in Intraoral Scanners. J. Dent. Res. 2024, 103, 1349–1357. [Google Scholar] [CrossRef]
- Arcuri, L.; Pozzi, A.; Lio, F.; Rompen, E.; Zechner, W.; Nardi, A. Influence of implant scanbody material, position and operator on the accuracy of digital impression for complete-arch: A randomized in vitro trial. J. Prosthodont. Res. 2020, 64, 128–136. [Google Scholar] [CrossRef]
- Gómez-Polo, M.; Álvarez, F.; Ortega, R.; Gómez-Polo, C.; Barmak, A.B.; Kois, J.C.; Revilla-León, M. Influence of the implant scan body bevel location, implant angulation and position on intraoral scanning accuracy: An in vitro study. J. Dent. 2022, 121, 104122. [Google Scholar] [CrossRef]
- Ender, A.; Mehl, A. Accuracy of complete-arch dental impressions: A new method of measuring trueness and precision. J. Prosthet. Dent. 2013, 109, 121–128. [Google Scholar] [CrossRef]
- Flügge, T.; van der Meer, W.J.; Gonzalez, B.G.; Vach, K.; Wismeijer, D.; Wang, P. The accuracy of different dental impression techniques for implant-supported dental prostheses: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S16), 374–392. [Google Scholar] [CrossRef]
- Schmidt, A.; Wöstmann, B.; Schlenz, M.A. Accuracy of digital implant impressions in clinical studies: A systematic review. Clin. Oral Implant. Res. 2022, 33, 573–585. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, B.; Song, H.; Wu, D.; Song, T. Accuracy of digital implant impressions obtained using intraoral scanners: A systematic review and meta-analysis of in vivo studies. Int. J. Implant Dent. 2023, 9, 48. [Google Scholar] [CrossRef]
- Albanchez-González, M.I.; Brinkmann, J.C.; Peláez-Rico, J.; López-Suárez, C.; Rodríguez-Alonso, V.; Suárez-García, M.J. Accuracy of Digital Dental Implants Impression Taking with Intraoral Scanners Compared with Conventional Impression Techniques: A Systematic Review of In Vitro Studies. Int. J. Environ. Res. Public Health 2022, 19, 2026. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Collins, A.M.; Coughlin, D.; Kirk, S. The Role of Google Scholar in Evidence Reviews and Its Applicability to Grey Literature Searching. PLoS ONE 2015, 10, e0138237. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Sheth, V.H.; Shah, N.P.; Jain, R.; Bhanushali, N.; Bhatnagar, V. Development and validation of a risk-of-bias tool for assessing in vitro studies conducted in dentistry: The QUIN. J. Prosthet. Dent. 2024, 131, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Liu, Y.; Huang, B.; Zhang, C.; Chen, Z.; Li, Z. Improved scanning accuracy with newly designed scan bodies: An in vitro study comparing digital versus conventional impression techniques for complete-arch implant rehabilitation. Clin. Oral Implant. Res. 2020, 31, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Mizumoto, R.M.; Yilmaz, B.; McGlumphy, E.A., Jr.; Seidt, J.; Johnston, W.M. Accuracy of different digital scanning techniques and scan bodies for complete-arch implant-supported prostheses. J. Prosthet. Dent. 2020, 123, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Moslemion, M.; Payaminia, L.; Jalali, H.; Alikhasi, M. Do Type and Shape of Scan Bodies Affect Accuracy and Time of Digital Implant Impressions? Eur. J. Prosthodont. Restor. Dent. 2020, 28, 18–27. [Google Scholar] [CrossRef]
- Motel, C.; Kirchner, E.; Adler, W.; Wichmann, M.; Matta, R.E. Impact of Different Scan Bodies and Scan Strategies on the Accuracy of Digital Implant Impressions Assessed with an Intraoral Scanner: An In Vitro Study. J. Prosthodont. 2020, 29, 309–314. [Google Scholar] [CrossRef]
- Revilla-León, M.; Fogarty, R.; Barrington, J.J.; Zandinejad, A.; Özcan, M. Influence of scan body design and digital implant analogs on implant replica position in additively manufactured casts. J. Prosthet. Dent. 2020, 124, 202–210. [Google Scholar] [CrossRef]
- Huang, R.; Liu, Y.; Huang, B.; Zhou, F.; Chen, Z.; Li, Z. Improved accuracy of digital implant impressions with newly designed scan bodies: An in vivo evaluation in beagle dogs. BMC Oral Health 2021, 21, 623. [Google Scholar] [CrossRef]
- Revilla-León, M.; Smith, Z.; Methani, M.M.; Zandinejad, A.; Özcan, M. Influence of scan body design on accuracy of the implant position as transferred to a virtual definitive implant cast. J. Prosthet. Dent. 2021, 125, 918–923. [Google Scholar] [CrossRef]
- Schmidt, A.; Dent, D.M.; Billig, J.W.; Schlenz, M.A.; Wöstmann, B.; Dent, M. The Influence of Using Different Types of Scan Bodies on the Transfer Accuracy of Implant Position: An In Vitro Study. Int. J. Prosthodont. 2021, 34, 254–260. [Google Scholar] [CrossRef]
- Yilmaz, B.; Gouveia, D.; Marques, V.R.; Diker, E.; Schimmel, M.; Abou-Ayash, S. The accuracy of single implant scans with a healing abutment-scanpeg system compared with the scans of a scanbody and conventional impressions: An in vitro study. J. Dent. 2021, 110, 103684. [Google Scholar] [CrossRef]
- Alvarez, C.; Domínguez, P.; Jiménez-Castellanos, E.; Arroyo, G.; Orozco, A. How the geometry of the scan body affects the accuracy of digital impressions in implant supported prosthesis. In vitro study. J. Clin. Exp. Dent. 2022, 14, e1008–e1014. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.T.; Kim, H.Y.; Song, S.Y.; Park, J.H.; Lee, J.Y. Accuracy of implant impression techniques with a scannable healing abutment. J. Prosthet. Dent. 2022, 128, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tsoi, J.K.H.; Lam, W.Y.H.; Chen, Z.; Pow, E.H.N. Does the geometry of scan bodies affect the alignment accuracy of computer-aided design in implant digital workflow: An in vitro study? Clin. Oral. Implant. Res. 2022, 33, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Meneghetti, P.C.; Li, J.; Borella, P.S.; Mendonça, G.; Burnett, L.H., Jr. Influence of scanbody design and intraoral scanner on the trueness of complete arch implant digital impressions: An in vitro study. PLoS ONE 2023, 18, e0295790. [Google Scholar] [CrossRef]
- Ramadan, R.E.; Razek, M.K.A.; Mohamed, F.S.; Fahmy, R.A.; Abd-Ellah, M.E. Positional transfer accuracy of titanium base implant abutment provided by two different scan body designs: An invitro study. BMC Oral Health 2023, 23, 746. [Google Scholar] [CrossRef]
- Shely, A.; Lugassy, D.; Rosner, O.; Zanziper, E.; Nissan, J.; Rachmiel, S.; Khoury, Y.; Ben-Izhack, G. The Influence of Laboratory Scanner versus Intra-Oral Scanner on Determining Axes and Distances between Three Implants in a Straight Line by Using Two Different Intraoral Scan Bodies: A Pilot In Vitro Study. J. Clin. Med. 2023, 12, 6644. [Google Scholar] [CrossRef]
- Uzel, S.M.; Guncu, M.B.; Aktas, G.; Arikan, H.; Reiss, N.; Turkyilmaz, I. Influence of the implant scan body modifications on trueness of digital impressions. J. Dent. Sci. 2023, 18, 1771–1777. [Google Scholar] [CrossRef]
- Alkindi, S.; Hamdoon, Z.; Aziz, A.M. Effect of different impression coping and scan body designs on the accuracy of conventional versus digital implant impressions: An in vitro study. J. Dent. 2024, 146, 105045. [Google Scholar] [CrossRef]
- Anwar, H.; Azer, A.; AboElHassan, R.G. Influence of a specially designed geometric device and modified scan bodies on the accuracy of a maxillary complete arch digital implant scan: An in vitro study. J. Prosthet. Dent. 2024, 131, 683.e1–683.e7. [Google Scholar] [CrossRef]
- Ashry, A.; Abdelhamid, A.M.; Ezzelarab, S.; Khamis, M.M. Effect of using scan body accessories and inter-implant distances on the accuracy of complete arch implant digital impressions: An in vitro study. J. Prosthodont. 2024, 1–9. [Google Scholar] [CrossRef]
- Lawand, G.; Ismail, Y.; Revilla-León, M.; Tohme, H. Effect of implant scan body geometric modifications on the trueness and scanning time of complete arch intraOral Implant. digital scans: An in vitro study. J. Prosthet. Dent. 2024, 131, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fang, H.; Yan, Y.; Geng, W. Accuracy of intraoral scanning using modified scan bodies for complete arch implant-supported fixed prostheses. J. Prosthet. Dent. 2024, 132, 994.e1–994.e8. [Google Scholar] [CrossRef] [PubMed]
- Michelinakis, G.; Apostolakis, D.; Nikolidakis, D.; Lapsanis, G. Influence of different scan body design features and intraoral scanners on the congruence between scan body meshes and library files: An in vitro study. J. Prosthet. Dent. 2024, 132, 454.e1–454.e11. [Google Scholar] [CrossRef] [PubMed]
- Park, G.S.; Chang, J.; Pyo, S.W.; Kim, S. Effect of scan body designs and internal conical angles on the 3-dimensional accuracy of implant digital scans. J. Prosthet. Dent. 2024, 132, 190.e1–190.e7. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, B.; Ge, R.; Zhang, C.; Zhang, H.; Wang, Y. Effect of a Novel ‘Scan Body’ on the In Vitro Scanning Accuracy of Full-Arch Implant Impressions. Int. Dent. J. 2024, 74, 847–854. [Google Scholar] [CrossRef]
- Eldabe, A.K.; Adel-Khattab, D.; Botros, K.H. Trueness of tooth modified scan bodies as a novel technique for edentulous full arch implant supported dental prosthesis: An in vivo prospective comparative study. BMC Oral Health 2025, 25, 29. [Google Scholar] [CrossRef]
- Farah, R.I.; Alresheedi, B.; Alazmi, S.; Al-Haj Ali, S.N. Evaluating the impact of scan body angulation and geometric attachments on the accuracy of complete-arch digital implant impressions: A comparison of two intraoral scanners. J. Prosthodont. 2025, 34, 174–181. [Google Scholar] [CrossRef]
- Pan, Y.; Dai, X.; Tsoi, J.K.; Lam, W.Y.; Pow, E.H. Effect of shape and size of implant scan body on scanning accuracy: An in vitro study. J. Dent. 2025, 152, 105498. [Google Scholar] [CrossRef]
- Wan, Q.; Limpuangthip, N.; Hlaing, N.H.M.M.; Hahn, S.; Lee, J.-H.; Lee, S.J. Enhancing scanning accuracy of digital implant scans: A systematic review on application methods of scan bodies. J. Prosthet. Dent. 2024, 132, 898.e1–898.e9. [Google Scholar] [CrossRef]
- Rutkūnas, V.; Gečiauskaitė, A.; Jegelevičius, D.; Vaitiekūnas, M. Accuracy of digital implant impressions with intraoral scanners. A systematic review. Eur. J. Oral Implant. 2017, 10 (Suppl. S1), 101–120. [Google Scholar]
- Sanda, M.; Miyoshi, K.; Baba, K. Trueness and precision of digital implant impressions by intraoral scanners: A literature review. Int. J. Implant Dent. 2021, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Pachiou, A.; Zervou, E.; Tsirogiannis, P.; Sykaras, N.; Tortopidis, D.; Kourtis, S. Characteristics of intraoral scan bodies and their influence on impression accuracy: A systematic review. J. Esthet. Restor. Dent. 2023, 35, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Polo, M.; Donmez, M.B.; Çakmak, G.; Yilmaz, B.; Revilla-León, M. Influence of implant scan body design (height, diameter, geometry, material, and retention system) on intraoral scanning accuracy: A systematic review. J. Prosthodont. 2023, 32, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Aragoneses, R.; Arroyo Valverde, E.M.; Gómez-Polo, M.; Kois, J.C. Classification of Scanning Errors of Digital Scans Recorded by Using Intraoral Scanners. J. Esthet. Restor. Dent. 2025, 37, 1363–1371. [Google Scholar] [CrossRef]
- Shetty, P.S.; Gangurde, A.P.; Chauhan, M.R.; Jaiswal, N.V.; Salian, P.R.; Singh, V. Accuracy of the digital implant impression with splinted and non-splinted intraoral scan bodies: A systematic review. J. Indian Prosthodont. Soc. 2025, 25, 3–12. [Google Scholar] [CrossRef]
| Study ID | Study Design | Scan Body Geometry | Sample Size | Measurement | Implants |
|---|---|---|---|---|---|
| Pan et al., 2022 [33] | In vitro | Cuboidal dome-shaped (both fabricated by the same manufacturer (ZfxTM Intrascan matchholder H4 and ZfxTM Evolution matchholder, Zimmer Biomet, Indiana, USA)) | 4 | Iterative-closest point (ICP) criteria | Six |
| Motel et al., 2020 [25] | In vitro | ELOS A/S, Gørløse, Denmark NT-Trading GmbH, Karlsruhe, Germany TeamZiereis, Baden-Württemberg, Germany | 10 | Local best fit | Three |
| Huang et al., 2021 [27] | In vivo | Scan body with extensional structure (a rigid bar) scan body without extensional structure | Best-fit algorithm | Two | |
| Huang et al., 2020 [22] | In vitro | Original scan body (Straumann, Basel, Switzerland)—CAD/CAM scan body without extensional structure—CAD/CAM scan body with extensional structure | 10 | Best-fit algorithm | Four |
| Revilla-León et al., 2020 [26] | In vitro | Elos Medtech, Gørløse, Denmark, Elos Accurate Intraoral Scan body Nt-Trading, Karlsruhe, Germany, 3D Guide Intraoral Scan body Dynamic Abutment (Talladium, Lleida, Spain), Intraoral scan body system with intraoral adaptor | 10 | Best-fit technique | Three |
| Revilla-León et al., 2021 [28] | In vitro | Elos accurate IO scanbody Brånemark system RP; Nobel Biocare Services AG Scan Body 3D Guide K Series; NT Digital Implant Technology | NA | NA | NA |
| Meneghetti et al., 2023 [34] | In vitro | SB1: Cylinder, with a trapezoidal in the top, with different surfaces. 14 mm high PEEK with a metal connection S.I.N., São Paulo, Brazil SB2: Cylinder with an angled flat surface, 9 mm high PEEK Neodent, Curitiba, Brazil SB3: Cylinder with diamond surfaces, 12 mm high PEEK Neodent, Curitiba, Brazil SB4: Prototype, rounded, 7 mm high 3D printed, grey resin, Custom SB5: Prototype, tri-angled flat surfaces, 7 mm high 3D printed, grey resin, Custom SB6: Prototype, bar 16 mm with a convex ball in the extremity 3D printed, grey resin, Custom SB7: Cylinder with an angled flat surface, 7 mm high PEEK S.I.N., São Paulo, Brazil | 10 | Using an ADD-ON for Blender called Object align/ICP align | Six |
| Ramadan et al., 2023 [35] | In vitro | One-piece SB (Elos Medtech) Two-piece Healing Abutment-Scan Peg (HA-SP; Neoss, Harrogate, HG1 2PW, United Kingdom) | 10 | Best-fit matching | One |
| Yilmaz et al., 2021 [30] | In vitro | Conventional intraoral scan body (CSB) (Neoss, Woodland Hills, CA, USA) A healing abutment-scanpeg system (HASP) | 10 | Local best-fit | One |
| Lawand et al., 2024 [41] | In vitro | CARES Mono Scanbody for a screw-retained abutment (nonmodified) Subtractively modified scan body Additively modified scan body | 15 | Reference best-fit algorithm | Two |
| Alvarez et al., 2022 [31] | In vitro | ELOS, MG, Ticare MG, and Talladium. Model 3a-B ELOS Medtech Denmark (ELOS), one piece, screwed in, a milled angulated side Mozo Grau S.A scan body, a milled pyramidal side, screwin placement, and two-piece clip-in system (MG) Mozo Grau S.A scan body, 12 milled sides, screw-in, one piece (Ticare MG) Talladium scan body (Talladium Spain), a milled side, magnetic placement, and 2 pieces | 10 | Best-fit | Six |
| Mizumoto et al., 2019 [23] | In vitro | AF (IO-Flo; Dentsply Sirona, 63457 Hanau, Germany) NT (Nt-Trading GmbH & Co KG, Karlsruhe, Baden-Württemberg, Germany) DE (DESS-USA, Lake Mary, FL 32746, USA) C3D (Core3Dcentres, Castle Hill, Australia) ZI (Zimmer Biomet Dental, Palm Beach Gardens, Florida 33410, USA) | 5 | Best-fit algorithm | Four |
| Jung et al., 2022 [32] | In vitro | Simple scan abutment (IHAB 50 06 H, Dentium, Gyeonggi-do, Republic of Korea) Scanning jig (SCJ I4565, Dentium, Gyeonggi-do, Republic of Korea) | 10 | Specific anatomic structures in the teeth adjacent to the implants were set as reference points, and the mean 3D linear intra-arch and interarch deviations were evaluated | Two |
| Moslemion et al., 2020 [24] | In vitro | DESS 14.005 NT-Trading E9.S3D4.300 Doowon B051 | 10 | Best-fit algorithm | Four |
| Schmidt et al., 2021 [29] | In vitro | 3D Guide, H-Series, NT Trading, Karlsruhe, Germany Cara H10/20, Kulzer, Hanau, Germany H1410, Medentika, Niefern-Öschelbronn, Germany | 10 | Reference system, which allowed determination of the exact x-, y-, and z-deviations | Four |
| Tan et al., 2022 [8] | In vitro | Medentika L-Series Scan body Second Generation (REF L1420) (Medentika) Straumann CARES Mono Scan body for implant-level scanning (REF 025.4915) (SM) Core 3D Scanbody, Straumann Bone level RC (compatible) (REF 2077) (C3D) Straumann RC Scan body (REF 025.4905) (SS) | 10 | Zero method | Ten |
| Li Y. et al., 2024 [42] | In vitro | Conventional scan bodies (CARES Mono Scanbody; Institute Straumann AG for IOS-C, Basel, Switzerland) Modified scan bodies (Digital Wings; Segma Medical Technology for IOSM, Beijing, China) | 10 | Best-fit algorithm | Six |
| Zhang et al., 2024 [45] | In vitro | Original scan bodies (group OS) Computer-aided design and computer-aided manufacturing (CAD/CAM) scan bodies without extension (group CS) CAD/CAM scan bodies with straight extension (group CSS) CAD/CAM scan bodies with arcuate extension (group CSA) | 10 | Best-fit algorithm | Four |
| Alkindi et al., 2024 [38] | In vitro | Short scan bodies (SSB) Long scan bodies (LSB) | 10 | Best-fit algorithm | Two |
| Park et al., 2024 [44] | In vitro | Conventional scan bodies with no vertical stop (nS) Experimental scan bodies with a vertical stop (S) | 10 | A computerized coordinate measuring machine (CMM: Contura; Zeiss) was used to probe the reference casts, and a computational software program (Calypso; Zeiss) was used to compute the 3D coordinates of the implant platform centroids and projection angles of the implant long axes. | Three |
| Pan et al., 2025 [48] | In vitro | Nine cylinders (⌀4.8 × 4 mm, ⌀4.8 × 8 mm, ⌀4.8 × 12 mm, ⌀5.5 × 4 mm, ⌀5.5 × 8 mm, ⌀5.5 × 12 mm, ⌀6.5 × 4 mm, ⌀6.5 × 8 mm, ⌀6.5 × 12 mm) Five cuboids (3 × 6 × 8 mm, 3 × 6 × 12 mm, 4 × 6 × 6 mm, 5 × 6 × 12 mm, 5 × 6 × 8 mm) Sphere (⌀8 mm) | 7 | Direct measurements are based on a physical standard reference point, which serves as the ground truth with established Cartesian coordinates, without resorting to virtual alignment procedures. | NA |
| Ashry et al., 2024 [40] | In vitro | Scan bodies without accessory parts—scan bodies with accessory parts | 20 | Best-fit algorithm | Four |
| Farah et al., 2025 [47] | In vitro | With geometrical attachments Without geometrical attachments | 20 intraoral scans (10 per scanner: 5 with geometric attachments, 5 without) | best-fit algorithm | Four |
| Michelinakis et al., 2024 [43] | In vitro | Straumann Cares Mono RN (STR) Paltop SP (PLT)—MIS SP V3 (MIS) TRI TV70 scan (TRI) | 10 | Best-fit algorithm | Four |
| Uzel et al., 2023 [37] | In vitro | Group 1: No modifications Group 2: A 2 mm × 3 mm slot was made on the proximal surface without damage to the occlusal surface Group 3: A 3 mm × 4 mm slot was made on the proximal surface without damage to the occlusal surface Group 4: A 3 mm × 6 mm slot was made including the occlusal and proximal surfaces | 10 | Best-fit registration program | Two |
| Shely et al., 2023 [36] | In vitro | MIS ISB (asymmetrical geometry, trapezoid with sharp angles with a larger surface area compared to the ZZ ISB, internal hex connection) (MIS) Zirkonzhan ISB (cylindrical with no angles/asymmetric geometry, internal hex connection) (ZZ) | 30 | Best-fit algorithm | Three |
| Eldabe et al., 2025 [46] | In vivo | Tooth-modified scan body (TMSB) Conventional scan body (CSB) | total sample size: 68 (2 scans per implant) | “N-point registration” and “global registration” algorithms | 4 (n = 1) and 5 implants (n = 6) |
| Anwar et al., 2024 [39] | In vitro | Implant level scan bodies (CopaSky; bredent medical, Senden, Germany) (NM) Modified scan bodies (round depressions were ground into the center of the buccal and palatal surfaces of the scan bodies without interfering with the coronal geometric bevel of the SB) (M) | 10 | Best-fit algorithm | Four |
| Geometry Type | Reported Impact on Accuracy | Key References |
|---|---|---|
| Cylindrical (various heights/diameters) | Best linear and angular trueness, especially with 5.5 mm diameter and 12 mm height | [8,48] |
| Cuboidal | Generally worse trueness and angular accuracy; affected by height and cross-section area | [48] |
| Spherical | Intermediate trueness; angular accuracy not measurable due to shape | [48] |
| With rigid bar extension | Improved trueness and angular deviation in both in vitro and in vivo settings | [27] |
| With extensional structure | Reduced mean deviation from ~119 µm to ~69 µm and angular error from ~0.75° to ~0.36° | [22] |
| Subtractive modification | Improved angular accuracy vs. non-modified and additive types | [41] |
| Additive modification | Degraded surface trueness; introduced irregularities | [41] |
| With surface facets (diamond, trapezoid, etc.) | Mixed impact; some improved trueness, others not significant | [26,34] |
| Healing abutment-scan peg (hybrid) | Comparable or slightly worse than one-piece bodies | [30,35] |
| Tooth-modified scan body | Significantly reduced 3D deviations and angular errors | [46] |
| Depression-type scan body | Improved surface trueness (from ~0.282 mm to ~0.229 mm) | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohajerani, R.; Djalalinia, S.; Alikhasi, M. The Effects of Scan Body Geometry on the Precision and the Trueness of Implant Impressions Using Intraoral Scanners: A Systematic Review. Dent. J. 2025, 13, 252. https://doi.org/10.3390/dj13060252
Mohajerani R, Djalalinia S, Alikhasi M. The Effects of Scan Body Geometry on the Precision and the Trueness of Implant Impressions Using Intraoral Scanners: A Systematic Review. Dentistry Journal. 2025; 13(6):252. https://doi.org/10.3390/dj13060252
Chicago/Turabian StyleMohajerani, Roksana, Shirin Djalalinia, and Marzieh Alikhasi. 2025. "The Effects of Scan Body Geometry on the Precision and the Trueness of Implant Impressions Using Intraoral Scanners: A Systematic Review" Dentistry Journal 13, no. 6: 252. https://doi.org/10.3390/dj13060252
APA StyleMohajerani, R., Djalalinia, S., & Alikhasi, M. (2025). The Effects of Scan Body Geometry on the Precision and the Trueness of Implant Impressions Using Intraoral Scanners: A Systematic Review. Dentistry Journal, 13(6), 252. https://doi.org/10.3390/dj13060252







