1. Introduction
Dental enamel is the outermost layer of tooth crowns, the hardest and most mineralized tissue in the human body. It is a composite material of approximately 95% mineral phase, predominantly hydroxyapatite, Ca
10(PO
4)
6(OH)
2; 1% organic phase, mainly formed by proteins and lipids; and 4% water. Its composition and structure enable dental enamel to withstand a wide range of physical and chemical demands in the oral environment, including significant compressive forces and pH fluctuations [
1]. The mineral phase of dental enamel is susceptible to dissolution in acid solutions (pH < 5.5), leading to a loss of calcium, phosphate, and other ions; a phenomenon known as demineralization [
2]. Saliva has the natural ability to remineralize dental enamel by supplying calcium and phosphate ions; however, this natural remineralization process is not enough when the pH in the oral cavity tends to remain acidic for long periods of time [
3]. Consequently, persistent acidic conditions in the oral cavity, mainly caused by dietary habits and oral bacteria, result in permanent demineralization of the dental enamel, causing alterations in its surface properties, facilitating bacterial adhesion on the teeth surface, and finally contributing to an increase in oral conditions such as caries or dental erosion [
4].
According to the World Health Organization, dental caries (also referred to as dental cavities or tooth decay) is the most prevalent noncommunicable disease, affecting 60–90% of school-age children and most adults. It is considered a major public health concern that consumes 5–10% of the healthcare budget in industrialized countries [
5]. Caries lesions usually begin with permanent demineralization of dental enamel; thus, various strategies for remineralization of dental enamel have been recently developed to enhance or preserve oral health. Treatments frequently promote early enamel remineralization by driving precipitation and solidification of minerals onto the enamel surface from ion clusters in saturated solutions [
6]. Among the different remineralization strategies, the use of fluorine-based compounds is one of the most common strategies [
7]. Topical application of fluoride products, such as sodium fluoride (NaF), has been demonstrated to promote remineralization of non-cavitated enamel lesions; however, fluoride ion-promoted remineralization is limited by the availability of calcium and phosphate ions in the microenvironment [
8]. Thus, enough calcium and phosphate ions must be present in the oral microenvironment for fluorine-based product treatments to function correctly and promote remineralization.
Two of the most widely used fluorine-based remineralizing agents in pediatric dentistry are (a) varnishes containing beta-tricalcium phosphate (β-TCP) along with sodium fluoride (NaF; 22,600 ppm) and (b) pastes containing casein phosphopeptide (CPP) and amorphous calcium phosphate (ACP) along with NaF, where casein phosphopeptide, a milk-derived protein, stabilizes calcium phosphate in solution and facilitates its binding to enamel. For both fluorine-based products, fluoride enhances remineralization in the form of NaF, contributing to caries prevention [
9]. A dentist applies β-TCP-F varnishes in dental practice, while CPP-ACP-F pastes can be applied daily at home. The general population can freely choose between these two alternatives, so it is important to further assess the effectiveness of these two widely used remineralizing agents to better establish and understand their benefits and limitations [
10].
In the case of β-TCP-F-containing varnishes, they function as calcium and phosphate ion reservoirs, regulating the transfer of these ions into the teeth surface and synergistically enhancing remineralization alongside fluoride ions. Furthermore, the interaction among these ions in the oral environment can favor the fluorapatite receptor, which enhances enamel’s resistance to demineralization. Moreover, NaF prevents undesirable reactions between calcium and phosphate ions during storage. Upon contact with saliva, the protective varnish barrier dissolves gradually, releasing its ions over time for effective tooth remineralization [
11,
12]. The United States Food and Drug Administration (FDA) approves the use of cavity varnishes to treat sensitive teeth, especially on exposed dentin tissue and the tooth root. Varnishes are also approved for application into cavities right before the insertion of restorative materials to prevent their penetration into the dentin tissue. Fluoride varnishes are recognized as an effective treatment not only for hypersensitivity but also in cases of patients with a history of caries. One of the most commonly used cavity varnishes in pediatric dentistry is the Vanish
® (St. Paul, MN, USA) β-TCP-F system, which is mainly used for three reasons: (1) its properties have been designed to provide sustained release of ions, not only to the teeth in close contact with the varnish, but also within the whole oral cavity; (2) it is supplemented with an innovative form of β-TCP-F; and (3) it is easy and quick to apply in the dental practice.
Proteins like caseins regulate the solubility of calcium and phosphate ions in biological systems [
13], and high concentrations of these ions with no regulation can lead to undesirable calcifications. Thus, fluorine-based pastes containing CPP and ACP can favor controlled remineralization, avoiding undesirable calcification side effects. Casein is the major protein present in milk. It can bind to calcium and phosphate ions, forming stable complexes that can be released, by partial enzymatic digestion, in the form of CPP sequences [
14]. The CPP-ACP-F systems have been demonstrated to prevent tooth enamel demineralization and dental erosion [
15,
16]. When the pH in the oral cavity decreases, the CPP-ACP-F system releases Ca
2+ and PO
43−, creating a supersaturated state of these ions around teeth [
17], which is then stabilized by CPP at 4.5–7 pH values, forming minerals consistent with fluorapatite in the presence of fluoride ions [
18]. The CPP-ACP-F system has been shown to delay biofilm formation and favor the crystallization of calcium phosphates [
17,
19,
20,
21,
22], inhibiting the demineralization of tooth enamel and consequently preventing tooth decay [
23].
Currently, there are different commercial gels, varnishes, and pastes aimed at promoting tooth enamel remineralization. Although many have demonstrated good efficacy, further research is still needed to support their use and evaluate their effects on the various complementary properties of tooth enamel. In the present study, we focused on comparing the efficacy of a varnish containing β-TCP-F and a paste containing CPP-ACP-F, as these are two representative products of the different enamel remineralization systems available on the market [
24].
This work aimed to comparatively evaluate the in vitro effect on the surface of demineralized human dental enamel of two of the most used fluorinated compounds in pediatric dentistry, that is, a varnish containing β-tricalcium phosphate with sodium fluoride (β-TCP-F) and a paste containing casein phosphopeptide-amorphous calcium phosphate with sodium fluoride (CPP-ACP-F). Different methods exist to study the in vitro demineralization and remineralization of dental enamel at different intervals, such as the cyclical pH model, which simulates pH daily cycles in the oral environment [
25,
26]. Thus, after initial demineralization, demineralized enamel tooth surfaces were daily treated with either β-TCP-F or CPP-ACP-F and subjected to 21–3 h demineralization–remineralization pH cycles for 5, 10, and 15 days; finally the hardness, roughness, and wettability of the enamel surfaces were studied at each time interval (5, 10, or 15 days) and compared with those of healthy enamel surfaces. Demineralized enamel surfaces that received no treatment, neither β-TCP-F nor CPP-ACP-F, were subjected to a daily pH demineralization–remineralization cycle and used as negative comparative controls. Furthermore, the presence of fluoride ions was daily measured in the aqueous environment where experimental treated surfaces were subjected to demineralizing–remineralizing pH cycles. Finally, the susceptibility of the experimental enamel surfaces to biofilm formation was studied using the cariogenic bacteria
Streptococcus mutans.
This study shows the varying efficacy of two widely used fluorine-based products in mitigating the effects of demineralization on human dental enamel surfaces, including susceptibility to biofilm formation. Thus, it represents a valuable tool for dentists to make evidence-based decisions.
2. Materials and Methods
2.1. Obtention of Human Dental Enamel Specimens
The enamel samples were obtained from lower retained third molars from 17- to 22-year-old patients who needed orthodontic or preventive treatment and to whom third molars extraction surgery was recommended. As retained third molars were not exposed to the oral cavity before extraction surgery, the risk of contamination was significantly reduced. The third molars studied were obtained from donors undergoing dental extraction procedures who had informed consent, through donations from maxillofacial surgeons from different private clinics. All molars were obtained over three months. This study was approved by the Ethics Committee of the Faculty of Higher Studies (FES) Iztacala (CE/FESI/032022/1499, approved on 1 January 2022) and followed the Declaration of Helsinki.
2.2. Preparation of Experimental Groups
This study used 60 human third molars with intact crowns and no structural defects (i.e., molars presented healthy tooth enamel). Third molars obtained were cut in half longitudinally in a mesiodistal direction with a diamond disk (Brasseler™ diamond 910 < 20,000 rpm, Savannah, GA, USA), under constant irrigation to obtain 120 working surfaces; periodontal debris was removed before teeth were sectioned. The working specimens were filled with pink wax in the internal area of the tooth (pulp chamber and root canals) to obtain a flat back-surface and stored in deionized, distilled water until experimental tests were performed. Following Ten Cate [
19], non-fluorinated prophylactic paste (Viarden Lab, Mission, TX, USA) prophylaxis was undertaken. Working surfaces were finished by covering the root and crown with acid-resistant varnish (Revlon™, different colors, New York, NY, USA), leaving a 3 × 6 mm
2 area exposed in each sample at the center of the lingual or buccal surface of the anatomic crown. Thirty working samples were left as obtained and used as healthy enamel controls (Group I: HE), while the remaining 90 surfaces were lesioned with demineralizing solution for 96 h according to the pH cycling model by Ten Cate and Duijsters [
21], one of the best models for reproducing in vivo oral conditions and the most widely used model to study the loss and gain of minerals on artificially lesioned enamel. Briefly, the 90 enamel working surfaces were immersed in a demineralizing solution (2.2 mM CaCl
2, 2.2 mM NaH
2PO
4, and 0.05 M CH
3COOH) for 96 h at 37 °C, while the pH was adjusted to 4.4 with 1M KOH solution. Before beginning the experimental phase, each sample was immersed in a solution of distilled, deionized, and demineralized water in a temperature-controlled chamber at 36 °C to prevent changes in the enamel’s mineral content.
Then, 30 working surfaces were daily treated with the paste containing casein phosphopeptide-amorphous calcium phosphate with NaF (Mi Paste Plus™ GC IBÉRICA Dental Products, S.L. Edificio Codesa 2 Playa de las Americas, 2, 1°, Of. 4, 28290 Las Rozas, Madrid, España); the other 30 working surfaces were treated with the varnish containing β-tricalcium phosphate with NaF (Clinpro™ White Varnish. St. Paul, MN, USA), following the manufacturer’s instructions; and the last 30 working surfaces were left as demineralized and used as untreated, lesioned, negative control enamel samples.
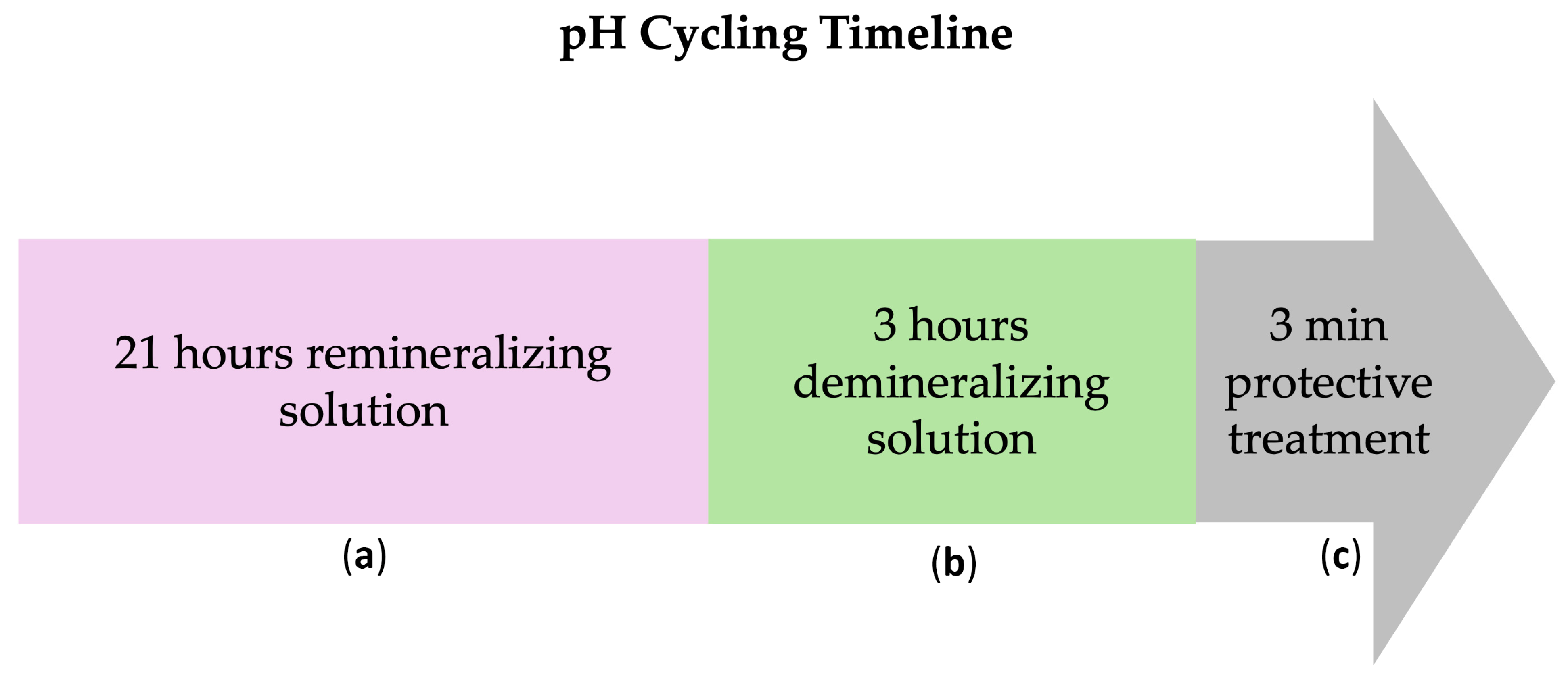
Subsequently, all working surfaces (either untreated or treated with β-TCP-F or CPP-ACP) were subjected to daily in vitro pH cycles, alternating demineralizing (pH = 4.4, for 21 h) and remineralizing (pH = 7, for 3 h) solutions, to mimic the in vivo oral conditions (
Figure 1). These cycles were performed daily for 5, 10, and 15 days using fresh pH solutions. At each measurement time (5, 10, and 15 days), 10 surfaces of each group were randomly chosen and immersed in deionized water at 37 °C, and their surface properties and susceptibility to biofilm formation were studied. Five days is equivalent to 1.2 months of remineralization treatment, 10 days to 2.5 months of remineralization treatment, and 15 days to 3.8 months of remineralization treatment. Considering this, a once-a-week application of the remineralization treatment can be used in cases of severe demineralization [
27,
28].
Working surfaces were categorized into three groups (
Figure 2) according to their treatment: Group II: initially lesioned and no treatment (IL, [n = 30]); Group III: initially lesioned and treated with β-tricalcium phosphate with NaF (β-TCP-F, [n = 30]); and Group IV: initially lesioned and treated with casein phosphopeptide-amorphous calcium phosphate with NaF (CPP-ACP-F, [n = 30]).
2.3. Fluoride Ion Content in the Demineralizing–Remineralizing pH Cycling Solutions
The amount of fluoride ions (ppm) in the demineralizing–remineralizing pH solutions was measured daily during the cyclical pH treatment using a Fluoride-Ion Selective Electrode (F-ISE) (Orion Star A-214®, Orion Research, Austin, TX, USA) and a pH/Ion 450/M® fluorimeter (Techno Scientific, Waltham, MA, USA). For electrode calibration, calibration curves from 0.250 to 2 μg/mL were constructed using Tissab II® solutions (Orion, Techno Scientific, Waltham, MA, USA), which hold the ions stable, raise the pH value, and release fluoride ions bound to metal ions. Measurements were performed at room temperature, in Tissab II® solution (Orion, Techno Scientific, Waltham, MA, USA), which keeps the ions stable, raises the pH value, and releases fluoride ions that are bound to the metal ions (iron, calcium, aluminum, etc.), preventing their interference in fluoride measurements. Fluoride concentration in the samples was determined by interpolating the calibration curves with a range of 2 to 0.25 μg/mL. Units of values reported correspond to millivolts.
To perform the measurements, the solutions were independently transferred to glass containers (Sigma-Merck, Darmstadt, Germany) using a dropper and mixed at room temperature (≈25 °C) with a magnetic stirrer. Reading was registered when the solution reached a stabilized state. The electrodes were rinsed with deionized water and dried with paper towels (Sanitas™, Madrid, Spain) between each measurement. New calibration curves were prepared daily to ensure the reproducibility of the results within ±2%.
2.4. Vickers Hardness
The Vickers hardness of 10 working surfaces from each group after 0, 5, 10, and 15 days of pH cycling treatment was determined using a durometer (Nano-Microindentador, NANOVEA Inc. Headquarters. Irvine, CA, USA; software Nanovea Micro Indentation Tester v1.8.3). Using a 20× objective lens, the indenter was placed perpendicularly to the working surface of each sample, and the assays were performed with the following conditions: approach speed: 50 μm/min; contact load: 50 mN; load: 10 N; loading rate: 5 N/min; and unloading rate: 5 N/min, with 10 notches per sample. The Vickers hardness number (VHN) was calculated using the Nanovea Micro Indentation Tester™ (NANOVEA Inc. Headquarters. Irvine, CA, USA) software [
29].
2.5. Surface Roughness
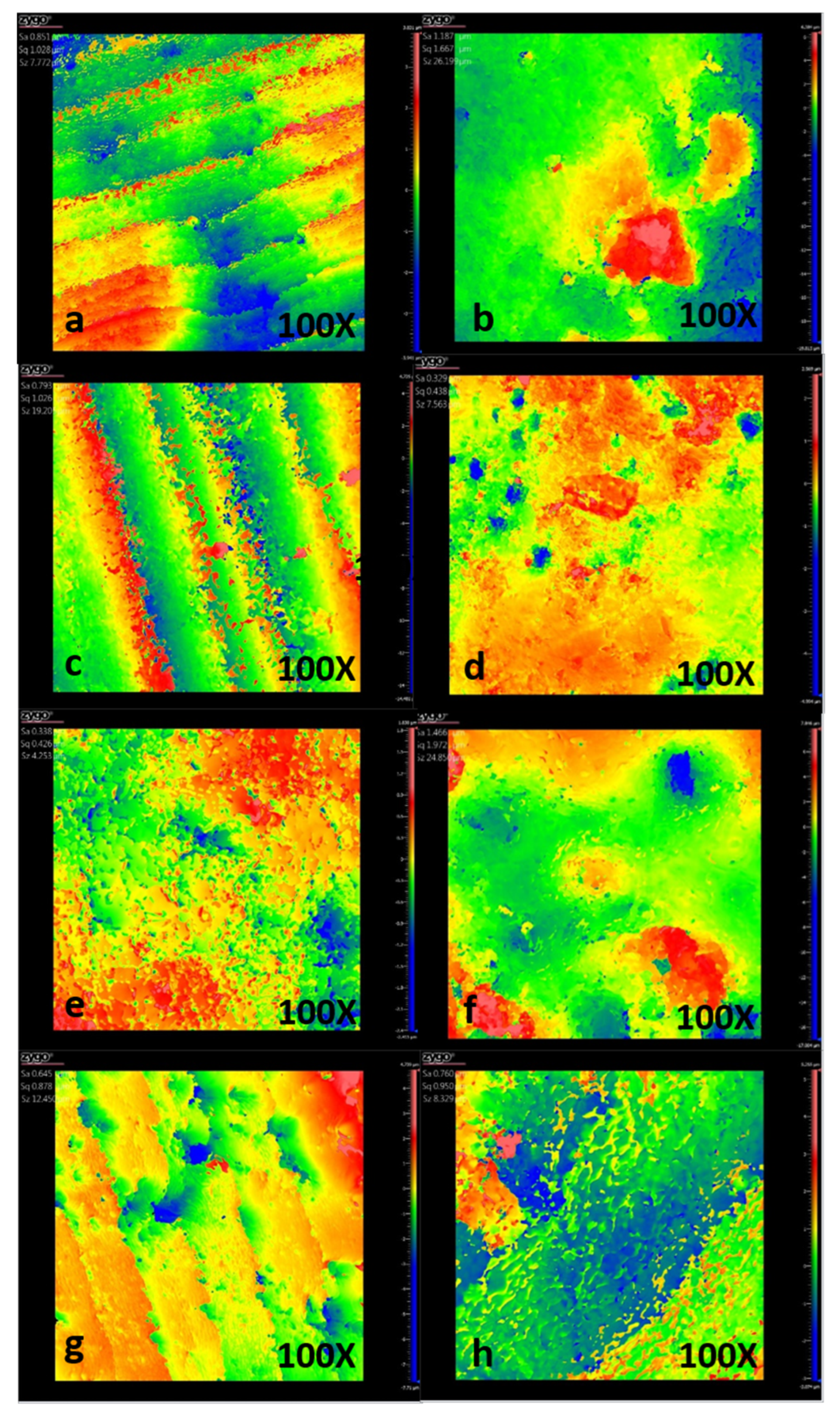
The roughness of the surfaces was measured using a ZYGO 3D-Nexview (Zygo, Middlefield, CT, USA) non-contact optical profilometer. Average roughness, Ra, which is defined as the arithmetic mean of the absolute values of the surface profile deviations from the mean line, was used to report the surface roughness. It measures the average distance between the surface’s peaks and valleys over a given evaluation length and is the most widely reported roughness value in materials science. For this, six roughness measurements (Ra) were randomly recorded on the 3 × 6 mm2 working surface of each sample, and the average of the profilometry data was obtained. Surface roughness measurements were recorded at 0, 5, 10, and 15 days of pH cycling treatment, and significant differences were calculated by comparing experimental treated groups’ measurements vs. Group I (Healthy Enamel; HE) and Group II (Initial Lesion, not treated; IL).
2.6. Wettability
The wettability of the surfaces was analyzed by measuring their water contact angle, using a goniometer (Dataphysics OCA-15EC. DataPhysics Instruments, Filderstadt, Germany). Two measurements were performed for each sample, placing a sessile drop of deionized water (4 μL) on the 3 × 6 mm2 working surface. The contact angle was determined using the SCA20_U software (V.5.0.38 build 5038), one second after the water droplet on the surface was stable, with 20 measurements performed for each sample in all groups, taking 10 contact angles on the left side and 10 on the right side as references.
2.7. Scanning Electron Microscopy
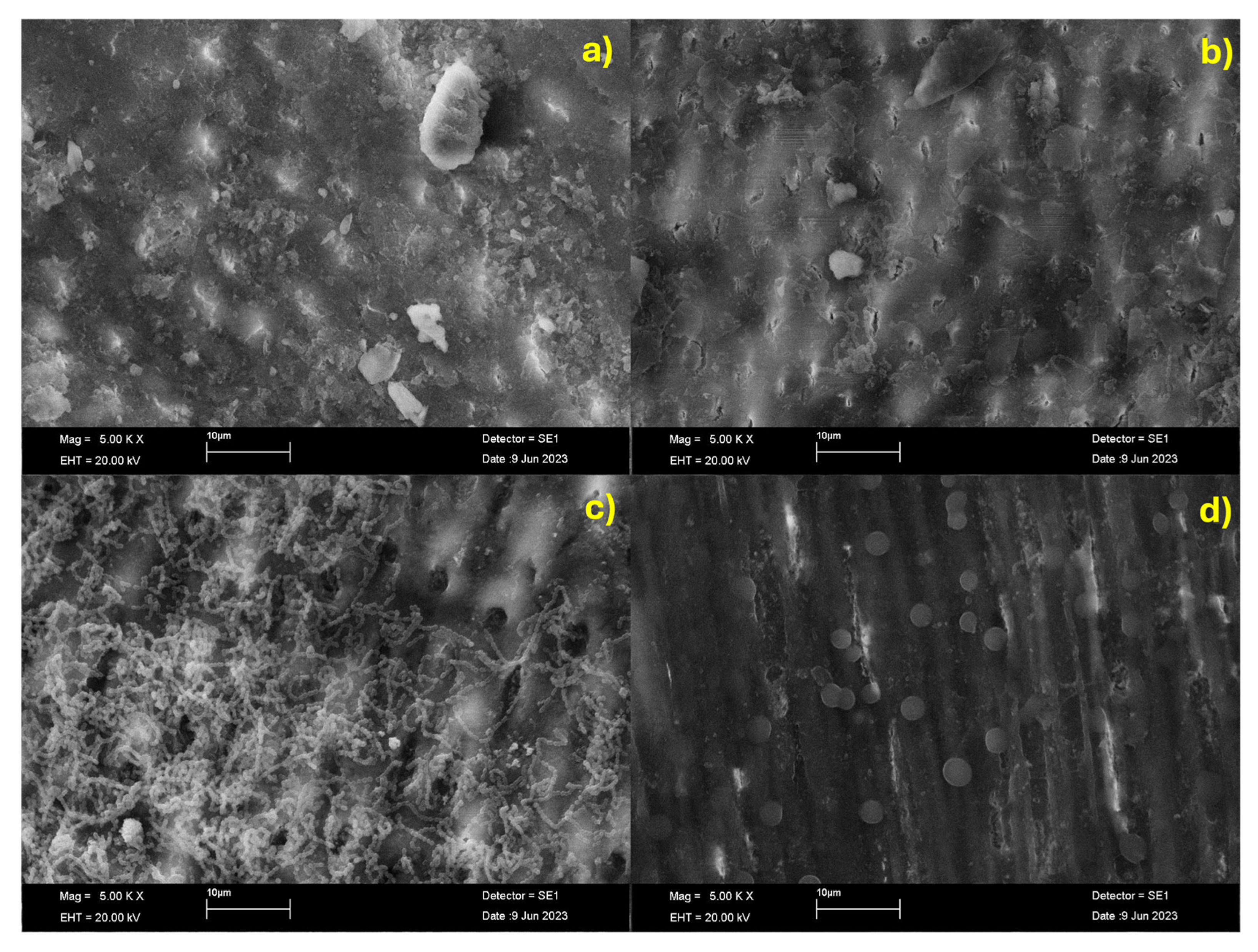
The samples were morphologically characterized by field emission scanning electron microscopy (FE-SEM, JEOL JSM-7600F, JEOL Ltd., Tokyo, Japan). Before SEM observation, samples were coated with a thin conductive carbon layer using a vacuum coater to avoid artifacts and ensure adequate electrical conductivity. Samples were analyzed under high vacuum conditions, at an acceleration voltage of 20 kV and a magnification of 5000×, using the secondary electron detector to obtain high-resolution images that allowed a representative and accurate surface topography evaluation.
2.8. Susceptibility to Streptococcus mutans Biofilm Formation
Biofilm formation of the cariogenic bacteria Streptococcus mutans (S. mutans; 25175™, ATCC®, Manassas, VA, USA) on dental enamel treated with β-TCP-F or CPP-ACP-F for 5, 10, and 15 days was evaluated by the MTT assay (Sigma-Aldrich. Merck KGaA, Darmstadt, Germany). For this, a pure culture of S. mutans was collected from a Petri dish prepared with HK agar (Trypticase Soy Agar, Brain Heart Infusion Agar, and Yeast, acquired from BBL®) enriched with menadione 1% v/v (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), hemin 1% v/v (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), and 5% of defibrinated sheep blood (Microlab Laboratory S.A. de C.V., Tlalnepantla, Mexico) and resuspended in Mycoplasma Broth Base (BBL®, Sigma. Saint Louis, MO, USA) enriched with menadione 1% v/v and hemin 1% v/v. A bacterial suspension of 1 × 109 cells/mL was obtained by adjusting the suspension to an optical density (OD) of 1 at λ = 600 nm, using a spectrophotometer (BioPhotometer D30. Fisher Scientific. 28108, Alcobendas, Madrid, Spain). Then, steam-sterilized surface samples (three from each group) were placed in 24-well culture plates, and 2 mL of a bacterial suspension at 1 × 107 cells/mL was inoculated in each well. Healthy dental enamel (Group I: HE) and demineralized dental enamel (Group II: IL) were used as positive and negative control groups, respectively. Inoculated samples were incubated for 24 h at 37 °C and 80 rpm in an orbital shaker (IKA® KS 130 basic, Staufen, Germany) under anaerobic conditions. After the incubation period, the samples were rinsed twice with steam-sterilized double-distilled water (dd-H2O) to detach loosely attached bacteria, transferred to a new culture well plate, and incubated with a 1:10 solution of MTT–culture broth for 3 h at 37 °C and 80 rpm, under dark and anaerobic conditions. Then, the formazan crystals metabolized by the viable bacterial attached on the samples were solubilized in a 1:1 solution of 2-propanol 99% (ISO; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) and dimethyl sulfoxide (DMSO; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany), and the OD was read at λ = 570 nm (Filter-MaxF5 multi-mode microplate reader; Molecular Devices, San Jose, CA, USA) to determine the amount of viable bacterial attached to the surfaces and, consequently, their susceptibility for biofilm formation.
2.9. Statistical Analysis
The Shapiro–Wilk test was performed to analyze the data distribution of the F-ISE variables (ppm of fluoride ions), hardness, and roughness. Non-parametric Kruskal–Wallis tests were performed for the F-ISE and hardness data, and multiple comparisons were made using Wilcoxon’s Rank and Dunn’s Test, respectively. Intergroup comparison for roughness measurements was performed with one-way analysis of variance (ANOVA) followed by pairwise comparison using Tukey’s honestly significant difference (HSD) post hoc test. Bacterial experiments were performed in triplicate, and the results were presented as the mean values (ME) ± the standard error of the mean (SEM); data were analyzed using the one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. In all tests, p < 0.05 was considered statistically significant. All statistical analyses and graphs were performed with the GraphPad Software Prism 8.0® (225 Franklin Street. Fl. 26, Boston, MA, USA).
4. Discussion
In the present study, we focused on evaluating the remineralizing effect of two fluoride-based treatments. In vitro models are essential for generating new knowledge that provides theoretical support for making initial predictions about the success of a new material, vehicle, or treatment. In vitro studies offer bioequivalence and predictions about the implementation of any product in clinical practice [
30,
31].
Significant advancements in remineralization therapies have focused on prolonging periods of supersaturation by delivering bioavailable calcium, phosphate, and fluoride ions to demineralized lesions, creating stable systems for ion delivery. To evaluate the efficacy of these strategies in vitro, researchers frequently employ methods like the cyclical pH model [
23,
25]. This model effectively simulates the fluctuating pH conditions of the oral cavity. It is based on alternating demineralizing (pH 4.4 for 21 h) and remineralizing (pH 7.0 for 3 h) solutions [
18,
21]. The cyclical pH method is a widely accepted approach for reproducing the demineralization and remineralization dynamics observed in vivo [
26]. In this study, we utilized this well-established cyclical pH model, chosen for its precise control and ability to minimize variability while requiring a smaller sample size. This model allowed us to investigate the in vitro effects of two prominent fluorine-based remineralizing agents in pediatric dentistry: β-TCP-F and CPP-ACP-F. These agents were delivered through two distinct vehicles: a paste containing CPP-ACP and 900 ppm sodium fluoride (Mi Paste Plus™) and a varnish containing β-TCP-F and 22,600 ppm sodium fluoride (Clinpro™ White Varnish), and their effects on human enamel were assessed over 5, 10, and 15 days.
Amorphous calcium phosphate (ACP) serves as a fundamental building block for mineralization in both bone and teeth. As a precursor to hydroxyapatite, ACP solutions are rich in calcium and phosphate ions, existing as highly hydrated clusters capable of delivering these ions to repair damaged enamel. The addition of casein phosphopeptide (CPP) further enhances the bioavailability of these ions. CPP, with its four to seven phosphate groups, can bind to ACP nanoclusters, effectively stabilizing and delivering them to the tooth surface. Crucially, the incorporation of fluoride further promotes the uptake of these ions by the enamel [
9].
In contrast, β-tricalcium phosphate (β-TCP-F) in the Clinpro™ White Varnish employs a different mechanism. Upon application, saliva breaks down the varnish matrix, providing the tooth with immediate access to calcium ions, phosphate groups, and a high concentration of sodium fluoride (22,600 ppm, equivalent to 50 mg). This varnish utilizes an alcohol-based solution of modified resins and β-TCP-F, and notably includes xylitol. Xylitol is recognized for its significant preventive properties against biofilm formation, primarily by inhibiting the accumulation of
Streptococcus mutans and
Lactobacillus acidophilus. While indicated for dentin hypersensitivity, its efficacy in treating initial caries lesions is also under investigation. Some research suggests that high concentrations of sorbitol and xylitol can form Ca
2+ polyol complexes in saturated calcium sulfate solutions, potentially influencing calcium bioavailability in saliva and promoting remineralization in deeper enamel layers. However, the manufacturer of the Clinpro™ White Varnish does not disclose the specific concentration of xylitol [
32]. Given the distinct mechanisms of action of CPP-ACP-F and β-TCP-F, this in vitro study compared their remineralizing potential on artificially induced enamel lesions using the cyclical pH model.
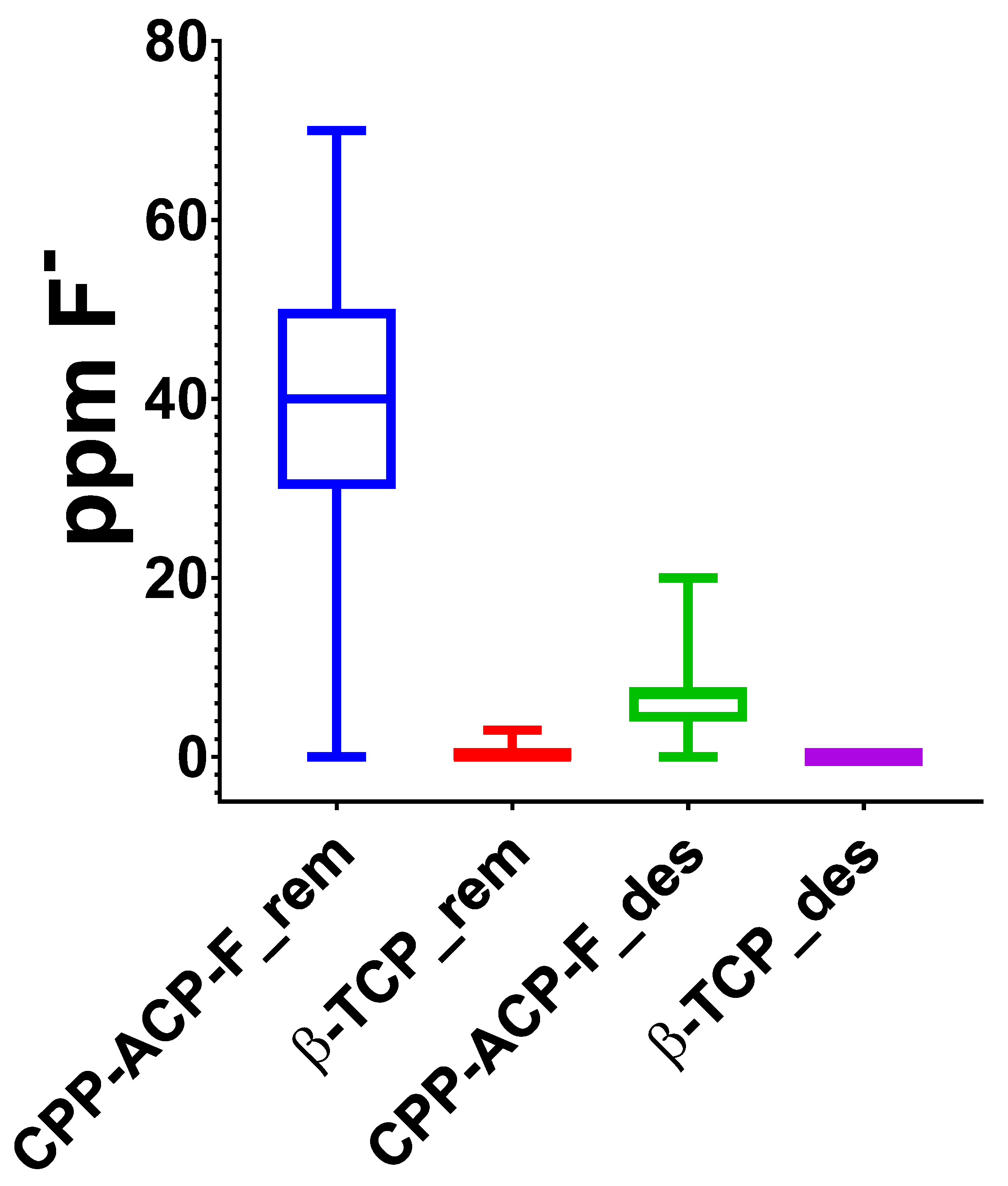
To comprehensively simulate the dynamic oral environment within our in vitro cyclical pH model, the concentration of fluoride ions in both the demineralizing and remineralizing solutions was meticulously measured daily throughout the 15-day treatment period. This approach provides a valuable insight into the potential systemic fluoride exposure that might occur due to the swallowing of saliva containing residual treatment agents. To our knowledge, no prior studies have reported such detailed longitudinal fluoride measurements in this type of experimental setup. Our analysis revealed a significantly higher average fluoride ion concentration (40 ppm) in the demineralizing solutions used for the varnish treatment (β-TCP-F) compared to the paste treatment (CPP-ACP-F). This finding directly correlates with the higher sodium fluoride concentration in the varnish (22,600 ppm) compared to the paste (900 ppm). While the high fluoride concentration in the varnish may contribute to its remineralizing efficacy in vitro, it also raises a crucial clinical concern. The potential for increased systemic fluoride absorption with varnish application warrants careful consideration, particularly in children aged 3 to 8 years during permanent dentition development, where excessive fluoride exposure can lead to dental fluorosis [
21].
Such findings were not reported in other in vitro research using a 96 h demineralization model that reported comparable efficacy between β-TCP-F and CPP-ACP-F. However, it is important to mention that different concentration ranges were employed in that study (30–90 mM) [
33]. Considering these variations in experimental parameters, future research stemming from our work could explore the impact of varying the concentrations of both treatments to determine if significant changes in the fluoride ion concentration and remineralization efficacy occur.
Currently, the effectiveness of remineralizing agents in treating early caries lesions in vitro [
18,
26] and managing erosive wear [
26] is well documented. While the literature supports the efficacy of CPP-ACP-F in dental remineralization, often enhanced by the addition of fluoride, our present study uniquely contributes to this understanding by specifically investigating the surface modifications induced on lesioned human enamel following treatment. To comprehensively evaluate the effectiveness of the remineralization treatments, we assessed both roughness and hardness parameters, as these are critical indicators of healthy enamel integrity and function [
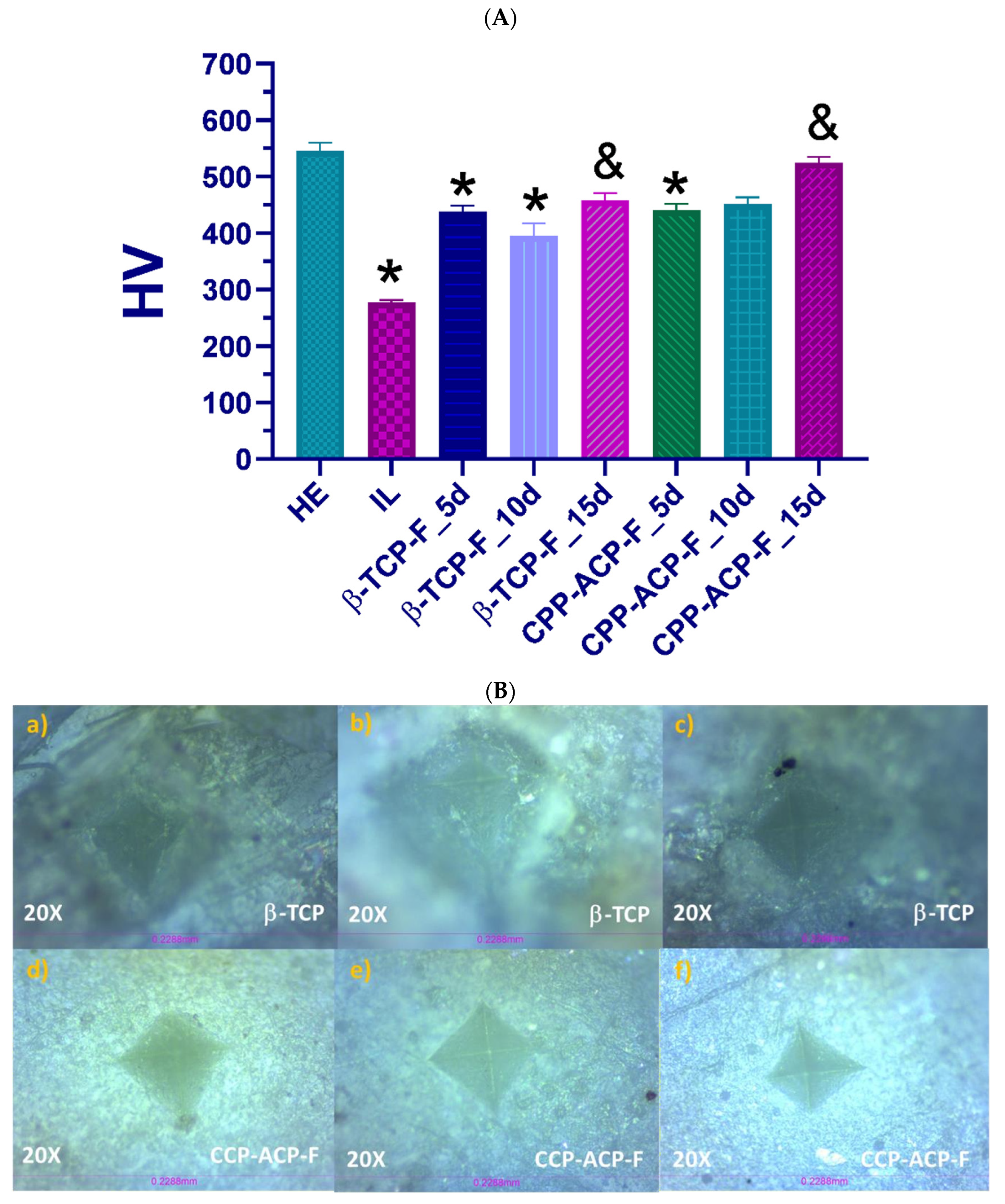
34]. Specifically, Vickers hardness testing was employed as an experimental method to determine the resistance of the enamel tissue to scratching or penetration under a controlled 10 N load across all experimental groups. Our initial measurements confirmed that the hardness values of healthy enamel were significantly higher than those of demineralized (lesioned) enamel. This reduction in hardness is likely a consequence of mineral ion loss during demineralization, leading to weakened surface bonds. Following the application of CPP-ACP-F and β-TCP-F to the demineralized human dental enamel and subsequent exposure to mineralizing–demineralizing pH cycling conditions for 5, 10, and 15 days (mimicking the oral environment), the enamel exhibited a partial recovery of its hardness. The CPP-ACP-F-treated samples showed a slightly greater increase in hardness compared to those treated with β-TCP-F, suggesting a potentially more effective remineralization of the enamel’s mechanical properties.
Our findings, demonstrating a partial recovery of enamel hardness with both treatments and a slightly greater increase with CPP-ACP-F compared to β-TCP-F, align with the results reported by Bhat et al. [
34], who also found CPP-ACP-F to be superior using the same treatment agents. While there were differences in the experimental design—Bhat et al. employed a longer 72 h demineralization process and a 28-day treatment duration—the most notable methodological distinction lies in the hardness assessment. They evaluated microhardness, while our study focused on macrohardness using a 10 N load (equivalent to 1.019 kg). Both microhardness and macrohardness, however, serve as indicators of the material’s resistance to indentation. Therefore, the consistency in the observed superiority of CPP-ACP-F across these two studies, despite variations in experimental parameters and hardness assessment techniques, provides a more robust and comprehensive understanding of the effects of these remineralizing agents on enamel.
Regarding the surface roughness of the samples, healthy dental enamel exhibited a lower roughness (Ra = 0.88 μm) compared to demineralized enamel (Ra = 1.33 μm). This increase in roughness in the demineralized group confirms the surface erosion and development of an incipient lesion resulting from exposure to the acidic environment (pH = 4.4) of the demineralizing solution [
4]. Following a 15-day treatment with CPP-ACP-F, the surface roughness of the lesioned enamel significantly decreased to Ra = 0.76 μm, effectively reaching levels comparable to those of healthy enamel. This substantial reduction strongly suggests that CPP-ACP-F was successful in remineralizing the damaged enamel surface. In contrast, the treatment with β-TCP-F resulted in only a modest reduction in the roughness of the lesioned enamel (Ra = 1.193 μm) after 15 days, potentially indicating a comparatively lower degree of remineralization achieved by β-TCP-F [
22]. This reduction in roughness observed with both treatments can be attributed to the adsorption and deposition of ions onto the irregular surface morphology of the lesioned enamel, effectively filling the demineralization defects.
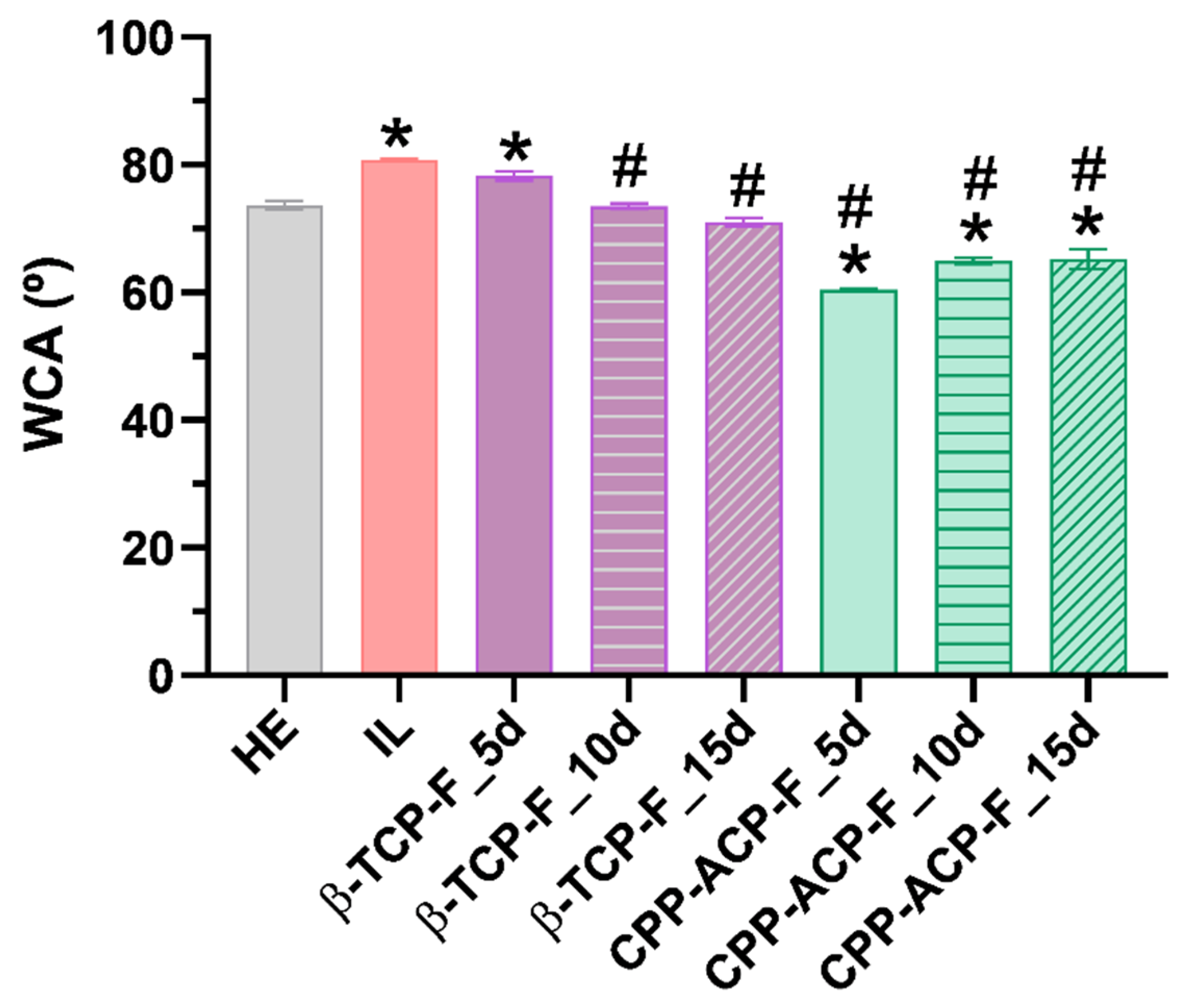
Wettability, a significant surface property influenced by surface energy and roughness, was evaluated in this study using deionized water to characterize the hydrophobic/hydrophilic nature of the enamel surfaces. Based on the classic surface classification (water contact angle (WCA) > 90° considered hydrophobic), all experimental surfaces exhibited a hydrophilic character. This finding supports the idea that the application of both studied products may enhance the enamel’s resistance to acidic attacks. However, applying Vogler’s definition [
35], which sets a biological context limit of WCA 65° to distinguish between hydrophilic and hydrophobic surfaces, reveals a more nuanced picture. The enamel surfaces treated with CPP-ACP-F paste are the only ones showing WCAs slightly below 65°, thus classifying them as hydrophilic under this biological context. This hydrophilic character could potentially correlate with the observed results regarding biofilm formation. The wettability of a surface is determined by the interplay of its microroughness, chemical composition, and intrinsic surface energy, and these properties are also known to influence bacterial adhesion [
36]. Generally, increased microroughness and wettability tend to promote bacterial adhesion.
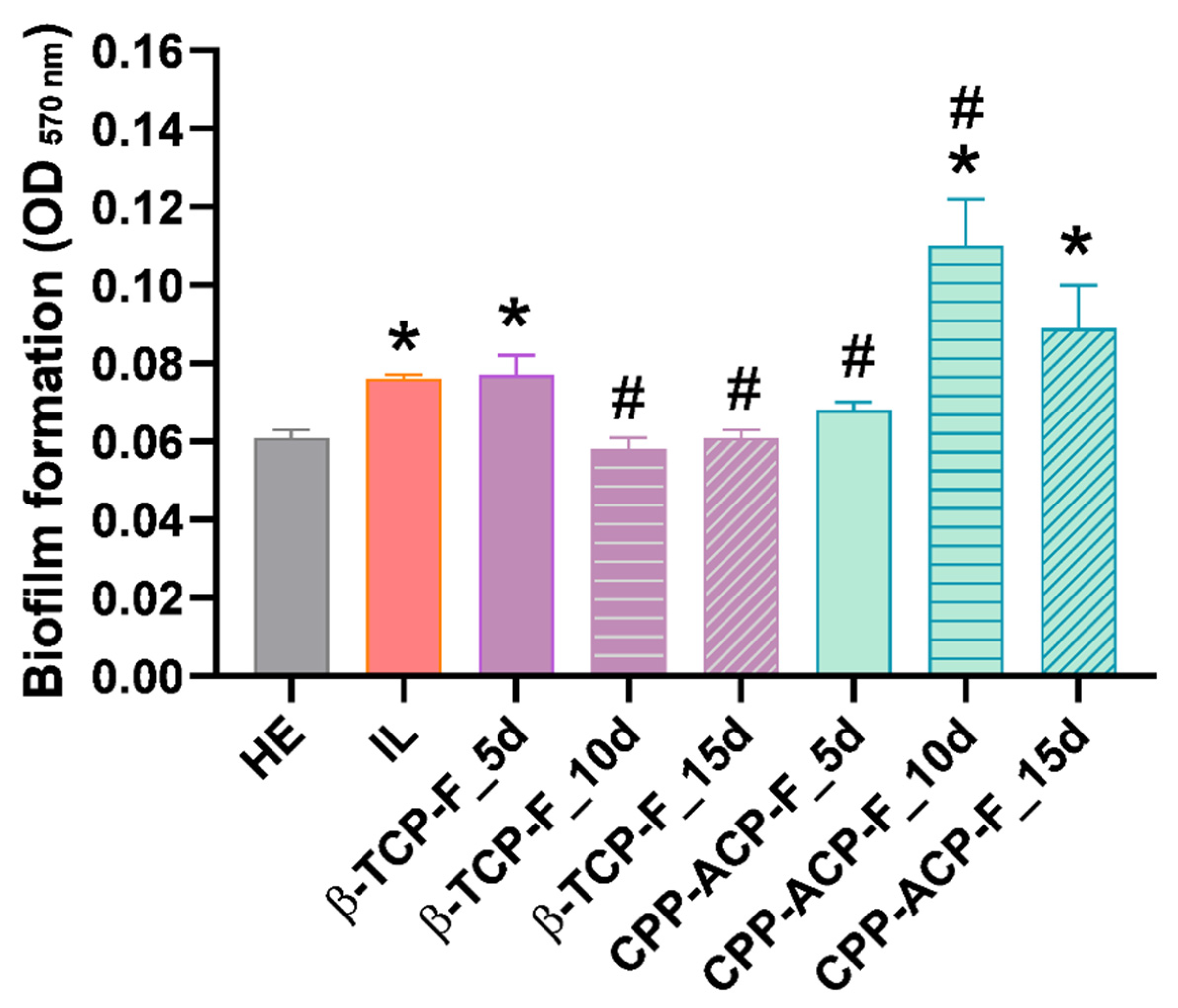
As expected, Streptococcus mutans biofilm formation was significantly higher on lesioned enamel surfaces compared to healthy enamel. Notably, a sustained protective effect against biofilm formation was observed following 15 days of treatment of the lesioned enamel with β-TCP-F, resulting in biofilm levels comparable to those on healthy enamel. This sustained reduction in biofilm is likely attributable to the similar surface properties, particularly wettability, achieved between the β-TCP-F-treated and healthy enamel. In contrast, while lesioned enamel surfaces treated with CPP-ACP-F initially showed a comparable anti-biofilm effect to healthy enamel after 5 days, this protection was not sustained at 10 and 15 days. The higher hydrophilic wettability of these CPP-ACP-F-treated surfaces, as previously discussed, may have facilitated the initial adhesion and subsequent spread of S. mutans, leading to enhanced colonization over time. Nonetheless, it is important to note that bacterial adhesion is a complex process that is also dependent on bacterial characteristics, such as size and cell envelope structure, and environmental factors, like pH, temperature, ions, and biomolecules.
Consistent with existing research [
16,
37], our findings indicate that both the CPP-ACP-F paste and β-TCP-F varnish treatments contribute to making acidic attacks from the oral environment less aggressive and enhance the remineralization of surface enamel, thereby preventing initial caries lesions. Supporting this, Tibba et al. [
38] evaluated the effect of Silver Diamine Fluoride (SDF) varnish (Riva Star) and sodium fluoride varnish on biofilm formation and other properties. Their results demonstrated a significant improvement in hardness with Riva Star application and a 25–35% reduction in oral biofilm formation with both Riva Star and NaF varnish. In our present investigation, we observed that β-TCP-F treatment for 10 and 15 days, and CPP-ACP-F treatment for 5 days, effectively protected the lesioned enamel surfaces against the initial biofilm growth of
S. mutans, achieving a similar level of protection as that observed on healthy dental enamel. This highlights the potential of both treatment modalities in mitigating early biofilm development, although the specific mechanisms and long-term efficacy may differ from those observed with SDF and NaF varnishes.
As our hardness results indicate, applying a fluorine-based varnish or paste to lesioned dental enamel is indeed very useful for the remineralization process, considerably increasing the enamel’s hardness values. This highlights the benefit of using a fluorinated and remineralizing compound compared to no treatment. The mechanism of CPP-ACP-F on the enamel surface is often compared to the remineralizing effect of saliva, as both deliver calcium and phosphate ions to the tooth surface, promoting mineral deposition. However, accurately replicating the complex dynamics of in vivo biofilm formation and its potential to directly influence ionic changes in the oral environment within an in vitro setting presents significant challenges. Therefore, the absence of a mature biofilm and the potentially different availability or dynamics of calcium and phosphate ions compared to the constant, low-level supersaturation provided by saliva in vivo might have influenced the extent to which the CPP-ACP-F system could exert its full remineralizing potential in our in vitro model [
39].
While the remineralizing effect of CPP-ACP-F on the enamel surface shares similarities with that of saliva, the complex in vivo environment, characterized by the presence of a biofilm that directly influences ionic exchanges, is challenging to fully replicate in vitro. The absence of this biofilm and the artificially optimized conditions in our experimental setup, potentially leading to a state of supersaturation of calcium and phosphate ions, might have influenced the remineralizing action of the CPP-ACP-F system compared to its performance under natural oral conditions. Recognizing these inherent limitations of our in vitro model, which does not account for factors such as variable saliva composition, dietary intake, hygiene habits, medication use, and the dynamic nature of the oral microbiota, our findings represent an initial yet promising step towards assessing the remineralizing effects of β-TCP-F and CPP-ACP-F. To gain a more realistic understanding of how these two products impact tooth enamel health, future research should focus on conducting evaluations in in vivo models and clinical studies that can better capture the complexities of the oral environment.