Periodontal Pathogens Correlate with Rheumatoid Arthritis Disease Parameters: A Systematic Review Based on Clinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Protocol and Registration
2.2. Search Strategy
2.3. Risk of Bias
3. Results
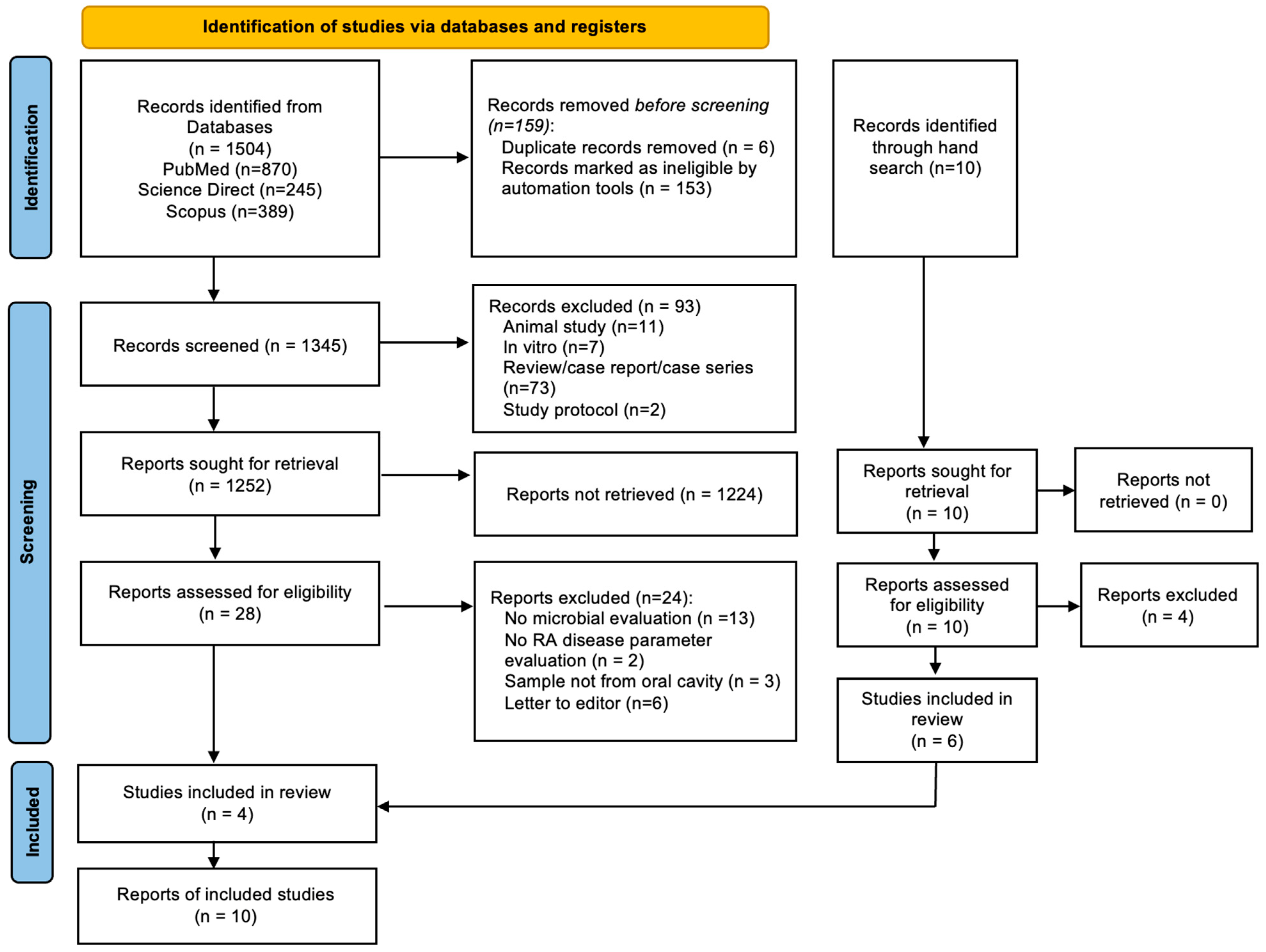
3.1. Overview Search Process
3.2. Studies Included
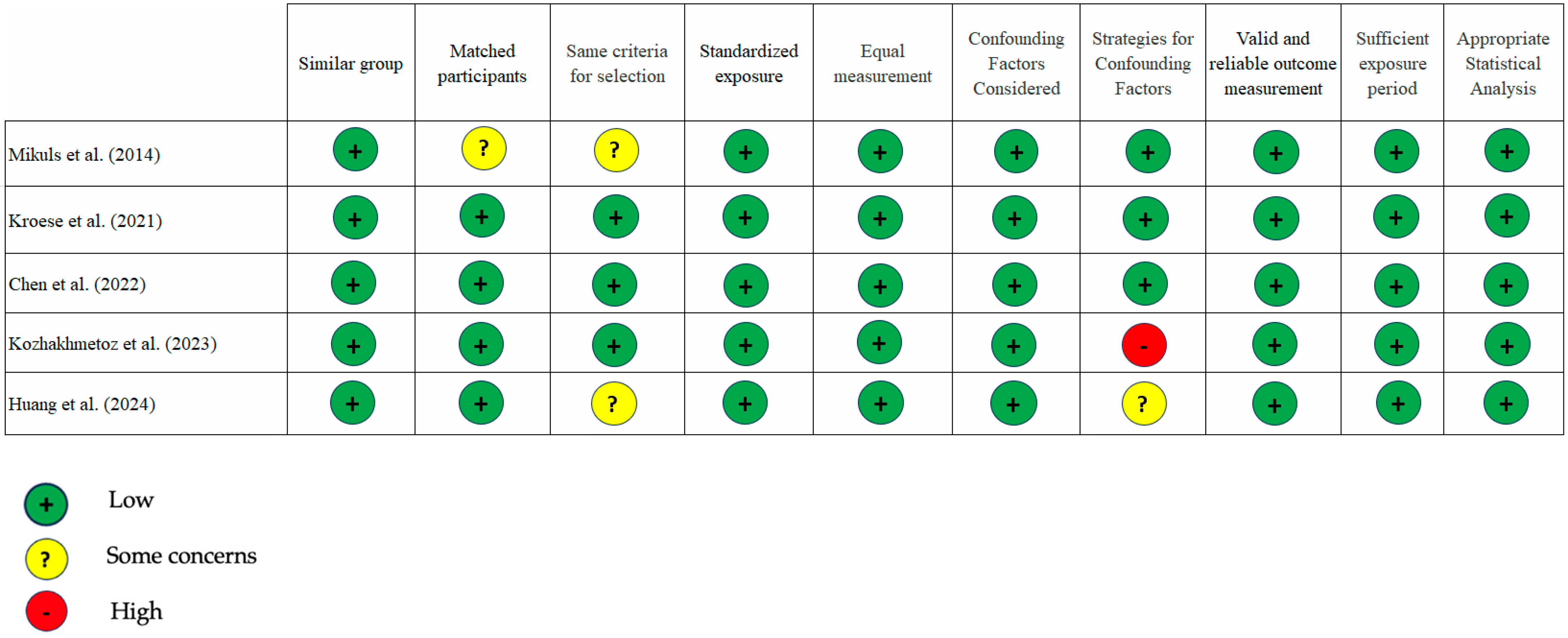
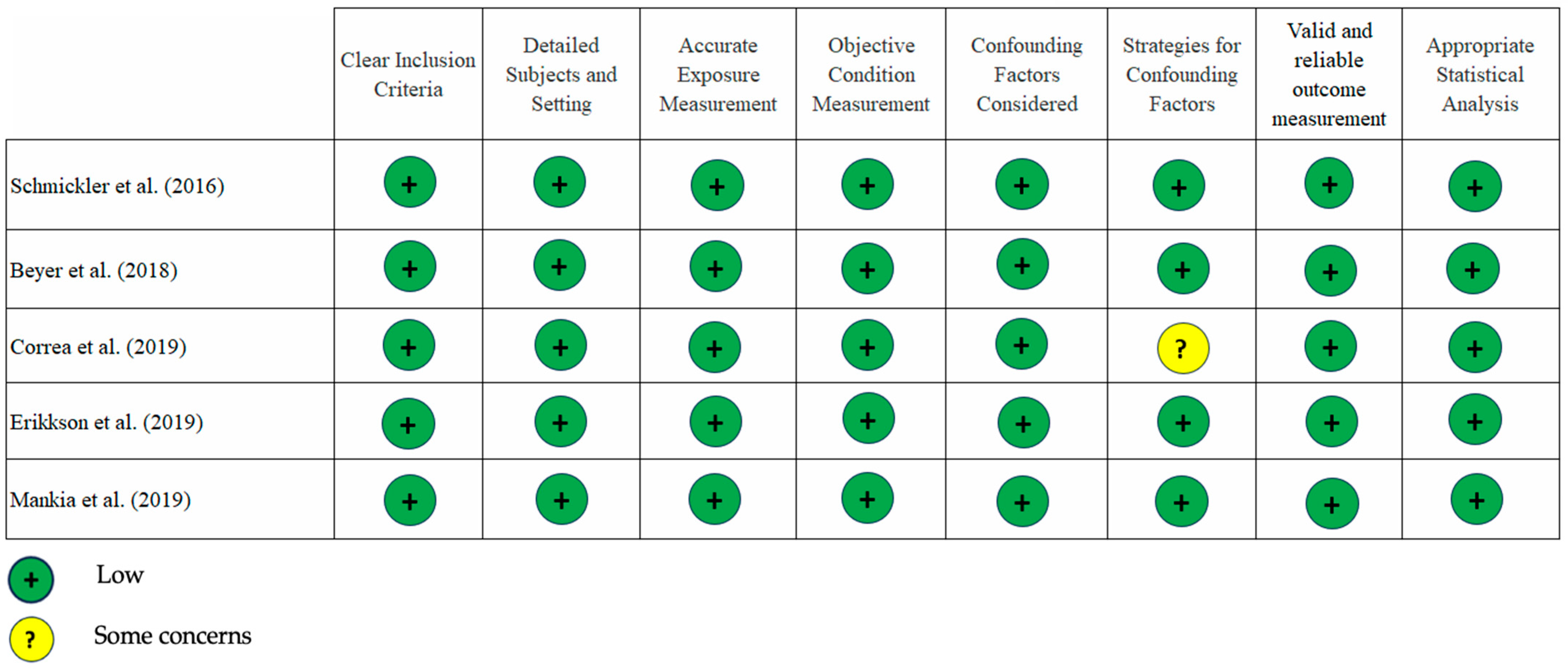
3.3. Quality of the Studies
3.4. The Description of Study Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| RA | Rheumatoid Arthritis |
| PD | Periodontal Disease |
| ACPA | Anti-Citrullinated Protein Antibodies |
| Anti-CCP2 | Anti-Cyclic Citrullinated Peptide |
| RF | Rheumatoid Factor |
| IgA | Imunoglobulin A |
| PAD | Peptidyl-Arginine Deiminase |
| PPAD | P. gingivalis Peptidyl-Arginine Deiminase |
| Ltx-A | Leukotoxin A |
| GCF | Gingival Crevicular Fluid |
| RT/qPCR | Real-Time/quantitative polymerase chain reaction |
| hs-CRP | High sensitivity-C-Reactive Protein |
| DAS-28 | Disease Activity Score-28 |
| NGS | Next Generation Sequencing |
| WGS | Whole Genome Sequencing |
| DNA | Deoxyribonucleic Acid |
| BMI | Body Mass Index |
References
- González-Febles, J.; Sanz, M. Periodontitis and Rheumatoid Arthritis: What Have We Learned about Their Connection and Their Treatment? Periodontology 2000 2021, 87, 181–203. [Google Scholar] [CrossRef] [PubMed]
- de Molon, R.S.; Rossa, C., Jr.; Thurlings, R.M.; Cirelli, J.A.; Koenders, M.I. Linkage of Periodontitis and Rheumatoid Arthritis: Current Evidence and Potential Biological Interactions. Int. J. Mol. Sci. 2019, 20, 4541. [Google Scholar] [CrossRef]
- Araújo, V.M.A.; Melo, I.M.; Lima, V. Relationship between Periodontitis and Rheumatoid Arthritis: Review of the Literature. Mediat. Inflamm. 2015, 2015, 259074. [Google Scholar] [CrossRef] [PubMed]
- Roszyk, E.; Puszczewicz, M. Role of Human Microbiome and Selected Bacterial Infections in the Pathogenesis of Rheumatoid Arthritis. Rheumatology 2017, 5, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.S.; Choi, Y.Y.; Lee, K.H. Relationship between Rheumatoid Arthritis and Periodontal Disease in Korean Adults: Data from the Sixth Korea National Health and Nutrition Examination Survey, 2013 to 2015. J. Periodontol. 2018, 90, 350–357. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Lee, K.H. Periodontitis as a Risk Factor for Rheumatoid Arthritis: A Matched-Cohort Study. Int. Dent. J. 2021, 71, 516–521. [Google Scholar] [CrossRef]
- Krutyhołowa, A.; Strzelec, K.; Dziedzic, A.; Bereta, G.P.; Łazarz-Bartyzel, K.; Potempa, J.; Gawron, K. Host and Bacterial Factors Linking Periodontitis and Rheumatoid Arthritis. Front. Immunol. 2022, 13, 980805. [Google Scholar] [CrossRef]
- Smit, M.; Westra, J.; Vissink, A.; Doornbos-van der Meer, B.; Brouwer, E.; van Winkelhoff, A. Periodontitis in Established Rheumatoid Arthritis Patients: A Cross-Sectional Clinical, Microbiological and Serological Study. Arthritis Res. Ther. 2012, 14, R222. [Google Scholar] [CrossRef]
- Konig, M.F.; Abusleme, L.; Reinholdt, J.; Palmer, R.J.; Teles, R.P.; Sampson, K.; Rosen, A.; Nigrovic, P.A.; Sokolove, J.; Giles, J.T.; et al. Aggregatibacter actinomycetemcomitans—Induced Hypercitrullination Links Periodontal Infection to Autoimmunity in Rheumatoid Arthritis. Sci. Transl. Med. 2016, 8, 369ra176. [Google Scholar] [CrossRef]
- Kobayashi, T.; Bartold, P.M. Periodontitis and Periodontopathic Bacteria as Risk Factors for Rheumatoid Arthritis: A Review of the Last 10 Years. Jpn. Dent. Sci. Rev. 2023, 59, 263–272. [Google Scholar] [CrossRef]
- Ahmadi, P.; Mahmoudi, M.; Kheder, R.K.; Faraj, T.A.; Mollazadeh, S.; Abdulabbas, H.S.; Esmaeili, S. Impacts of Porphyromonas gingivalis Periodontitis on Rheumatoid Arthritis Autoimmunity. Int. Immunopharmacol. 2023, 118, 109936. [Google Scholar] [CrossRef] [PubMed]
- Bingham, C.O.; Moni, M. Periodontal Disease and Rheumatoid Arthritis. Curr. Opin. Rheumatol. 2013, 25, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Kobayashi, T.; Ito, S.; Yokoyama, T.; Komatsu, Y.; Abe, A.; Murasawa, A.; Yoshie, H. Antibody Responses to Periodontopathic Bacteria in Relation to Rheumatoid Arthritis in Japanese Adults. J. Periodontol. 2011, 82, 1433–1441. [Google Scholar] [CrossRef]
- Corrêa, J.D.; Fernandes, G.R.; Calderaro, D.C.; Mendonça, S.M.S.; Silva, J.M.; Albiero, M.L.; Cunha, F.Q.; Xiao, E.; Ferreira, G.A.; Teixeira, A.L.; et al. Oral Microbial Dysbiosis Linked to Worsened Periodontal Condition in Rheumatoid Arthritis Patients. Sci. Rep. 2019, 9, 8379. [Google Scholar] [CrossRef]
- Laugisch, O.; Wong, A.; Sroka, A.; Kantyka, T.; Koziel, J.; Neuhaus, K.; Sculean, A.; Venables, P.J.; Potempa, J.; Möller, B.; et al. Citrullination in the Periodontium—A Possible Link between Periodontitis and Rheumatoid Arthritis. Clin. Oral Investig. 2015, 20, 675–683. [Google Scholar] [CrossRef]
- Goh, C.E.; Kopp, J.; Papapanou, P.N.; Molitor, J.A.; Demmer, R.T. Association between Serum Antibodies to Periodontal Bacteria and Rheumatoid Factor in the Third National Health and Nutrition Examination Survey. Arthritis Rheumatol. 2016, 68, 2384–2393. [Google Scholar] [CrossRef]
- Martu, M.A.; Solomon, S.M.; Sufaru, I.G.; Jelihovschi, I.; Martu, S.; Rezus, E.; Surdu, A.E.; Onea, R.M.; Grecu, G.P.; Foia, L. Study on the Prevalence of Periodontopathogenic Bacteria in Serum and Subgingival Bacterial Plaque in Patients with Rheumatoid Arthritis. Rev. Chim. 2017, 68, 1946–1950. [Google Scholar] [CrossRef]
- Lopez-Oliva, I.; Paropkari, A.D.; Saraswat, S.; Serban, S.; Yonel, Z.; Sharma, P.; de Pablo, P.; Raza, K.; Filer, A.; Chapple, I.; et al. Dysbiotic Subgingival Microbial Communities in Periodontally Healthy Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2018, 70, 1008–1013. [Google Scholar] [CrossRef]
- Manoil, D.; Bostanci, N.; Mumcu, G.; Can, M.; Direskeneli, H.; Belibasakis, G.N. Novel and Known Periodontal Pathogens Residing in Gingival Crevicular Fluid Are Associated with Rheumatoid Arthritis. J. Periodontol. 2020, 92, 359–370. [Google Scholar] [CrossRef]
- Eriksson, K.; Lundmark, A.; Delgado, L.F.; Hu, Y.O.O.; Fei, G.; Lee, L.; Fei, C.; Catrina, A.I.; Jansson, L.; Andersson, A.F.; et al. Salivary Microbiota and Host-Inflammatory Responses in Periodontitis Affected Individuals with and without Rheumatoid Arthritis. Front. Cell. Infect. Microbiol. 2022, 12, 841139. [Google Scholar] [CrossRef]
- Kroese, J.M.; Brandt, B.W.; Buijs, M.J.; Crielaard, W.; Lobbezoo, F.; Loos, B.G.; van Boheemen, L.; van Schaardenburg, D.; Zaura, E.; Volgenant, C.M.C. The Oral Microbiome in Early Rheumatoid Arthritis Patients and Individuals at Risk Differs from Healthy Controls. Arthritis Rheumatol. 2021, 73, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Rethlefsen, M.L.; Kirtley, S.; Waffenschmidt, S.; Ayala, A.P.; Moher, D.; Page, M.J.; Koffel, J.B. PRISMA-S: An Extension to the PRISMA Statement for Reporting Literature Searches in Systematic Reviews. Syst. Rev. 2021, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Moola, S.; Munn, Z.; Tufunaru, C.; Aromataris, E.; Sears, K.; Sfetc, R.; Currie, M.; Lisy, K.; Qureshi, R.; Mattis, P.; et al. Systematic Reviews of Aetiology and Risk. JBI Ebooks 2024. [Google Scholar] [CrossRef]
- Mikuls, T.R.; Payne, J.B.; Yu, F.; Thiele, G.M.; Reynolds, R.J.; Cannon, G.W.; Markt, J.; McGowan, D.; Kerr, G.S.; Redman, R.S.; et al. Periodontitis And Porphyromonas gingivalis in Patients with Rheumatoid Arthritis. Arthritis Rheumatol. 2014, 66, 1090–1100. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Hung, W.-C.; Chou, Y.-H.; Lai, C.-H.; Peng, P.; Jhou, P.-S.; Tsai, M.-R.; Sheu, J.J.-C.; Yen, J.-H. Subgingival microbiome in rheumatoid arthritis patients with periodontitis. Int. J. Mol. Sci. 2022, 23, 9883. [Google Scholar] [CrossRef]
- Kozhakhmetov, S.; Babenko, D.; Issilbayeva, A.; Nurgaziyev, M.; Kozhakhmetova, S.; Meiramova, A.; Akhmetova, Z.; Kunz, J.; Ainabekova, B.; Marotta, F.; et al. Oral Microbial Signature of Rheumatoid Arthritis in Female Patients. J. Clin. Med. 2023, 12, 3694. [Google Scholar] [CrossRef]
- Huang, Y.; Ni, S. Aggregatibacter actinomycetemcomitans with Periodontitis and Rheumatoid Arthritis. Int. Dent. J. 2023, 74, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Schmickler, J.; Rupprecht, A.; Patschan, S.; Patschan, D.; Müller, G.A.; Haak, R.; Mausberg, R.F.; Schmalz, G.; Kottmann, T.; Ziebolz, D. Cross-Sectional Evaluation of Periodontal Status and Microbiologic and Rheumatoid Parameters in a Large Cohort of Patients with Rheumatoid Arthritis. J. Periodontol. 2017, 88, 368–379. [Google Scholar] [CrossRef]
- Beyer, K.; Zaura, E.; Brandt, B.W.; Buijs, M.J.; Brun, J.G.; Crielaard, W.; Bolstad, A.I. Subgingival Microbiome of Rheumatoid Arthritis Patients in Relation to Their Disease Status and Periodontal Health. PLoS ONE 2018, 13, e0202278. [Google Scholar] [CrossRef]
- Eriksson, K.; Fei, G.; Lundmark, A.; Benchimol, D.; Lee, L.; Hu, Y.O.O.; Kats, A.; Saevarsdottir, S.; Catrina, A.I.; Klinge, B.; et al. Periodontal Health and Oral Microbiota in Patients with Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 630. [Google Scholar] [CrossRef]
- Mankia, K.; Cheng, Z.; Do, T.; Hunt, L.; Meade, J.; Kang, J.; Clerehugh, V.; Speirs, A.; Tugnait, A.; Hensor, E.M.A.; et al. Prevalence of Periodontal Disease and Periodontopathic Bacteria in Anti–Cyclic Citrullinated Protein Antibody–Positive At-Risk Adults without Arthritis. JAMA Netw. Open 2019, 2, e195394. [Google Scholar] [CrossRef] [PubMed]
- Mikuls, T.R.; Payne, J.B.; Deane, K.D.; Thiele, G.M. Autoimmunity of the Lung and Oral Mucosa in a Multisystem Inflammatory Disease: The Spark That Lights the Fire in Rheumatoid Arthritis? J. Allergy Clin. Immunol. 2016, 137, 28–34. [Google Scholar] [CrossRef]
- de Smit, M.J.; Rahajoe, P.S.; Raveling-Eelsing, E.; Lisotto, P.; Harmsen, H.J.M.; Kertia, N.; Vissink, A.; Westra, J. Influence of Oral Microbiota on the Presence of IgA Anti-Citrullinated Protein Antibodies in Gingival Crevicular Fluid. Front. Oral Health 2022, 3, 904711. [Google Scholar] [CrossRef] [PubMed]
- Kralik, P.; Ricchi, M. A Basic Guide to Real Time PCR in Microbial Diagnostics: Definitions, Parameters, and Everything. Front. Microbiol. 2017, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Garibyan, L.; Avashia, N. Research Techniques Made Simple: Polymerase Chain Reaction (PCR). J. Investig. Dermatol. 2013, 133, e6. [Google Scholar] [CrossRef]
- Hilt, E.E.; Ferrieri, P. Next Generation and Other Sequencing Technologies in Diagnostic Microbiology and Infectious Diseases. Genes 2022, 13, 1566. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 319–338. [Google Scholar] [CrossRef]
- Martinez-Martinez, R.E.; Abud-Mendoza, C.; Patiño-Marin, N.; Rizo-Rodríguez, J.C.; Little, J.W.; Loyola-Rodríguez, J.P. Detection of Periodontal Bacterial DNA in Serum and Synovial Fluid in Refractory Rheumatoid Arthritis Patients. J. Clin. Periodontol. 2009, 36, 1004–1010. [Google Scholar] [CrossRef]
- Reichert, S.; Haffner, M.; Keyßer, G.; Schäfer, C.; Stein, J.M.; Schaller, H.-G.; Wienke, A.; Strauss, H.; Heide, S.; Schulz, S. Detection of Oral Bacterial DNA in Synovial Fluid. J. Clin. Periodontol. 2013, 40, 591–598. [Google Scholar] [CrossRef]
- Témoin, S.; Chakaki, A.; Askari, A.; El-Halaby, A.; Fitzgerald, S.; Marcus, R.E.; Han, Y.W.; Bissada, N.F. Identification of Oral Bacterial DNA in Synovial Fluid of Patients with Arthritis with Native and Failed Prosthetic Joints. JCR J. Clin. Rheumatol. 2012, 18, 117–121. [Google Scholar] [CrossRef]
- Rosenstein, E.D.; Greenwald, R.A.; Kushner, L.J.; Weissmann, G. Hypothesis: The Humoral Immune Response to Oral Bacteria Provides a Stimulus for the Development of Rheumatoid Arthritis. Inflammation 2004, 28, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Hojo, K.; Nagaoka, S.; Ohshima, T.; Maeda, N. Bacterial Interactions in Dental Biofilm Development. J. Dent. Res. 2009, 88, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liu, J.; Merritt, J.; Qi, F. A YadA-like Autotransporter, Hag1 in Veillonella atypica Is a Multivalent Hemagglutinin Involved in Adherence to Oral streptococci, Porphyromonas gingivalis, and Human Oral Buccal Cells. Mol. Oral Microbiol. 2015, 30, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Ueki, A.; Goto, K.; Kaku, N.; Ueki, K. Aminipila butyrica Gen. Nov., Sp. Nov., a Strictly Anaerobic, Arginine-Decomposing Bacterium Isolated from a Methanogenic Reactor of Cattle Waste. Int. J. Syst. Evol. Microbiol. 2018, 68, 443–448. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Efimov, B.A.; Kondova, I.; Ouwerling, B.; Chaplin, A.V.; Shcherbakova, V.A.; Langermans, J.A.M. Peptococcus simiae Sp. Nov., Isolated from Rhesus Macaque Faeces and Emended Description of the Genus Peptococcus. Int. J. Syst. Evol. Microbiol. 2016, 66, 5187–5191. [Google Scholar] [CrossRef]
- Cai, L.; Zhu, H.; Mou, Q.; Wong, P.Y.; Lan, L.; Ng, C.W.K.; Lei, P.; Cheung, M.K.; Wang, D.; Wong, E.W.Y.; et al. Integrative Analysis Reveals Associations between Oral Microbiota Dysbiosis and Host Genetic and Epigenetic Aberrations in Oral Cavity Squamous Cell Carcinoma. NPJ Biofilms Microbiomes 2024, 10, 39. [Google Scholar] [CrossRef]
- Scher, J.U.; Ubeda, C.; Equinda, M.; Khanin, R.; Buischi, Y.; Viale, A.; Lipuma, L.; Attur, M.; Pillinger, M.H.; Weissmann, G.; et al. Periodontal Disease and the Oral Microbiota in New-Onset Rheumatoid Arthritis. Arthritis Rheum. 2012, 64, 3083–3094. [Google Scholar] [CrossRef]
- Erciyas, K.; Sezer, U.; Üstün, K.; Pehlivan, Y.; Kısacık, B.; Şenyurt, S.; Tarakçıoğlu, M.; Onat, A. Effects of Periodontal Therapy on Disease Activity and Systemic Inflammation in Rheumatoid Arthritis Patients. Oral Dis. 2012, 19, 394–400. [Google Scholar] [CrossRef]
- Polak, D. Is Aggregatibacter actinomycetemcomitans the Missing Link between Periodontitis and Rheumatoid Arthritis? Oral Dis. 2017, 24, 1148–1149. [Google Scholar] [CrossRef]
- Volkov, M.; Dekkers, J.; Loos, B.G.; Bizzarro, S.; Huizinga, T.W.J.; Praetorius, H.A.; Toes, R.E.M.; van der Woude, D. Comment on “Aggregatibacter actinomycetemcomitans—Induced Hypercitrullination Links Periodontal Infection to Autoimmunity in Rheumatoid Arthritis”. Sci. Transl. Med. 2018, 10, aan8349. [Google Scholar] [CrossRef]
- Li, Y.; Guo, R.; Oduro, P.K.; Sun, T.; Chen, H.; Yi, Y.; Zeng, W.; Wang, Q.; Leng, L.; Yang, L.; et al. The Relationship between Porphyromonas Gingivalis and Rheumatoid Arthritis: A Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 956417. [Google Scholar] [CrossRef] [PubMed]
- Rahajoe, P.S.; de Smit, M.J.; Raveling-Eelsing, E.; du Teil Espina, M.; Stobernack, T.; Lisotto, P.; Harmsen, H.J.M.; van Dijl, J.M.; Kertia, N.; Vissink, A.; et al. No Obvious Role for Suspicious Oral Pathogens in Arthritis Development. Int. J. Environ. Res. Public Health 2021, 18, 9560. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.C. Rheumatology; Mosby/Elsevier: Philadelphia, PA, USA, 2015. [Google Scholar]
- Rahajoe, P.S.; Smit, M.; Schuurmans, G.; Raveling-Eelsing, E.; Kertia, N.; Vissink, A.; Westra, J. Increased IgA Anti-Citrullinated Protein Antibodies in the Periodontal Inflammatory Exudate of Healthy Individuals Compared to Rheumatoid Arthritis Patients. J. Clin. Periodontol. 2020, 47, 552–560. [Google Scholar] [CrossRef] [PubMed]
| No. | Author (Country) Study Type | Population/ Condition | Microbial Sampling Source/Assay | Rheumatoid Parameter/Disease Activity | Main Findings |
|---|---|---|---|---|---|
| 1. | Mikuls et al. [24] USA 2014 Case–control | 1. RA (n = 287); age 59 ± 12; m/f 63%/37%, BMI: 30 ± 7 kg/m2 2. OA (n = 330) age 59 ± 11; m/f 60%/40%, BMI: 32 ± 7 kg/m2 | Biofilm subgingiva PCR | Anti-CCP-2 ≥ 5 units/mL RA-PD vs. RA non PD = 90% (170 ± 128 unit/mL) vs. 82% (131 ± 126 unit/mL) RF > 15 IU/mL RA-PD vs. RA non PD = 86% (390 ± 633 IU/mL) vs. 72% (185 ± 379 IU/mL) | 1. PD patients have a higher chance of being RF positive (p = 0.006) and concentrations of RF and anti-CCP-2 (p < 0.05) compared to those without PD. 2. The levels of antibody against F. nucleatum significantly higher in RA cases (p = 0.018). 3. The anti–P. gingivalis statistically significant and weakly correlate with anti–CCP-2 (r = 0.14, p = 0.022) and RF (r = 0.19, p = 0.001) 4. Over-expression of ACPAs of patients with subgingival P. gingivalis, regardless of smoking status. |
| 2. | Schmickler, et al. [28] Germany 2016 Cross-sectional | 1. RA (n = 168); age 58.4 ± 7.9; m/f: 18.5%/81.5% 2. Healthy patients (n = 168); age 56.8 ± 6.8; m/f: 39.9%/60.1% | Biofilm subgingiva RT-PCR | RF positive Positive: ≥20 U/mL Negative: <20 U/mL RF positivity: 35% aCCP Positive: ≥5 U/mL Negative: <5 U/mL aCCP positivity: 44% | 1. F. nucleatum (p < 0.001) and P. micra (p < 0.001) more prevalent in RA group. 2. There were no significant differences in the concentration of P. gingivalis (p = 0.06) and F. nucleatum (p = 0.06) in aCCP-positive patients and P. intermedia (p = 0.08) in RF-positive patients. |
| 3. | Beyer et al. [29] Norway 2018 Cross-sectional | Chronic RA (n = 78), aged 57 ± 11.5; were divided into 4 subgroups: 1. Gingivitis (n = 14) 2. Mild periodontitis (n = 7) 3. Moderate periodontitis (n = 43) 4. Severe periodontitis (n = 14) | Biofilm subgingiva Sequencing qPCR | ACPA positive: >3 U/mL RF posisitve: >25 IU/mL | 1. The most predominant genera: Fusobacterium, Prevotella, Corynebacterium, Actinomyces, Leptotrichia, Selenomonas, Veillonella, and Treponema. 2. Higher CRP in subjects with subgingival P. gingivalis (p = 0.009). 3. Microbial related to higher RA disease duration, disease activity, prednisolone dose, and ESR: Corynebacterium, Veillonella, Actinomyces, Leptotrichia, Streptococcus and Neisseria. |
| 4. | Correa et al. [14] Brazil 2019 Cross-sectional | 1. RA with PD (n = 21); m/f: 66%/34%; 53 ± 10.4 2. RA non PD (n = 21); m/f: 46.52%/53.48%; 50 ± 11.1 3. Non CP (control) (n = 27); m/f: 63.5%/36.5%; 42.8 ± 14 4. CP (control) (n = 20); m/f: 52%/48%; 46.5 ± 12.3 | Biofilm subgingiva Next Generation Sequencing (NGS) | ACPA positive: ≥5 U/mL RA non-CP vs. RA + CP: 33% vs. 85.7% DAS28 (tender and swollen joints) RA non-CP vs. RA + CP: 3.5 ± 1.2 vs. 3.7 ± 1.5 | 1. Fretibacterium fastidiosum, Parvimonas micra and Anaeroglobus geminatus were correlated with augmented numbers of swollen (rho = 0.35) and tender joints (rho = 0.30, p < 0.05). 2. Elevated numbers of Prevotella spp., A. actinomycetemcommitans, and P.micra in RA + PD. |
| 5. | Eriksson et al. [30] Sweden 2019 Cross-sectional | 1. RA with no/mild PD (n = 10); aged: 50 + 14; m/f: 0%/100%; BMI26 ± 6.8 2. RA with moderate/severe PD (n = 30); aged: 64 ± 7.8; m/f: 17%/83%; BMI: 24 ± 5.6 | Biofilm subgingiva Sequencing qPCR | ACPA positive: 3 E/mL RF positive: 20 E/mL No/mild periodontitis ACPA-positive (50%) RF-positive (50%) Moderate/severe periodontitis ACPA-positive (86%) RF-positive (73%) | 1. Prevotella oris and Porphyromonas sp. are abundant in mild/non PD group. 2. Moderate/severe PD includes numerous bacteria: Desulfobulbus sp. prevotella, NA 92, Bulleidia, Capnocytophaga, and Tannerrella. 3. There were no significant differences in the percentage of P. gingivalis (p = 0.66) between the moderate/severe PD group and the no/mild PD group. 4. The prevalence of ACPA positive was substantially higher (p = 0.032) in RA patients with moderate/severe PD compared to those with no/mild PD. |
| 6. | Mankia et al. [31] United Kingdom 2019 Cross-sectional | 1. Healthy control (n = 32); aged: 49.4 ± 15.3; m/f: 46%/54% 2. aCCP + at-risk (n = 48); aged: 51.9 ± 11.4; m/f: 35%/65% 3. Early RA (n = 26); aged: 54.4 + 16.7; m/f: 46%/54% Divided into 4 subgroups according to clinical periodontal status: 1. PD (n = 12) 2. Healthy (n = 10) 3. RA with PD (n = 14) 4. RA without PD (n = 3) | Biofilm subgingiva Shotgun metagenomic analysis | IgG anti CCP+ Positive: ≥3 times upper limit of normal range (BioRad) | There is a higher occurrence of PD and P. gingivalis in CCP+ at risk individuals. |
| 7. | Kroese et al. [21] Netherland 2021 Case–control | 1. Patients with early RA (n = 50); aged: 52.1 + 13.2; m/f: 22%/78% 2. Individual at risk of developing RA (n = 50); aged: 51.4 + 10.3; m/f: 24%/76%. 3. Healthy control (n = 50) aged: 51.2 + 11.0; m/f: 24%/76%. | Subgingival, saliva, tongue coating Sequencing | ACPA Positive: >10.0 kU/liter RF Positive: >5.0 kU/ liter ACPA positive Early RA/at risk of RA/healthy control: 62%/48%/0% RF positive Early RA/at risk of RA/healthy control: 74%/92%/0% Both ACPA and RF positive Early RA/at risk of RA/healthy control: 76%/100%/0% | 1. There were no microbial significant differences in subgingival plaque, saliva, and tongue coating between ACPA (+) and (−) individual at risk of RA. 2. Prevotella salivae, Veillonella (p = 0.012) and Prevotella (p = 0.022) were more abundant in the early RA group and at-risk individual. 3. Neisseria flavescens/subflava (p = 0.02), Porphyromonas pasteri/sp._oral_taxon_278 (p = 0.01), and Veillonella parvula were more abundant in the healthy control group. |
| 8. | Chen et al. [25] Taiwan 2022 Case–control | 1. AM (age, gender, DM statuses) (n = 42; 21 RA, 21 control); aged 57.57 + 8.06, 67.19 + 8.73; m/f: 23.81%/76.19%. 2. PD (n = 24; 12 RA, 12 control); aged 59.00 + 7.8, 58.5 + 7.86; m/f: 25%/75%. 3. PH (n = 12; 6 RA, 6 control); aged: 54.50 + 11.66, 53.33 + 11.04; m/f: 16.67%/83.33%. | Biofilm subgingiva Sequencing | ACPA (Elia Kit CCP) ACPA level: AM (RA/control) vs. PD (RA/control) vs. PH (RA/control): 54.50/1.00 vs. 97.50/0.80 vs. 142.5/1.20 Positive: >10U/mL AM vs. PD vs. PH: 71.43% vs. 83.33% vs. 0 RF (N Latex RF Kit) Positive:>8 IU/mL AM (RA/control) vs. PD (RA/control) vs. PH (RA/control): 80.95%/9.52% vs. 91.67%/8.33% vs. 83.33%/16.67% | 1. A. butyrica and P. simiae positively correlated with the levels of ACPA in AM + PD patients. 2. A. butyrica and P. simiae, have a potential role in the development of RA through the induction of ACPA production by hypercitrullination. |
| 9. | Kozhakhmetov et al. [26] Kazakhtan 2023 Case–control | 1. RA (n = 75); aged: 46 (mean); BMI: 24.5 (mean) 2. Control (n = 114); aged 43 (mean); BMI: 25.5 (mean) | Surface tongue, gums, tonsils, plaque Sequencing | ACPA positive: 57.3% RF positive: 76.0% | 1. Prevotella_9 positively correlated with ACPA and RF (p < 0.001) 2. Oscillospiraceae UCG-005, Leptotrichia spp., Leptotrichia wadei, Neisseria baciliformis positively correlated with of ACPA (p < 0.05) 3. Treponema sp. canine oral taxon 087 positively correlated RF (p < 0.05) |
| 10. | Huang et al. [27] 2023 Case–control | 1. RA (n = 67); aged: 50.5% + 8.17; m/f:25.4%/74.6%; BMI: 23.1 + 2.83 2. RA + PD (n = 48); aged 49.9 + 8.74; m/f: 22.9%/77.1%; BMI: 24.2 + 3.63 | Saliva and buccal epithelial cells (BECs) PCR | Anti-CCP level (U/mL): RA vs. RA + PD: 130.2 + 112.5 vs. 182.1 + 107.8 Anti-CCP positivity: RA vs. RA + PD: 59.7% vs. 81.3% RF concentration level (IU/mL): RA vs. RA + PD: 273.2 + 102.6 vs. 365.5 + 142.6 RF positivity: RA vs. RA + PD: 68.7% vs. 83.3% ACPA concentration level (IU/mL): RA vs. RA + PD: 498.2 + 598.7 vs. 778.1 + 842.0 ACPA positivity: RA vs. RA + PD: 56.7% vs. 75% | The A actinomycetemcomitans were positively correlated with anti-CCP (p = 0.008), RF concentration (p < 0.001), ACPA (p = 0.006). |
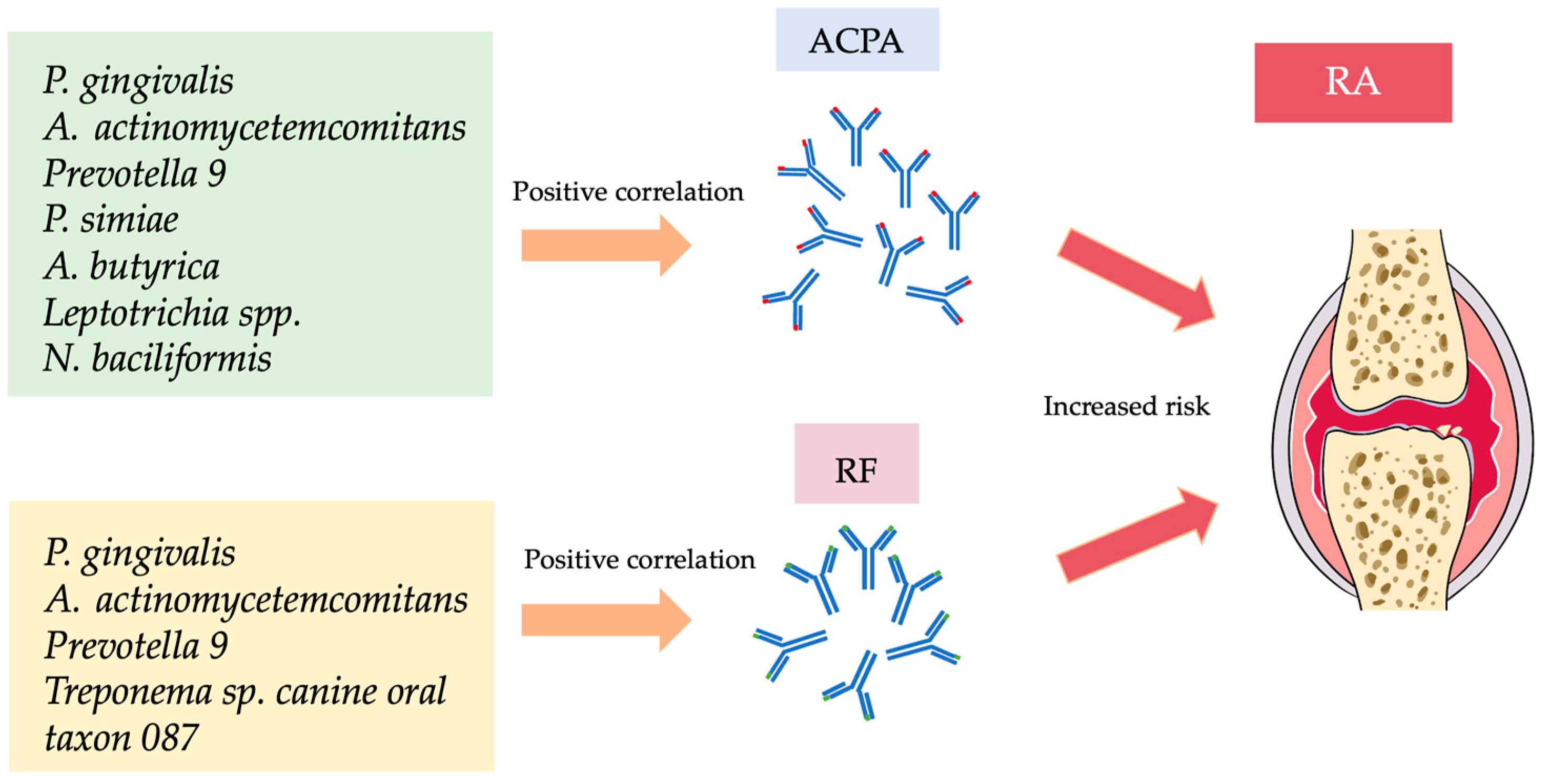
| Bacteria | ACPA | Anti-CCP2 | RF | Author |
|---|---|---|---|---|
| Porphyromonas gingivalis | √ | √ | Mikuls et al. (2014) [24] | |
| Porphyromonas gingivalis | √ | Eriksson et al. (2019) [30] | ||
| Aminipila butyrica | √ | Chen et al. (2022) [25] | ||
| Peptococcus simiae | √ | Chen et al. (2022) [25] | ||
| Prevotella_9 | √ | √ | Kozhakhmetov et al. (2023) [26] | |
| Leptotrichia spp. | √ | Kozhakhmetov et al. (2023) [26] | ||
| Oscillospiraceae UCG-005 | √ | Kozhakhmetov et al. (2023) [26] | ||
| Leptotrichia wadei | √ | Kozhakhmetov et al. (2023) [26] | ||
| Neisseria baciliformis | √ | Kozhakhmetov et al. (2023) [26] | ||
| Treponema sp. canine oral taxon 087 | √ | Kozhakhmetov et al. (2023) [26] | ||
| Aggregatibacter actinomycetemcomitans | √ | √ | √ | Huang et al. (2023) [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astuti, L.; Masulili, S.L.C.; Gunardi, I.; Sulijaya, B.; Soeroso, Y. Periodontal Pathogens Correlate with Rheumatoid Arthritis Disease Parameters: A Systematic Review Based on Clinical Studies. Dent. J. 2025, 13, 214. https://doi.org/10.3390/dj13050214
Astuti L, Masulili SLC, Gunardi I, Sulijaya B, Soeroso Y. Periodontal Pathogens Correlate with Rheumatoid Arthritis Disease Parameters: A Systematic Review Based on Clinical Studies. Dentistry Journal. 2025; 13(5):214. https://doi.org/10.3390/dj13050214
Chicago/Turabian StyleAstuti, Luki, Sri Lelyati Chaidar Masulili, Indrayadi Gunardi, Benso Sulijaya, and Yuniarti Soeroso. 2025. "Periodontal Pathogens Correlate with Rheumatoid Arthritis Disease Parameters: A Systematic Review Based on Clinical Studies" Dentistry Journal 13, no. 5: 214. https://doi.org/10.3390/dj13050214
APA StyleAstuti, L., Masulili, S. L. C., Gunardi, I., Sulijaya, B., & Soeroso, Y. (2025). Periodontal Pathogens Correlate with Rheumatoid Arthritis Disease Parameters: A Systematic Review Based on Clinical Studies. Dentistry Journal, 13(5), 214. https://doi.org/10.3390/dj13050214






