Abstract
Background/Objectives: The correlation between cardiovascular diseases, particularly infective endocarditis, and oral disorders such as periodontitis and dental caries has been widely discussed in the scientific literature. In this mapping review, we aim to examine the available evidence on the link between these conditions, focusing on the pathogenetic mechanisms that underlie the development of endocarditis in patients with oral diseases. Methods: A systematic search was conducted across three major databases—PubMed, Scopus, and ScienceDirect—as well as grey literature in Google Scholar. Relevant articles were selected based on inclusion and exclusion criteria, focusing primarily on systematic reviews. The data extracted included study characteristics, main outcomes, and risk-of-bias evaluations. Results: A total of 13 systematic reviews were included in this mapping review. The findings suggest there is a significant connection between periodontal disease, dental caries, and the incidence of infective endocarditis. The evidence highlights that oral bacteria, particularly Streptococcus species, can enter the bloodstream during daily activities and invasive dental procedures, contributing to the development of endocarditis in susceptible individuals. However, the role of antibiotic prophylaxis in preventing endocarditis following dental procedures remains controversial. Conclusions: This review reinforces the importance of oral health in preventing cardiovascular complications, especially infective endocarditis. Although antibiotic prophylaxis may reduce the risk in high-risk individuals, further research is needed to clarify its effectiveness. Enhanced awareness of and education on the shared risks between oral and cardiovascular health could improve prevention strategies and patient outcomes.
1. Introduction
Over the years, scientific studies have highlighted the correlation between oral diseases and systemic conditions, particularly cardiovascular diseases and periodontitis. In fact, epidemiological evidence suggests there is a consistent association between periodontitis and an increased risk of developing cardiovascular disease [1].
Cardiovascular diseases (CVDs) are a group of disorders that affect the heart and/or the body’s entire vascular system. Most cardiovascular diseases are chronic conditions that persist for extended periods. However, in some cases, they may manifest as acute episodes, such as myocardial infarction and stroke, which occur suddenly due to the obstruction of blood vessels supplying the brain or heart [2].
Cardiovascular diseases occur more frequently in individuals who smoke, have hypertension, have elevated cholesterol levels (particularly low-density lipoprotein cholesterol (LDL-C)), are overweight, do not engage in physical activity, and/or suffer from diabetes [3].
Among cardiovascular diseases, endocarditis most commonly has a bacterial aetiology and can present in conjunction with other infectious conditions such as periodontitis or dental caries, sharing certain pathogens or risk factors.
The term periodontal disease refers to a group of inflammatory conditions that affect both the superficial and deep supporting tissues of the tooth [4]. In the former case, gingivitis is present; however, when the inflammation extends beyond the gingival region and involves the underlying areas that constitute the supporting tissues of the tooth, periodontitis or chronic periodontitis occurs, progressively destroying the tooth-supporting structures. It typically manifests as a worsening of gingivitis and, if left untreated, leads to tooth mobility and eventual tooth loss. Other symptoms are rare, except when abscesses develop; in these cases, pain and swelling are common. Diagnosis is based on inspection, periodontal probing, and intraoral radiographs. Treatment involves scaling below the gum line and a home hygiene regimen [5].
Caries is a dental disease characterised, in its initial phase, by the destruction of the enamel caused by certain bacterial species, particularly Streptococcus mutans [6]. If left untreated, caries progresses to affect the deeper layers of the tooth, extending to the dentine and eventually reaching the pulp and roots. In addition to significant painful symptoms, untreated caries can lead to the loss of the affected tooth and may require antibiotics and surgery. Cariogenic disease is a complex and multifactorial condition that, despite the implementation of various preventive methods, remains one of the most common diseases among humans. The development of dental caries is due to the interaction between genetic and environmental factors, which influence each other [7].
Several mechanisms have been proposed to explain the correlation between oral infections and cardiovascular conditions such as endocarditis. Among these, chronic inflammation caused by periodontitis or caries may induce systemic inflammatory responses, promoting endothelial dysfunction and creating a favourable environment for microbial colonisation. Furthermore, repeated episodes of transient bacteraemia—triggered by daily oral activities or invasive dental procedures—enable oral bacteria, particularly Streptococcus species, to reach and adhere to damaged cardiac tissues. The virulence factors of these bacteria, combined with an impaired immune response or pre-existing cardiac lesions, may facilitate the formation of vegetations on heart valves, ultimately leading to infective endocarditis. These mechanisms underline the importance of exploring the pathogenic interplay between oral and cardiovascular health, as discussed in detail in the following sections [8].
The aim of this mapping review is to examine the available scientific literature on the correlation between cardiovascular diseases, particularly endocarditis, and oral disorders, with a focus on periodontitis and, secondarily, cariogenic disease [9].
This review will focus on the analysis of existing evidence regarding the correlations between periodontal disease, caries, and endocarditis. The objective of the review is to identify the common pathogenetic mechanisms between endocarditis and oral disorders, with particular emphasis on the effects of chronic inflammation, epigenetic modifications, and immune responses. The review will also examine the links between bacteraemia caused by oral infections and the worsening of cardiac conditions such as endocarditis. An additional aim of this review will be to map the areas of research explored and identify gaps in the existing literature. This will enable the suggestion of future directions for further investigation into the connections between oral health and cardiovascular diseases, with a particular focus on new potential therapeutic approaches.
2. Materials and Methods
2.1. Protocol and Registration
This mapping review was prepared according to the guidelines of the SANRA (Scale for the Assessment of Narrative Review Articles) [10]. We decided not to register the review protocol, despite it being prepared prior to conducting the database search.
2.2. Eligibility Criteria
All studies examining the correlations between cardiovascular diseases, particularly endocarditis, and oral disorders were considered potentially eligible. Special attention was given to systematic literature reviews. Given the vast scope of this topic, focusing on systematic reviews was a strategic decision aimed at reducing the volume of information, allowing us to concentrate on the aspects and results that had already been extensively evaluated in previous reviews, without wasting time and resources synthesising the results of each individual study linking oral medicine with cardiovascular diseases (forming part of a considerable number of studies). No restrictions were applied based on the year of publication or language, provided that an abstract in English was available.
The exclusion criteria applied included: (i) narrative reviews, editorials, letters to the editor, and case reports; (ii) studies that did not address the association between oral diseases and endocarditis or cardiovascular diseases; (iii) duplicate publications; and (iv) reviews lacking sufficient methodological transparency or a clear description of the included studies. Only systematic reviews were considered for inclusion in order to ensure a high-quality synthesis of evidence.
2.3. Information Sources
For the preparation of this review, three databases were used: PubMed, Scopus, and ScienceDirect. Additionally, grey literature searches were conducted on Google Scholar. Potentially eligible articles were also sought within the reference lists of literature reviews on the correlations between cardiovascular diseases and oral disorders. The search was conducted between the 1st February 2025 and 10th February 2025, with the last update of the identified records made on 14th February 2025.
2.4. Search
The research questions that guided the selection of and search for studies were as follows:
What is the scientific evidence on the correlations between endocarditis and oral disorders, particularly periodontitis and dental caries?
How do common inflammatory, genetic, and epigenetic mechanisms contribute to the relationship between these conditions and oral health?
What gaps exist in the literature regarding the interaction between oral health and endocarditis, and what future directions could be explored to further our understanding of this connection?
The authors responsible for the study search used the following keywords in PubMed: Search: (oral OR dental OR parodontal OR caries OR periodontitis) AND (endocarditis) Sort by: Most Recent (“mouth”[MeSH Terms] OR “mouth”[All Fields] OR “oral”[All Fields] OR (“dental health services”[MeSH Terms] OR (“dental”[All Fields] AND “health”[All Fields] AND “services”[All Fields]) OR “dental health services”[All Fields] OR “dental”[All Fields] OR “dentally”[All Fields] OR “dentals”[All Fields]) OR (“parodont”[All Fields] OR “parodontal”[All Fields] OR “parodontitis”[All Fields]) OR (“carie”[All Fields] OR “dental caries”[MeSH Terms] OR (“dental”[All Fields] AND “caries”[All Fields]) OR “dental caries”[All Fields] OR “caries”[All Fields]) OR (“periodontal”[All Fields] OR “periodontally”[All Fields] OR “periodontically”[All Fields] OR “periodontics”[MeSH Terms] OR “periodontics”[All Fields] OR “periodontic”[All Fields] OR “periodontitis”[MeSH Terms] OR “periodontitis”[All Fields] OR “periodontitides”[All Fields])) AND (“endocarditis”[MeSH Terms] OR “endocarditis”[All Fields] OR “endocarditides”[All Fields]).
Translations
oral: “mouth”[MeSH Terms] OR “mouth”[All Fields] OR “oral”[All Fields]
dental: “dental health services”[MeSH Terms] OR (“dental”[All Fields] AND “health”[All Fields] AND “services”[All Fields]) OR “dental health services”[All Fields] OR “dental”[All Fields] OR “dentally”[All Fields] OR “dentals”[All Fields]
parodontal: “parodont”[All Fields] OR “parodontal”[All Fields] OR “parodontitis”[All Fields]
caries: “carie”[All Fields] OR “dental caries”[MeSH Terms] OR (“dental”[All Fields] AND “caries”[All Fields]) OR “dental caries”[All Fields] OR “caries”[All Fields]
periodontitis: “periodontal”[All Fields] OR “periodontally”[All Fields] OR “periodontically”[All Fields] OR “periodontics”[MeSH Terms] OR “periodontics”[All Fields] OR “periodontic”[All Fields] OR “periodontitis”[MeSH Terms] OR “periodontitis”[All Fields] OR “periodontitides”[All Fields]
endocarditis: “endocarditis”[MeSH Terms] OR “endocarditis”[All Fields] OR “endocarditides”[All Fields].
During the search process, filters were applied when available in order to refine the results and include review articles. In PubMed, the filter “Systematic review” was selected to prioritise high-quality evidence. No language restrictions were applied, as long as an abstract was available in English. The search was limited to human studies.
A total of 3963 records were identified in PubMed. In Scopus and ScienceDirect, the following keywords were used: TITLE-ABS-KEY ((oral OR dental OR periodontal OR caries OR periodontitis) AND (endocarditis)). Consequently, 5587 records were identified through Scopus, and 36,821 records were found using ScienceDirect.
The search for eligible articles and reports was a collaborative effort between two reviewers, M.D. and D.S. After reaching a consensus on the eligibility criteria, keywords, and selected databases, the primary reviewers independently conducted the search for articles and reports [11]. They meticulously recorded the number of articles retrieved for each keyword and from each designated database. Duplicate studies identified across different databases were systematically removed using EndNote 8 software (Philadelphia, PA, USA).
The authors carefully carried out the removal of study overlaps that could not be processed using EndNote during the screening phase [12]. Subsequently, the two reviewers proceeded with screening and inclusion of studies, engaging in comparative analyses and constructive discussions to determine which studies to include in the mapping review [13].
2.5. Synthesis of Results
The studies deemed eligible were read, and the key information was noted and used to draft this mapping review.
The data to be extracted from the included articles were predetermined by the two reviewers and included the first author of the study, the publication date, the country where the research was conducted, the number of studies included, the registration protocol number, the main outcome with the principal result, the presence of meta-analysis, and any evaluation of the risk of bias. The data were extracted and recorded in a dedicated table.
2.6. Risk of Bias
The risk of bias in the individual systematic reviews was assessed by two authors (M.D. and A.B.). The ROBIS (Risk of Bias in Systematic Reviews) was used as an assessment tool specifically developed to assess the risk of bias in systematic reviews. Studies with a high risk of bias were excluded from the review [14].
The distinction between bias within the review process (meta-bias) and bias in the primary studies included in the review is critical. A systematic review may be considered to have a low risk of bias even if all the included primary studies have a high risk of bias, provided that the review appropriately assesses the risk of bias in the primary studies before drawing its conclusions [14].
3. Results
The search conducted using the databases yielded a total of 9550 articles. After duplicates were removed using EndNote X8 software, the number of articles reduced to 6889. Applying inclusion and exclusion criteria based on titles, abstracts, or keywords, we identified approximately 54 articles. Given the abundance of studies, it was deemed appropriate to exclude articles considered to be of lesser qualitative significance and to focus solely on systematic reviews.
A full-text assessment was subsequently conducted. During this phase, 44 studies were excluded for the following main reasons:
- ✓
- A total of 27 did not specifically address the correlation between endocarditis and oral diseases but rather discussed broader cardiovascular implications or unrelated infections (A);
- ✓
- Five lacked sufficient methodological transparency, either due to the absence of protocol registration or the presence of poorly defined protocols and unclear inclusion criteria (B);
- ✓
- Six were duplicate systematic reviews or had substantial overlaps with previously published reviews, which the reviews in question effectively updated (C);
- ✓
- Five were not systematic reviews but clinical guidelines, position papers, or survey-based studies (D) (Table 1).
 Table 1. Excluded studies.
Table 1. Excluded studies.
As a result, 11 systematic reviews were included in the final analysis.
The entire selection process is represented in the flow chart (Figure 1).
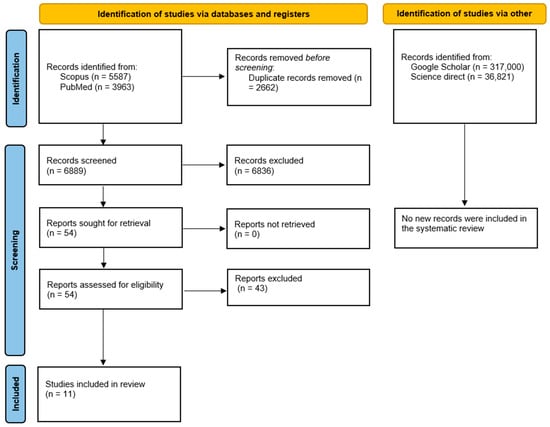
Figure 1.
Flow chart for the mapping review.
The results were extracted and presented in Table 2.

Table 2.
Data extraction table showing the information regarding the papers and the main results of the systematic reviews. Not all included studies provided data for the meta-analyses.
In the 11 systematic reviews included, a total of approximately 157 primary studies were analysed. However, due to overlaps between reviews, many of these studies were included in more than one review. For example, common references were identified in the reviews by Sperotto et al. (2024) [68], Cahill et al. (2017) [161] and Lafaurie et al. (2019) [127], González Navarro et al. (2017) [50], and others, particularly regarding the incidence of bacteraemia and the effects of antibiotic prophylaxis. This overlap introduces a potential source of bias, as some studies may be over-represented in the evidence synthesis. Fortunately, the included reviews provided detailed lists of the primary studies analysed, limiting the possibility of the precise quantification of duplications, as the total number of primary studies included in the review was 101 (Lacassin et al., 1995 [59]; Strom et al., 1998 [60]; Porat et al., 2008 [61]; Chen et al., 2015 [62]; Chen et al., 2018 [63]; Tubiana et al., 2017 [64]; Thornhill et al., 2023 [65]; Thornhil et al., 2022 [66]; Thornhill et al., 2024 [67]; Keller et al., 2017 [69];van den Brink et al., 2017 [70]; Bates et al., 2017 [71]; Sakai Bizmark et al., 2017 [72]; Garg et al., 2019 [73]; Quan et al., 2020 [74]; Vähäsarja et al., 2020 [75]; Bikdeli et al., 2013 [76]; DeSimone et al., 2015 [77]; Toyoda et al., 2017 [78]; Sun et al., 2017 [79]; Thornhill et al., 2022 [80]; Rogers et al., 2008 [81]; Pasquali et al., 2015 [82]; Pant et al., 2015 [83]; Thornhill et al., 2017 [84]; DeSimone et al., 2021 [85]; Mackie et al., 2016 [86]; Knirsch et al., 2020 [87]; Weber et al., 2022 [88]; Krul et al., 2015 [89]; Duval et al., 2012 [90]; Dayer et al., 2015 [91]; Shah et al., 2020 [92]; Zegri-Reiriz et al., 2018 [94]; Thornhill et al., 2018 [95]; Khairat, 1966 [97]; Shanson et al., 1985 [98]; Maskell et al., 1986 [99]; Shanson et al., 1987 [100]; Vergis et al., 2001 [101]; Lockhart et al., 2004 [102]; Diz Dios et al., 2006 [103]; Abu-Ta’a et al., 2008 [104]; Anitua et al., 2008 [105]; Lockhart et al., 2008 [106]; Asi et al., 2010 [107]; Esposito et al., 2010 [108]; Siddiqi et al., 2010 [109]; Chandramohan et al., 2011 [110]; Maharaj et al., 2012 [111]; DuVall et al., 2013 [112]; Limeres Posse et al., 2016 [113]; Van der Meer et al., 1992 [116]; Pourmoghaddas et al., 2018 [118]; Ali et al., 2017 [119]; Cantekin et al., 2015 [120]; Cantekin et al., 2013 [121]; Suma et al., 2011[122]; Siahi-Benlarbi et al., 2010 [123]; da Fonseca et al., 2009 [124]; Tasioula et al., 2008 [125]; Stecksén-Blicks et al., 2004 [126]; Snanson et al., 1978 [128]; Roberts et al., 1987 [129]; Hall et al., 1993 [130]; Hall et al., 1996 [131]; Wahlmann et al., 1999 [132]; de Souza et al., 2016 [134]; Deppe et al., 2007 [135]; Wu et al., 2008 [136]; Hakeberg et al., 1999 [137]; Nakamura et al., 2011 [138]; Bratel et al., 2011 [139]; Lockhart et al., 2009 [141]; Bahrani-Mougeot et al., 2008 [142]; Heimdahl et al., 1980 [143]; Rajasuo et al., 2004 [144]; Roberts et al., 2006 [145]; Tomás et al., 2007 [146]; Roberts et al., 1998 [147]; Benítez-Páez et al., 2013 [148]; Maharaj et al., 2012 [149]; Peterson et al., 1976 [150]; Rahn et al., 1995 [151]; Sefton et al., 1990 [152]; Hall et al., 1996 [153]; Tuna et al., 2012 [154]; Sweet et al., 1978 [155]; Cannell et al., 1991 [156];Aitken et al., 1995 [157]; Josefsson et al., 1985 [158]; Tomás et al., 2008 [159]; Piñeiro et al., 2010 [160]; Imperiale and Horwitz, 1990 [162]; Baltch et al., 1982 [163]; Coulter, et al., 1990 [164]; Head et al., 1984 [165];; Horstkotte et al., 1987 [166]; Thornhill et al., 2011 [167]; Desimone et al., 2012 [168]; and Salam et al., 2014 [169]).
The primary outcomes evaluated in the included systematic reviews were as follows:
- ✓
- Incidence of IE following dental procedures;
- ✓
- Association between oral conditions (periodontitis and dental caries) and IE;
- ✓
- Bacteraemia induced by dental procedures;
- ✓
- Effectiveness of AP in preventing IE;
- ✓
- Antimicrobial resistance patterns;
- ✓
- Impact of oral health interventions in high-risk cardiac patients.
These outcomes are summarised in Table 3 and further discussed in the Discussion section. In total, 8 out of 11 reviews included outcomes related to antibiotic prophylaxis, while 4 out of 11 specifically quantified its effectiveness. Only 1 out of 11 reviews included data on caries-related infections, focusing specifically on children with congenital heart disease (CHD).

Table 3.
Summary of results from the included systematic reviews on infective endocarditis, oral conditions, and antibiotic prophylaxis.
Among the reviews that reported data on AP, the most commonly studied regimens included oral or intravenous amoxicillin with clavulanic acid, and oral azithromycin for penicillin-allergic patients. According to Lafaurie et al. (2019) [127], azithromycin showed greater efficacy in reducing bacteraemia compared to clindamycin. Amoxicillin was typically administered as a single 2 g oral dose 30–60 minutes prior to the procedure, whereas intravenous regimens were reserved for hospitalised or high-risk patients undergoing surgery.
Barbosa-Ribeiro et al. (2024) observed high levels of resistance to clindamycin, metronidazole, and rifampicin in E. faecalis, highlighting the importance of local antibiogram data [24]. Only four of the included reviews provided direct comparisons between antibiotic regimens, and no clear consensus emerged from the literature regarding the optimal agent or dosage.
Preventive antibiotics were discussed primarily in relation to invasive procedures [170] and not as a routine treatment for caries or mild periodontal therapy.
Regarding caries, only one review (Karikoski et al., 2021) addressed this condition, showing that there is a significantly higher prevalence of dental caries among children with congenital heart disease (CHD) [117]. However, this review did not evaluate the role of antibiotics in caries management as a preventive strategy for IE. No systematic review supported the routine use of antibiotic therapy for non-invasive caries treatment (e.g., restorations).
As for periodontal treatment, several reviews (Kussainova et al., 2025 [58]; Lafaurie et al., 2019 [127]; González Navarro et al., 2017 [140]) considered procedures such as scaling and periodontal surgery in the context of bacteraemia risk. However, the findings consistently showed no significant association between non-invasive periodontal treatment and an increased risk of IE (e.g., Kussainova et al. reported an OR of 0.69; p = 0.41 for periodontal therapy) [58]. Consequently, routine antibiotic prophylaxis for periodontal therapy is not supported by current evidence, unless the procedure is invasive and the patient is considered high-risk.
Age-related findings were limited. Only one review (Karikoski et al., 2021) specifically focused on children with congenital heart disease and reported a higher caries burden and associated IE risk [117]. Other reviews included populations with heterogeneous age ranges but did not stratify findings by age group, making it difficult to assess whether age modifies the association between oral diseases and IE.
Risk of Bias
The risk of bias for systematic reviews was determined using the ROBIS tool, and for each factor, it was evaluated as “low”, “high”, or “unclear”. The three phases of the evaluation process were as follows: Phase 1—the evaluation of the relevance of the research question (PICO); Phase 2—the identification of the critical points of the review process; and Phase 3—the evaluation of the overall risk of bias of the review. All data related to the risk of bias are reported in Table 4.

Table 4.
Risk of bias.
The main critical issues related to the individual revisions are as follows:
- ✓
- Sperotto et al., 2024 [68]: Regarding the identification and selection of studies (?), the start or end dates of the review were not specified.
- ✓
- Friedlander and Couto-Souza, 2023 [93]: Regarding the study eligibility criteria (?), the protocol number with which the systematic review was registered was not reported. Regarding data collection and study appraisal (?), the risk of bias was not formally assessed using an appropriate scale or tool. Regarding the dentification and selection of studies (?), the selection was performed using only one database (PubMed).
- ✓
- Albakri et al., 2022 [96]: Regarding data collection and study appraisal (?), the risk of bias was not formally assessed using an appropriate scale or tool.
- ✓
- Karikoski et al., 2021 [117]: Regarding the dentification and selection of studies (?), the systematic review by Karikoski et al. (2021) [117] did not report registration of a review protocol in any public registry.
- ✓
- González Navarro et al., 2017 [140]: Regarding the dentification and selection of studies (?), the systematic review by González Navarro et al. did not report registration of a review protocol in any public registry. Regarding data collection and study appraisal (?), the risk of bias was not formally assessed using an appropriate scale or tool.
- ✓
- Cahill et al., 2017 [161]: Regarding the identification and selection of studies (?), the systematic review by Cahill et al. did not report registration of a review protocol in any public registry. Regarding data collection and study appraisal (?), the risk of bias was not formally assessed using an appropriate scale or tool.
4. Discussion
4.1. Association Between Endocarditis and Periodontitis
Endocarditis is an inflammation of the thin inner lining of the heart (endocardium) and the heart valves. Endocarditis most commonly affects the heart valves but can also form in shunts or other abnormal connections between the heart chambers [171].
In most cases, endocarditis is caused by an infection, although in a smaller percentage of cases, it has a non-infectious aetiopathogenesis. Infective endocarditis is generally of bacterial origin, but other pathogens, such as fungi, can also trigger the inflammatory process [172].
Bacterial endocarditis occurs when microorganisms from other parts of the body, such as the skin, oral cavity, intestines, or urinary tract, spread through the bloodstream and reach the heart [173].
Under normal conditions, the immune system recognises and defends the body against infectious agents, which—even if they reach the heart—would typically be harmless, passing through without causing an infection. However, if the cardiac structures are damaged, due to rheumatic fever, congenital defects, or other diseases, they may be susceptible to microbial invasion. In these conditions, it is easier for bacteria that have entered the body through the bloodstream to settle in the inner lining of the heart, overcoming the normal immune response to infections [174]. When the conditions are ideal, the infectious agents can organise themselves into aggregations known as “vegetations” (characteristic lesions of bacterial endocarditis) at the site of infection, whether it be a heart valve or other cardiac structures, including implanted devices [175]. There is a risk that these cell masses behave similarly toward blood clots, blocking the blood supply to organs, leading to heart failure or triggering a stroke [176].
Under microscopic examination, these vegetations reveal the presence of microcolonies of infecting microorganisms, embedded in a network of platelets, fibrin, and a few inflammatory cells [176].
The interaction between predisposing factors in the host and the inability of the immune system to eradicate the infectious agent from the endocardium makes the patient susceptible to infection. Bacterial endocarditis occurs when infectious agents enter the bloodstream and manage to “stick” to the heart tissue, multiplying in damaged or surgically implanted heart valves. This damaged tissue in the endocardium provides the ideal environment for the infecting agents to settle: the cardiac surface offers them the support they need to adhere and proliferate [177].
Not all bacteria that enter the bloodstream can cause endocarditis. Only infectious agents that have a tropism for valvular structures and endocardial tissues, meaning those that can interact with the surface of the heart lining and abnormal valves can potentially lead to the clinical picture of endocarditis [178].
If endocarditis is neglected, inflammation can damage or destroy endocardial tissue or heart valves, leading to life-threatening complications. If a heart defect is present, certain medical procedures can lead to transient bacteraemia, potentially responsible for endocarditis; these include tonsillectomy, adenoidectomy, intestinal and respiratory surgery, cystoscopy, bronchoscopy, colonoscopy, etc. There is also a risk of endocarditis when a patient undergoes certain dental procedures. In fact, the earliest studies on bacteraemia associated with the oral activity focused on the transient effects of daily oral activity as well as clinical interventions [80].
Microorganisms can spread through the blood and reach the heart via various pathways, some of which are related to dental and oral health activities, including daily actions such as brushing teeth, chewing, or other activities involving the mouth that can allow bacteria to enter the bloodstream [179], especially in the presence of periodontitis or gingivitis. In fact, microorganisms can spread from pre-existing infections, such as endodontic abscesses (a clear complication of penetrating caries in the pulp chamber) [180] or during periodontal disease, circulating in the bloodstream and settling in the endocardium if they encounter a favourable environment [181].
Alternatively, they can spread through dental medical procedures, such as tooth extractions, root planing, and scaling, which can introduce bacteria into the bloodstream, particularly if they cause bleeding [182].
4.2. Correlation with Periodontitis
Numerous studies have assessed the correlation between infective endocarditis and periodontitis, demonstrating that the association between these two conditions is primarily due to the spread of bacteria present in the oral cavity through the bloodstream [183].
It is well known that infective endocarditis is a very aggressive disease with high morbidity and mortality, and its connection with oral bacteria has raised continuous concerns among dentists, patients, and cardiologists [184]. The microbiota of the mouth is extremely diverse, with specific sites in the mouth such as the tongue, palate, cheek, teeth, and periodontal pockets each harbouring their own microbiota [185]. However, the main microorganisms responsible for the formation of dental plaque, which can lead to periodontitis, are oral streptococci belonging to the viridans group (such as Streptococcus mutans and Streptococcus sanguis) [186]. Since these streptococci form part of the dental plaque, they can enter the bloodstream, causing bacteraemia through daily activities such as chewing or brushing teeth [187].
A recently proposed causal model suggests that early bacteraemia can influence the endothelial surface of the heart for many years, promoting thickening of the valve and making it susceptible to vegetation from a subsequent infection, which could rapidly evolve into a fulminant infection [188]. In most studies, significantly higher rates of bacteraemia in patients with periodontitis compared to healthy patients have indicated that periodontitis serves as a gateway for oral streptococci to enter the bloodstream [189].
Periodontal infections are inflammatory diseases. Inflammation of the periodontal tissue leads to the deepening of the gingival sulcus and the formation of periodontal pockets, acting as a reservoir for a large number of microorganisms. Periodontal infections affect the oral tissues, and bacteria can enter the bloodstream through the inflamed, ulcerated sulcus and the epithelium of the pocket, as well as the adjacent gingival microcirculation [190].
Invasive dental procedures and routine daily activities such as chewing and tooth brushing are significant predisposing factors with respect to microorganisms entering the bloodstream [191]. Additionally, recent studies have shown that smoking increases the frequency and severity of periodontal disease. Smoking is associated with poor oral hygiene, and thus smokers tend to have greater amounts of plaque and tartar accumulation, along with increased susceptibility to bacterial growth [192]. Smoking also impacts periodontal disease through various systemic effects, such as reduced chemotaxis and phagocytosis by both oral and peripheral neutrophils as well as decreased antibody production, which favours the breakdown of periodontal tissues. Consequently, smokers with chronic periodontitis experience greater attachment loss and bone loss, more involvement of the root furcations, and deeper pockets, leading to an increase in bacteraemia induced by periodontitis [193].
It has been observed that effective treatment of periodontal infections is important in reducing local inflammation and bacteraemia. Furthermore, poor periodontal health seems to increase the risk of cardiovascular diseases. Indeed, good oral hygiene and prophylaxis for high-risk patients undergoing dental procedures are included among the various recommendations of the American Heart Association and the European Society of Cardiology [194].
In light of these findings, there has been strong interest in the role of the oral microbiota in cardiovascular diseases, making this topic a subject of ongoing study, especially regarding how antimicrobial treatments can generate drug-resistant mutant bacteria, which has become a significant social problem [195].
4.3. Correlation with Dental Caries
Although periodontitis has been studied more extensively in relation to infective endocarditis [118], the potential role of dental caries and its complications must be taken into consideration. Dental caries, especially if untreated, can progress to pulpitis and subsequently apical periodontitis, both of which are associated with local inflammatory responses with possible systemic implications. These infections can lead to abscess formation and contribute to transient or prolonged episodes of bacteraemia, particularly with the involvement of pathogens such as Streptococcus mutans [196], Lactobacillus spp. [197], and Enterococcus faecalis [198], which have been implicated in cases of infective endocarditis.
Some epidemiological studies have identified a higher prevalence of dental caries and untreated pulp infections in patients with congenital heart disease and a history of endocarditis [199]. The caries formation process itself may not directly cause endocarditis, but it creates a portal of entry for bacteria into the bloodstream, especially when accompanied by inadequate oral hygiene or during procedures such as endodontic therapy [200] and tooth extractions [201]. Furthermore, caries-related pulp necrosis often requires surgical intervention, increasing the risk of bacteraemia in susceptible individuals.
Therefore, although the evidence linking dental caries to endocarditis is less robust than that for periodontitis, the literature suggests that untreated caries and secondary infections should be considered significant risk factors, particularly for high-risk cardiac patients. This highlights the importance of early diagnosis, prevention, and management of caries as part of cardiovascular risk reduction strategies.
4.4. Evidence from the Literature
The results of a systematic review conducted by Kussainova et al. in 2025 identified an increased incidence of infective endocarditis following invasive dental procedures, such as tooth extractions and oral surgery [202], with the post-extraction socket and surgical wounds being more susceptible to bacteraemia caused by bacteria such as Streptococci, which are also responsible for endocarditis [58]. The main causative pathogens involved are Staphylococcus aureus (33.4%) and Streptococcus species, including S. bovis and viridans (32.0%), followed by enterococci, according to the data reported by Budea et al. (2022) [28].
The peak of bacteraemia occurs approximately five minutes after the dental procedure and decreases over time. The procedures associated with the highest incidence of bacteraemia, according to Martins et al. (2024) [21], are tooth extractions (62–66%), followed by scaling and root planing (36–44%), and oral health procedures such as dental prophylaxis and dental probing (27–28%) [203].
However, in a 2017 publication, González Navarro et al. [140] noted that antimicrobial prophylaxis prior to invasive dental procedures does not prevent bacteraemia but may reduce its extent and persistence [140].
Furthermore, in a 2022 publication, Albakri et al. [96] suggested that post-procedure bacteraemia may not be a reliable marker for infective endocarditis, pointing out that there are few studies investigating a direct association between antibiotic prophylaxis and infective endocarditis [96].
It is generally thought that most cases of bacterial endocarditis caused by oral bacteria are likely the result of frequent bacteraemia arising from daily activities such as tooth brushing, especially in the case of periodontitis, as supported by a 2017 study by Cahill et al. [161], although some cases may still stem from infrequent invasive dental procedures.
Moreover, the effectiveness of antibiotic prophylaxis for the prevention of infective endocarditis following invasive dental procedures remains unclear.
In fact, another systematic review of the literature conducted by Sperotto et al. (2024) highlighted that the use of antibiotic prophylaxis is associated with a reduced risk of infective endocarditis in high-risk individuals following invasive dental procedures, while no association was found for those at low/unknown risk [68].
Concerning endodontic procedures [204], especially in the presence of persistent endodontic infections, the most effective drugs according to the meta-analysis conducted by Barbosa-Ribeiro et al. (2024) [24] remain amoxicillin and clavulanic acid, followed by amoxicillin and benzylpenicillin, while there remain significant resistances against E. faecalis to clindamycin, gentamicin, metronidazole, and rifampicin [24]. The use of antibiotic prophylaxis is also strongly suggested in cases of bicuspid aortic valve or mitral valve prolapse [93].
While intravenous amoxicillin plus clavulanic acid may be administered to high-risk patients undergoing more invasive dental procedures requiring general anaesthesia, for penicillin-allergic patients, oral azithromycin has shown greater efficacy in reducing bacteraemia compared to clindamycin, according to the data reported by Lafaurie et al. in 2019 [127].
Bergadà-Pijuan et al. (2023) [114], however, argue that the evidence supporting or discouraging the use of antibiotic prophylaxis before dental procedures as a prevention measure for infective endocarditis is very limited [115].
Furthermore, there is no definitive scientific evidence, as stated by Lockhart et al. in 2019 [133], suggesting that performing dental procedures before cardiac surgery, such as cardiac valve surgery or left-ventricular assist device implantation, reduces the risk of mortality or comorbidities such as endocarditis.
In a 2021 publication, Karikoski et al. [117] explored the potential implications for children with congenital heart disease who had developed endocarditis and carious lesions, investigating the prevalence of carious lesions and concluding that there is a correlation between carious lesions and congenital heart failure. Additionally, the risk of infective endocarditis is increased among children with congenital heart failure, for whom endocarditis prophylaxis is recommended.
Several factors can predispose children with congenital heart failure to developing caries during their early years. Congenital disorders may affect enamel, predisposing the children to caries due to hypomineralisation defects [205]. Increased food intake to meet energy needs leads to the consumption of sugars and sugary liquids, often supplemented with additional meals at night, as well as a greater predisposition to infections. Diuretic use also leads to reduced salivation and a buffering effect on the acidity produced by bacteria [117].
4.5. Endocarditis Prophylaxis for At-Risk Patients Undergoing Non-Surgical Periodontal Treatment: Role of Amoxicillin
The use of AP to prevent IE in patients undergoing dental procedures remains a subject of ongoing debate, particularly in the context of non-surgical periodontal treatments such as scaling and root planing. Although a direct causal relationship between procedure-induced transient bacteraemia and the onset of IE has not been definitively established, a substantial body of evidence supports the notion that manipulations involving gingival or periapical tissues may introduce oral bacteria into the bloodstream, posing a potential risk for individuals with specific predisposing cardiac conditions (Albakri et al., 2022 [96]; Rutherford et al., 2022 [115]; and Lafaurie et al., 2019 [127]).
4.5.1. Risk Associated with Non-Surgical Periodontal Procedures
Several studies have demonstrated that non-surgical periodontal procedures—particularly in regard to patients with active gingival inflammation or periodontitis—can induce bacteraemia at frequencies comparable to those observed following dental extractions. For instance, the study by Albakri et al. from 2022 [96] reported that periodontal probing and scaling were associated with bacteraemia rates ranging from 10% among patients with gingivitis to 40% among those with periodontitis, while tartar removal (i.e., supragingival and subgingival scaling) resulted in a bacteraemia prevalence of 24.5%. According to Albakri et al. [96], these variations are influenced by the severity of the underlying periodontal disease.
Conversely, the meta-analysis by Kussainova et al. from 2025 [58] found no significant association between invasive dental procedures and the development of infective endocarditis. Specifically, the pooled odds ratios (OR) were as follows: scaling—OR, 1.00; 95% CI, 0.85–1.18; p = 1.00; I2 = 0%; endodontic treatment—OR, 1.04; 95% CI, 0.73–1.49; p = 0.82; I2 = 0%; periodontal treatment—OR, 0.69; 95% CI, 0.28–1.67; p = 0.41; I2 = 69%.
Among the studies included in Kussainova et al.’s review [58], only that conducted by Lacassin et al. (1995) [59] reported a trend toward an increased risk associated with scaling and root canal therapy.
These findings indicate that even non-surgical procedures such as scaling and periodontal probing can induce transient bacteraemia, which represents a theoretical risk for infective endocarditis in predisposed patients. Consequently, such interventions may reasonably be classified as invasive procedures in the context of IE prevention for high-risk individuals.
4.5.2. International Guidelines and Indications for Prophylaxis (Amoxicillin)
Current guidelines from the American Heart Association (AHA) [206] and the European Society of Cardiology (ESC) [194] recommend AP exclusively for patients at high risk—such as individuals with prosthetic heart valves, a history of IE, or complex congenital heart disease—who undergo dental procedures involving the manipulation of gingival tissues or the periapical region of teeth. Non-surgical periodontal treatments, particularly in the presence of active inflammation or gingival bleeding, clearly fall within this definition.
Most international protocols recommend a single dose of 2 g of oral amoxicillin without clavulanic acid, administered 30–60 minutes prior to the procedure, as reported in trials included in the reviews by Rutherford et al. (2022) [115], Lafaurie et al. (2019) [127] and Bergadà-Pijuan et al. (2023) [114].
While it is well established that antibiotic prophylaxis reduces post-procedural bacteraemia—as shown in the systematic review by Albakri et al. from 2022 [96], which reported an approximate 49% reduction in bacteraemia risk (Risk Ratio: 0.51, 95% CI: 0.45–0.58; p < 0.0001)—other studies suggest that this reduction does not necessarily translate into a decreased risk of developing IE. Supporting this view is the Cochrane systematic review by Rutterford et al. (2022) [115], which included non-surgical periodontal procedures (such as supragingival and subgingival scaling as well as curettage) among the evaluated interventions, all of which are potentially associated with transient bacteraemia.
In detail, Rutterford et al. evaluated the administration of amoxicillin (2–3 g) or other beta-lactam antibiotics 30–60 minutes before the procedure compared with a placebo or no prophylaxis in regard to patients with ha high risk of developing IE. The only study included in the review was one by Van der Meer et al. from 1992 [116], which found that prophylaxis did not result in a statistically significant reduction in the risk of endocarditis among patients undergoing scaling or similar treatments (OR 1.62; 95% CI 0.57–4.57). However, a detailed analysis of associated procedures revealed that 13% of IE cases had undergone subgingival scaling, and 25% had received scaling combined with polishing (root surface debridement), and, notably, 43% of the control patients underwent the same procedures without developing IE. These findings, while not conclusive, highlight a possible association between such interventions and bacteraemia, even in the absence of demonstrated efficacy of antibiotic prophylaxis in preventing endocarditis in this context.
It is also important to note that oral amoxicillin combined with clavulanic acid has not shown any additional benefit in this setting [116].
In patients with penicillin allergy, oral azithromycin appears to be more effective than clindamycin in reducing bacteraemia, as reported by Lafaurie et al. (2019) [127]. Specifically, Lafaurie et al. documented that pre-procedural use of amoxicillin, azithromycin, and clindamycin (as per AHA protocols) led to reductions in bacteraemia risk of 59%, 49%, and 11%, respectively, compared to no prophylaxis. Aggregated estimates also showed that two antibiotics not included in AHA protocols—namely, moxifloxacin and intravenous amoxicillin–clavulanic acid—were associated with significant reductions in bacteraemia following invasive dental procedures amounting to 41% and 99%, respectively [127].
4.6. Future Research Perspectives
A key area of research, still widely debated yet presenting notable gaps and uncertainties, is antibiotic prophylaxis and how it may reduce the risk of endocarditis in patients with periodontal disease—particularly in the context of evaluating the effectiveness of antibiotic regimens and resistance patterns based on the specific types of oral bacteria involved. Optimising antibiotic prophylaxis for high-risk patients undergoing invasive dental procedures should play a crucial role in public health, reducing both complications and antibiotic resistance.
Innovative directions for future studies could include the development of personalised prophylactic strategies based on individual risk profiles, taking into account comorbidities, the composition of the oral microbiota, and genetic predisposition [207]. This would allow for the design of targeted antibiotic protocols rather than universal approaches, thereby minimising both the risk of infective endocarditis and the emergence of antibiotic resistance [208].
Another promising line of investigation concerns the role of epigenetic and inflammatory markers as predictors of systemic complications related to oral infections [209]. Longitudinal studies evaluating these markers could help identify patients at higher risk of cardiovascular events and guide early intervention strategies [210].
Moreover, more attention should be given to specific high-risk populations, such as children with congenital heart disease, elderly patients with comorbidities, immunocompromised individuals, and patients with “intermediate-risk” cardiac conditions (e.g., bicuspid aortic valve and mitral valve prolapse), whose classification may need to be revised based on recent evidence.
Additionally, the implementation of real-world data platforms [211] and multi-omics approaches [212] could enhance our understanding of the oral–systemic interface and the impact of oral health interventions on cardiovascular outcomes [212].
Finally, public health strategies and educational programs targeted at preventing oral diseases in at-risk cardiac patients should be developed and evaluated [213]. These may include oral hygiene campaigns, antimicrobial stewardship programs in dentistry, and interdisciplinary clinical pathways involving both cardiologists and dental professionals [214].
Recent advances in artificial intelligence (AI) provide innovative opportunities to enhance adherence to antibiotic prophylaxis protocols for the prevention of IE. Among these innovations, the implementation of AI tools such as Large Language Models (LLMs) has gained particular attention due to their efficiency and accessibility, facilitating their adoption by dental healthcare professionals. In this context, a study conducted by Rewthamrongsris et al. in 2025 [215] assessed the accuracy of seven recent LLMs in recommending antibiotic prophylaxis for dental procedures as a preventive strategy against IE. These models may serve as accessible instruments for rapidly retrieving detailed information and clinical protocols for physicians and dentists.
The cited study revealed that the evaluated LLMs achieved an accuracy ranging from 68.57% to 80%, indicating that these models are not yet optimally trained to address highly specific questions within the medical–dental domain. Additionally, inconsistencies, difficulties in managing complex question structures, and occasional contradictions were observed. The authors concluded that although LLMs exhibit promising potential, they should currently be regarded as supplementary tools that necessitate careful evaluation and verification by healthcare professionals, particularly in complex clinical decision-making contexts.
Future research should focus on the domain-specific training of LLMs, their integration with electronic health records, and the development of AI-assisted clinical decision support systems specifically tailored to dental care and the prevention of infective endocarditis [216]. Such advancements may contribute to reducing variability in antibiotic-prescribing practices and to promoting evidence-based clinical approaches [215].
4.7. Limitations of the Review
As this review focused exclusively on systematic reviews, a considerable number of original studies were excluded, which may have limited the variety of evidence considered. Observational, clinical, or cohort studies that could have provided complementary information were excluded.
The data extraction process primarily focused on the main outcomes of the systematic reviews. However, potential secondary or more detailed outcomes may not have been included, limiting the depth of the analysis.
The search was conducted using three main databases (PubMed, Scopus, and ScienceDirect) and Google Scholar for grey literature. However, the exclusive use of these tools may have excluded studies published in other journals or narrower databases, which could contain relevant evidence.
5. Conclusions
This mapping review examined the existing literature on the correlation between cardiovascular diseases, particularly endocarditis, and oral disorders, with a specific focus on periodontitis and dental caries. The results suggest that there is a significant connection between oral health and cardiovascular health, highlighting how oral infections, particularly periodontitis, can contribute to bacteraemia and promote the development of cardiovascular complications, including infective endocarditis.
Antibiotic prophylaxis for preventing infective endocarditis, especially in high-risk patients, remains a controversial topic. While some systematic reviews have highlighted the effectiveness of prophylaxis in specific contexts, its universal applicability is still under discussion, with studies suggesting the need for further research to clarify its real effectiveness.
Author Contributions
Conceptualisation, M.D., P.L. and C.G.; methodology, M.D., C.G. and D.S.; software, A.M. and E.L.; search strategy, M.D., D.S. and P.L.; screening, M.D., D.S. and P.L.; resources, M.D. and E.L.; data curation, M.D. and A.M.; writing—original draft preparation, M.D. and P.L.; writing—review and editing, M.D. and A.B.; supervision, A.B., G.I. and L.L.M.; project administration, A.B. and L.L.M.; funding acquisition, G.I., A.B. and L.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80. [Google Scholar]
- Mannoh, I.; Hussien, M.; Commodore-Mensah, Y.; Michos, E.D. Impact of social determinants of health on cardiovascular disease prevention. Curr. Opin. Cardiol. 2021, 36, 572–579. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Niemiec, B.A. Periodontal disease. Top. Companion Anim. Med. 2008, 23, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Highfield, J. Diagnosis and classification of periodontal disease. Aust. Dent. J. 2009, 54 (Suppl. 1), S11–S26. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Favaloro, E.J.; Sanchis-Gomar, F.; Lippi, G. Periodontitis, coronary heart disease and myocardial infarction: Treat one, benefit all. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2020, 31, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.; Gajagowni, S.; Qadeer, Y.; Wang, Z.; Virani, S.S.; Meurman, J.H.; Krittanawong, C. Oral Health and Cardiovascular Disease. Am. J. Med. 2024, 137, 304–307. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Caione, A.; Guido, A.L.; Martella, A.; Paiano, R.; Pandurino, A. Knowledge base support for dynamic information system management. Inf. Syst. E-Bus. Manag. 2016, 14, 533–576. [Google Scholar] [CrossRef]
- Longo, A.; Zappatore, M.; Martella, A.; Rucco, C. Enhancing Data Education with Datathons: An Experience with Open Data on Renewable Energy Systems. In Proceedings of the 1st ACM SIGMOD International Workshop on Data Systems Education: Bridging Education Practice with Education Research, Philadelphia, PA, USA, 12–17 June 2022; Association for Computing Machinery: New York, NY, USA, 2022; pp. 26–31. [Google Scholar]
- Somma, A.; Benedictis, A.D.; Zappatore, M.; Martella, C.; Martella, A.; Longo, A. Digital Twin Space: The Integration of Digital Twins and Data Spaces. In Proceedings of the IEEE International Conference on Big Data, Sorrento, Italy, 15–18 December 2023; pp. 4017–4025. [Google Scholar]
- Whiting, P.; Savović, J.; Higgins, J.P.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Dave, M.; Tattar, R. Antimicrobial resistance genes in the oral microbiome. Evid.-Based Dent. 2025, 26, 42–43. [Google Scholar] [CrossRef]
- Zorman, M.J.; Vibhishanan, J.; Dangas, K.; Castle, J.; Li, K.H.C.; Coronelli, M.; Eastwick-Jones, K.; Swan, A.; Johnson, N.; Choksey, A.; et al. Valve Thrombosis and Antithrombotic Therapy After Bioprosthetic Mitral Valve Replacement: A Systematic Review and Meta-Analysis. Eur. Heart J. Cardiovasc. Pharmacother. 2025, 11, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Barda, B.; Schindler, C.; Bernasconi, E.; Bongiovanni, M. Breaking the Dogma of Intravenous Treatment for Infective Endocarditis: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 7518. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Bruno, A.; Lombardo, M.; Muti, P. Echocardiographic Assessment of Mitral Valve Prolapse Prevalence before and after the Year 1999: A Systematic Review. J. Clin. Med. 2024, 13, 6160. [Google Scholar] [CrossRef]
- Mourad, A.; Nwafo, N.; Skalla, L.; Holland, T.L.; Jenkins, T.C. Oral Versus Intravenous Antibiotic Therapy for Staphylococcus aureus Bacteremia or Endocarditis: A Systematic Review and Meta-Analysis of Randomized, Controlled Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2025, 80, 29–36. [Google Scholar] [CrossRef]
- Veldurthy, S.; Shrivastava, D.; Majeed, F.; Ayaz, T.; Munir, A.; Haider, A.; Mylavarapu, M. Incidence of Infective Endocarditis Post-TPVR with MELODY Valve in Pediatric Patients: A Systematic Review and Meta-Analysis. Curr. Cardiol. Rev. 2025, 21, e1573403X1324878. [Google Scholar] [CrossRef]
- Martins, C.C.; Lockhart, P.B.; Firmino, R.T.; Kilmartin, C.; Cahill, T.J.; Dayer, M.; Occhi-Alexandre, I.G.P.; Lai, H.; Ge, L.; Thornhill, M.H. Bacteremia following different oral procedures: Systematic review and meta-analysis. Oral Dis. 2024, 30, 846–854. [Google Scholar] [CrossRef]
- Monaci, C.; Nair, A.N.; Gilukara, S.S.; Tummala, T.; Shreenithi, J.; Fatima, S.; Gupta, R.; Sabu, N.; Nagra, H.M.; Colca Herrera, A.V.; et al. Clinical Profiles and Outcomes of Prosthesis-Specific Infective Endocarditis Subsequent to Transcatheter Versus Surgical Aortic Valve Replacement: A Systematic Review and Meta-Analysis. Cureus 2024, 16, e59398. [Google Scholar] [CrossRef]
- Brown, A.; Jefferson, H.L.; Daley, P.; Kent, W.D.; Webster, D.; Adams, C. Partial oral versus full intravenous antibiotic treatment of endocarditis in people who inject drugs: A systematic review. J. Assoc. Med. Microbiol. Infect. Dis. 2024, 8, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ribeiro, M.; Gomes, B.; Arruda-Vasconcelos, R.; Monteiro, I.A.; Costa, M.J.F.; Sette-de-Souza, P.H. Antibiotic Resistance Profile of Clinical Strains of Enterococci from Secondary/Persistent Endodontic Infections: What do We Know? A Systematic Review of Clinical Studies. J. Endod. 2024, 50, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Nor, M.A.; Ogedegbe, O.J.; Barbarawi, A.; Ali, A.I.; Sheikh, I.M.; Yussuf, F.M.; Adam, S.M.; Hassan, O.A.; Tabowei, G.; Jimoh, A.; et al. Systemic Lupus Erythematosus and Cardiovascular Diseases: A Systematic Review. Cureus 2023, 15, e39284. [Google Scholar] [CrossRef]
- Diz Dios, P.; Monteiro, L.; Pimolbutr, K.; Gobbo, M.; France, K.; Bindakhil, M.; Holmes, H.; Sperotto, F.; Graham, L.; Turati, F.; et al. World Workshop on Oral Medicine VIII: Dentists’ compliance with infective endocarditis prophylaxis guidelines for patients with high-risk cardiac conditions: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, 757–771. [Google Scholar] [CrossRef] [PubMed]
- Lean, S.S.H.; Jou, E.; Ho, J.S.Y.; Jou, E.G.L. Prophylactic antibiotic use for infective endocarditis: A systematic review and meta-analysis. BMJ Open 2023, 13, e077026. [Google Scholar] [CrossRef] [PubMed]
- Budea, C.M.; Bratosin, F.; Bogdan, I.; Bota, A.V.; Turaiche, M.; Tirnea, L.; Stoica, C.N.; Csep, A.N.; Feciche, B.; Pescariu, S.A.; et al. Clinical Presentation and Risk Factors of Infective Endocarditis in the Elderly: A Systematic Review. J. Pers. Med. 2023, 13, 296. [Google Scholar] [CrossRef]
- Araújo Júnior, A.G.; Costa, M.; Silva, F.R.P.; Arcanjo, D.D.R.; Moura, L.; Oliveira, F.A.A.; Soares, M.J.S.; Quelemes, P.V. Amoxicillin-Resistant Streptococci Carriage in the Mouths of Children: A Systematic Review and Meta-Analysis. Pathog. 2022, 11, 1114. [Google Scholar] [CrossRef]
- Hussein, H.; Montesinos-Guevara, C.; Abouelkheir, M.; Brown, R.S.; Hneiny, L.; Amer, Y.S. Quality appraisal of antibiotic prophylaxis guidelines to prevent infective endocarditis following dental procedures: A systematic review. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022, 134, 562–572. [Google Scholar] [CrossRef]
- Shimizu, K.; Horinishi, Y.; Sano, C.; Ohta, R. Infection Route of Parvimonas micra: A Case Report and Systematic Review. Healthcare 2022, 10, 1727. [Google Scholar] [CrossRef]
- Alifragki, A.; Kontogianni, A.; Protopapa, I.; Baliou, S.; Ioannou, P. Infective Endocarditis by Pasteurella Species: A Systematic Review. J. Clin. Med. 2022, 11, 5037. [Google Scholar] [CrossRef]
- Sindoni, A.; Valeriani, F.; Protano, C.; Liguori, G.; Romano Spica, V.; Vitali, M.; Gallè, F. Health risks for body pierced community: A systematic review. Public Health 2022, 205, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Talha, K.M.; Dayer, M.J.; Thornhill, M.H.; Tariq, W.; Arshad, V.; Tleyjeh, I.M.; Bailey, K.R.; Palraj, R.; Anavekar, N.S.; Rizwan Sohail, M.; et al. Temporal Trends of Infective Endocarditis in North America from 2000 to 2017—A Systematic Review. Open Forum Infect. Dis. 2021, 8, ofab479. [Google Scholar] [CrossRef] [PubMed]
- Wald-Dickler, N.; Holtom, P.D.; Phillips, M.C.; Centor, R.M.; Lee, R.A.; Baden, R.; Spellberg, B. Oral Is the New IV. Challenging Decades of Blood and Bone Infection Dogma: A Systematic Review. Am. J. Med. 2022, 135, 369–379.e361. [Google Scholar] [CrossRef]
- Franconieri, F.; Join-Lambert, O.; Creveuil, C.; Auzou, M.; Labombarda, F.; Aouba, A.; Verdon, R.; de La Blanchardière, A. Rothia spp. infective endocarditis: A systematic literature review. Infect. Dis. Now 2021, 51, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Hidalgo, L.; de Alarcón, A.; López-Cortes, L.E.; Luque-Márquez, R.; López-Cortes, L.F.; Gutiérrez-Valencia, A.; Gil-Navarro, M.V. Enterococcus faecalis Endocarditis and Outpatient Treatment: A Systematic Review of Current Alternatives. Antibioticcs 2020, 9, 657. [Google Scholar] [CrossRef]
- Martí-Carvajal, A.J.; Dayer, M.; Conterno, L.O.; Gonzalez Garay, A.G.; Martí-Amarista, C.E. A comparison of different antibiotic regimens for the treatment of infective endocarditis. Cochrane Database Syst. Rev. 2020, 5, CD009880. [Google Scholar] [CrossRef]
- Cummins, J.; McCarthy, M.; Esterman, A.; Karve, A.; Lee, A. Knowledge and Compliance of Dentists’ and Dental Students’ with Respect to Relevant Guidelines for Prescribing Antibiotic Prophylaxis for the Prevention of Infective Endocarditis: A Systematic Review. J. Evid.-Based Dent. Pract. 2020, 20, 101311. [Google Scholar] [CrossRef]
- Vahabi, A.; Gül, F.; Garakhanova, S.; Sipahi, H.; Sipahi, O.R. Pooled analysis of 1270 infective endocarditis cases in Turkey. J. Infect. Dev. Ctries. 2019, 13, 93–100. [Google Scholar] [CrossRef]
- Khan, A.; Aslam, A.; Satti, K.N.; Ashiq, S. Infective endocarditis post-transcatheter aortic valve implantation (TAVI), microbiological profile and clinical outcomes: A systematic review. PLoS ONE 2020, 15, e0225077. [Google Scholar] [CrossRef]
- Napolitani, M.; Troiano, G.; Bedogni, C.; Messina, G.; Nante, N. Kocuria kristinae: An emerging pathogen in medical practice. J. Med. Microbiol. 2019, 68, 1596–1603. [Google Scholar] [CrossRef]
- Nakatani, S.; Ohara, T.; Ashihara, K.; Izumi, C.; Iwanaga, S.; Eishi, K.; Okita, Y.; Daimon, M.; Kimura, T.; Toyoda, K.; et al. JCS 2017 Guideline on Prevention and Treatment of Infective Endocarditis. Off. J. Jpn. Circ. Soc. 2019, 83, 1767–1809. [Google Scholar] [CrossRef]
- Abarbanell, G.; Tepper, N.K.; Farr, S.L. Safety of contraceptive use among women with congenital heart disease: A systematic review. Congenit. Heart Dis. 2019, 14, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Singh Gill, A.; Morrissey, H.; Rahman, A. A Systematic Review and Meta-Analysis Evaluating Antibiotic Prophylaxis in Dental Implants and Extraction Procedures. Medicina 2018, 54, 95. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.A.; Walsh, W.F.; Costello, B.; McLellan, A.J.A.; Brown, A.; Reid, C.M.; Tran, L.; Maguire, G.P. Medical Management of Rheumatic Heart Disease: A Systematic Review of the Evidence. Cardiol. Rev. 2018, 26, 187–195. [Google Scholar] [CrossRef]
- Al-Omari, A.; Cameron, D.W.; Lee, C.; Corrales-Medina, V.F. Oral antibiotic therapy for the treatment of infective endocarditis: A systematic review. BMC Infect. Dis. 2014, 14, 140. [Google Scholar] [CrossRef] [PubMed]
- Glenny, A.M.; Oliver, R.; Roberts, G.J.; Hooper, L.; Worthington, H.V. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst. Rev. 2013, Cd003813. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Worthington, H.V. Interventions for replacing missing teeth: Antibiotics at dental implant placement to prevent complications. Cochrane Database Syst. Rev. 2013, 13, CD004152. [Google Scholar] [CrossRef]
- Tepper, N.K.; Paulen, M.E.; Marchbanks, P.A.; Curtis, K.M. Safety of contraceptive use among women with peripartum cardiomyopathy: A systematic review. Contraception 2010, 82, 95–101. [Google Scholar] [CrossRef]
- Swedish Council on Health Technology. SBU Systematic Review Summaries. In Antibiotic Prophylaxis for Surgical Procedures: A Systematic Review; Swedish Council on Health Technology Assessment (SBU) Swedish Council on Health Technology Assessment: Stockholm, Sweden, 2010. [Google Scholar]
- Esposito, M.; Worthington, H.V.; Loli, V.; Coulthard, P.; Grusovin, M.G. Interventions for replacing missing teeth: Antibiotics at dental implant placement to prevent complications. Cochrane Database Syst. Rev. 2010, 7, CD004152. [Google Scholar] [CrossRef]
- Oliver, R.; Roberts, G.J.; Hooper, L.; Worthington, H.V. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst. Rev. 2008, Cd003813. [Google Scholar] [CrossRef]
- Esposito, M.; Grusovin, M.G.; Talati, M.; Coulthard, P.; Oliver, R.; Worthington, H.V. Interventions for replacing missing teeth: Antibiotics at dental implant placement to prevent complications. Cochrane Database Syst. Rev. 2008, 3, CD004152. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Loven, B.; Brennan, M.T.; Fox, P.C. The evidence base for the efficacy of antibiotic prophylaxis in dental practice. J. Am. Dent. Assoc. 2007, 138, 458–474. [Google Scholar] [CrossRef]
- Oliver, R.; Roberts, G.J.; Hooper, L. Penicillins for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst. Rev. 2004, CD003813. [Google Scholar] [CrossRef]
- Esposito, M.; Coulthard, P.; Oliver, R.; Thomsen, P.; Worthington, H.V. Antibiotics to prevent complications following dental implant treatment. Cochrane Database Syst. Rev. 2003, 3, CD004152. [Google Scholar] [CrossRef]
- Kussainova, Z.; Kulmaganbetov, M.; Abiltayev, A.; Bulegenov, T.; Salikhanov, I. Risk of Infective Endocarditis Following Invasive Dental Procedures: A Systematic Review and Meta-Analysis. Public Health Rev. 2024, 45, 1607684. [Google Scholar] [CrossRef]
- Lacassin, F.; Hoen, B.; Leport, C.; Selton-Suty, C.; Delahaye, F.; Goulet, V.; Etienne, J.; Briançon, S. Procedures associated with infective endocarditis in adults. A case control study. Eur. Heart J. 1995, 16, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Abrutyn, E.; Berlin, J.A.; Kinman, J.L.; Feldman, R.S.; Stolley, P.D.; Levison, M.E.; Korzeniowski, O.M.; Kaye, D. Dental and cardiac risk factors for infective endocarditis. A population-based, case-control study. Ann. Intern. Med. 1998, 129, 761–769. [Google Scholar] [CrossRef]
- Porat Ben-Amy, D.; Littner, M.; Siegman-Igra, Y. Are dental procedures an important risk factor for infective endocarditis? A case-crossover study. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 269–273. [Google Scholar] [CrossRef]
- Chen, P.C.; Tung, Y.C.; Wu, P.W.; Wu, L.S.; Lin, Y.S.; Chang, C.J.; Kung, S.; Chu, P.H. Dental Procedures and the Risk of Infective Endocarditis. Medicine 2015, 94, e1826. [Google Scholar] [CrossRef]
- Chen, T.T.; Yeh, Y.C.; Chien, K.L.; Lai, M.S.; Tu, Y.K. Risk of Infective Endocarditis After Invasive Dental Treatments: Case-Only Study. Circulation 2018, 138, 356–363. [Google Scholar] [CrossRef]
- Tubiana, S.; Blotière, P.O.; Hoen, B.; Lesclous, P.; Millot, S.; Rudant, J.; Weill, A.; Coste, J.; Alla, F.; Duval, X. Dental procedures, antibiotic prophylaxis, and endocarditis among people with prosthetic heart valves: Nationwide population based cohort and a case crossover study. BMJ 2017, 358, j3776. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Crum, A.; Campbell, R.; Stone, T.; Lee, E.C.; Bradburn, M.; Fibisan, V.; Dayer, M.; Prendergast, B.D.; Lockhart, P.; et al. Temporal association between invasive procedures and infective endocarditis. Heart 2023, 109, 223–231. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Gibson, T.B.; Yoon, F.; Dayer, M.J.; Prendergast, B.D.; Lockhart, P.B.; O’Gara, P.T.; Baddour, L.M. Antibiotic Prophylaxis Against Infective Endocarditis Before Invasive Dental Procedures. J. Am. Coll. Cardiol. 2022, 80, 1029–1041. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Gibson, T.B.; Yoon, F.; Dayer, M.J.; Prendergast, B.D.; Lockhart, P.B.; O’Gara, P.T.; Baddour, L.M. Endocarditis, invasive dental procedures, and antibiotic prophylaxis efficacy in US Medicaid patients. Oral Dis. 2024, 30, 1591–1605. [Google Scholar] [CrossRef] [PubMed]
- Sperotto, F.; France, K.; Gobbo, M.; Bindakhil, M.; Pimolbutr, K.; Holmes, H.; Monteiro, L.; Graham, L.; Hong, C.H.L.; Sollecito, T.P.; et al. Antibiotic Prophylaxis and Infective Endocarditis Incidence Following Invasive Dental Procedures: A Systematic Review and Meta-Analysis. JAMA Cardiol. 2024, 9, 599–610. [Google Scholar] [CrossRef]
- Keller, K.; von Bardeleben, R.S.; Ostad, M.A.; Hobohm, L.; Munzel, T.; Konstantinides, S.; Lankeit, M. Temporal Trends in the Prevalence of Infective Endocarditis in Germany Between 2005 and 2014. Am. J. Cardiol. 2017, 119, 317–322. [Google Scholar] [CrossRef] [PubMed]
- van den Brink, F.S.; Swaans, M.J.; Hoogendijk, M.G.; Alipour, A.; Kelder, J.C.; Jaarsma, W.; Eefting, F.D.; Groenmeijer, B.; Kupper, A.J.F.; Ten Berg, J.M. Increased incidence of infective endocarditis after the 2009 European Society of Cardiology guideline update: A nationwide study in the Netherlands. Eur. Heart J. 2017, 3, 141–147. [Google Scholar] [CrossRef]
- Bates, K.E.; Hall, M.; Shah, S.S.; Hill, K.D.; Pasquali, S.K. Trends in infective endocarditis hospitalisations at United States children’s hospitals from 2003 to 2014: Impact of the 2007 American Heart Association antibiotic prophylaxis guidelines. Cardiol. Young 2017, 27, 686–690. [Google Scholar] [CrossRef]
- Sakai Bizmark, R.; Chang, R.R.; Tsugawa, Y.; Zangwill, K.M.; Kawachi, I. Impact of AHA’s 2007 guideline change on incidence of infective endocarditis in infants and children. Am. Heart J. 2017, 189, 110–119. [Google Scholar] [CrossRef]
- Garg, P.; Ko, D.T.; Bray Jenkyn, K.M.; Li, L.; Shariff, S.Z. Infective Endocarditis Hospitalizations and Antibiotic Prophylaxis Rates Before and After the 2007 American Heart Association Guideline Revision. Circulation 2019, 140, 170–180. [Google Scholar] [CrossRef]
- Quan, T.P.; Muller-Pebody, B.; Fawcett, N.; Young, B.C.; Minaji, M.; Sandoe, J.; Hopkins, S.; Crook, D.; Peto, T.; Johnson, A.P.; et al. Investigation of the impact of the NICE guidelines regarding antibiotic prophylaxis during invasive dental procedures on the incidence of infective endocarditis in England: An electronic health records study. BMC Med. 2020, 18, 84. [Google Scholar] [CrossRef]
- Vähäsarja, N.; Lund, B.; Ternhag, A.; Götrick, B.; Olaison, L.; Hultin, M.; Krüger Weiner, C.; Naimi-Akbar, A. Incidence of infective endocarditis caused by viridans group streptococci in Sweden—Effect of cessation of antibiotic prophylaxis in dentistry for risk individuals. J. Oral Microbiol. 2020, 12, 1768342. [Google Scholar] [CrossRef] [PubMed]
- Bikdeli, B.; Wang, Y.; Kim, N.; Desai, M.M.; Quagliarello, V.; Krumholz, H.M. Trends in hospitalization rates and outcomes of endocarditis among Medicare beneficiaries. J. Am. Coll. Cardiol. 2013, 62, 2217–2226. [Google Scholar] [CrossRef]
- DeSimone, D.C.; Tleyjeh, I.M.; Correa de Sa, D.D.; Anavekar, N.S.; Lahr, B.D.; Sohail, M.R.; Steckelberg, J.M.; Wilson, W.R.; Baddour, L.M. Incidence of Infective Endocarditis Due to Viridans Group Streptococci Before and After the 2007 American Heart Association’s Prevention Guidelines: An Extended Evaluation of the Olmsted County, Minnesota, Population and Nationwide Inpatient Sample. Mayo Clin. Proc. 2015, 90, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, N.; Chikwe, J.; Itagaki, S.; Gelijns, A.C.; Adams, D.H.; Egorova, N.N. Trends in Infective Endocarditis in California and New York State, 1998–2013. JAMA 2017, 317, 1652–1660. [Google Scholar] [CrossRef]
- Sun, L.C.; Lai, C.C.; Wang, C.Y.; Wang, Y.H.; Wang, J.Y.; Hsu, Y.L.; Hu, Y.L.; Wu, E.T.; Lin, M.T.; Sy, L.B.; et al. Risk factors for infective endocarditis in children with congenital heart diseases—A nationwide population-based case control study. Int. J. Cardiol. 2017, 248, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Crum, A.; Rex, S.; Campbell, R.; Stone, T.; Bradburn, M.; Fibisan, V.; Dayer, M.J.; Prendergast, B.D.; Lockhart, P.B.; et al. Infective endocarditis following invasive dental procedures: IDEA case-crossover study. Health Technol. Assess. 2022, 26, 1–86. [Google Scholar] [CrossRef]
- Rogers, A.M.; Schiller, N.B. Impact of the first nine months of revised infective endocarditis prophylaxis guidelines at a university hospital: So far so good. J. Am. Soc. Echocardiogr. 2008, 21, 775. [Google Scholar] [CrossRef]
- Pasquali, S.K.; He, X.; Mohamad, Z.; McCrindle, B.W.; Newburger, J.W.; Li, J.S.; Shah, S.S. Trends in endocarditis hospitalizations at US children’s hospitals: Impact of the 2007 American Heart Association Antibiotic Prophylaxis Guidelines. Am. Heart J. 2012, 163, 894–899. [Google Scholar] [CrossRef]
- Pant, S.; Patel, N.J.; Deshmukh, A.; Golwala, H.; Patel, N.; Badheka, A.; Hirsch, G.A.; Mehta, J.L. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J. Am. Coll. Cardiol. 2015, 65, 2070–2076. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Gibson, T.B.; Cutler, E.; Dayer, M.J.; Chu, V.H.; Lockhart, P.B.; O’Gara, P.T.; Baddour, L.M. Antibiotic Prophylaxis and Incidence of Endocarditis Before and After the 2007 AHA Recommendations. J. Am. Coll. Cardiol. 2018, 72, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- DeSimone, D.C.; Lahr, B.D.; Anavekar, N.S.; Sohail, M.R.; Tleyjeh, I.M.; Wilson, W.R.; Baddour, L.M. Temporal Trends of Infective Endocarditis in Olmsted County, Minnesota, Between 1970 and 2018: A Population-Based Analysis. Open Forum Infect. Dis. 2021, 8, ofab038. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.S.; Liu, W.; Savu, A.; Marelli, A.J.; Kaul, P. Infective Endocarditis Hospitalizations Before and After the 2007 American Heart Association Prophylaxis Guidelines. Can. J. Cardiol. 2016, 32, 942–948. [Google Scholar] [CrossRef]
- Knirsch, W.; Schuler, S.K.; Christmann, M.; Weber, R. Time-trend population analysis of the clinical and epidemiologic effect on pediatric infective endocarditis after change of antibiotic prophylaxis guidelines. Infection 2020, 48, 671–678. [Google Scholar] [CrossRef]
- Weber, C.; Luehr, M.; Petrov, G.; Misfeld, M.; Akhyari, P.; Tugtekin, S.M.; Diab, M.; Saha, S.; Elderia, A.; Lichtenberg, A.; et al. Impact of the 2009 ESC Guideline Change on Surgically Treated Infective Endocarditis. Ann. Thorac. Surg. 2022, 114, 1349–1356. [Google Scholar] [CrossRef]
- Krul, M.M.; Vonk, A.B.; Cornel, J.H. Trends in incidence of infective endocarditis at the Medical Center of Alkmaar. Neth. Heart J. Mon. J. Neth. Soc. Cardiol. Neth. Heart Found. 2015, 23, 548–554. [Google Scholar] [CrossRef][Green Version]
- Duval, X.; Delahaye, F.; Alla, F.; Tattevin, P.; Obadia, J.F.; Le Moing, V.; Doco-Lecompte, T.; Celard, M.; Poyart, C.; Strady, C.; et al. Temporal trends in infective endocarditis in the context of prophylaxis guideline modifications: Three successive population-based surveys. J. Am. Coll. Cardiol. 2012, 59, 1968–1976. [Google Scholar] [CrossRef]
- Dayer, M.J.; Jones, S.; Prendergast, B.; Baddour, L.M.; Lockhart, P.B.; Thornhill, M.H. Incidence of infective endocarditis in England, 2000–2013: A secular trend, interrupted time-series analysis. Lancet 2015, 385, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.S.V.; McAllister, D.A.; Gallacher, P.; Astengo, F.; Rodríguez Pérez, J.A.; Hall, J.; Lee, K.K.; Bing, R.; Anand, A.; Nathwani, D.; et al. Incidence, Microbiology, and Outcomes in Patients Hospitalized with Infective Endocarditis. Circulation 2020, 141, 2067–2077. [Google Scholar] [CrossRef]
- Friedlander, A.H.; Couto-Souza, P.H. Recent infective endocarditis research findings suggest dentists prescribe prophylactic antibiotics for patients having a bicuspid aortic valve or mitral valve prolapse. Med. Oral Patol. Oral Cir. Bucal 2023, 28, e567–e571. [Google Scholar] [CrossRef]
- Zegri-Reiriz, I.; de Alarcón, A.; Muñoz, P.; Martínez Sellés, M.; González-Ramallo, V.; Miro, J.M.; Falces, C.; Gonzalez Rico, C.; Kortajarena Urkola, X.; Lepe, J.A.; et al. Infective Endocarditis in Patients with Bicuspid Aortic Valve or Mitral Valve Prolapse. J. Am. Coll. Cardiol. 2018, 71, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, M.H.; Jones, S.; Prendergast, B.; Baddour, L.M.; Chambers, J.B.; Lockhart, P.B.; Dayer, M.J. Quantifying infective endocarditis risk in patients with predisposing cardiac conditions. Eur. Heart J. 2018, 39, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Albakri, A.; Ahsan, A.; Vengal, M.; Ramacham Parambathu, A.K.; Majeed, A.; Siddiq, H. Antibiotic prophylaxis before invasive dental procedures for patients at high risk of infective endocarditis—A systematic review. Indian J. Dent. Res. 2022, 33, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Khairat, O. An effective antibiotic cover for the prevention of endocarditis following dental and other post-operative bacteraemias. J. Clin. Pathol. 1966, 19, 561–566. [Google Scholar] [CrossRef]
- Shanson, D.C.; Akash, S.; Harris, M.; Tadayon, M. Erythromycin stearate, 1.5 g, for the oral prophylaxis of streptococcal bacteraemia in patients undergoing dental extraction: Efficacy and tolerance. J. Antimicrob. Chemother. 1985, 15, 83–90. [Google Scholar] [CrossRef]
- Maskell, J.P.; Carter, J.L.; Boyd, R.B.; Williams, R.J. Teicoplanin as a prophylactic antibiotic for dental bacteraemia. J. Antimicrob. Chemother. 1986, 17, 651–659. [Google Scholar] [CrossRef]
- Shanson, D.C.; Shehata, A.; Tadayon, M.; Harris, M. Comparison of intravenous teicoplanin with intramuscular amoxycillin for the prophylaxis of streptococcal bacteraemia in dental patients. J. Antimicrob. Chemother. 1987, 20, 85–93. [Google Scholar] [CrossRef]
- Vergis, E.N.; Demas, P.N.; Vaccarello, S.J.; Yu, V.L. Topical antibiotic prophylaxis for bacteremia after dental extractions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2001, 91, 162–165. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Brennan, M.T.; Kent, M.L.; Norton, H.J.; Weinrib, D.A. Impact of amoxicillin prophylaxis on the incidence, nature, and duration of bacteremia in children after intubation and dental procedures. Circulation 2004, 109, 2878–2884. [Google Scholar] [CrossRef]
- Diz Dios, P.; Tomás Carmona, I.; Limeres Posse, J.; Medina Henríquez, J.; Fernández Feijoo, J.; Alvarez Fernández, M. Comparative efficacies of amoxicillin, clindamycin, and moxifloxacin in prevention of bacteremia following dental extractions. Antimicrob. Agents Chemother. 2006, 50, 2996–3002. [Google Scholar] [CrossRef]
- Abu-Ta’a, M.; Quirynen, M.; Teughels, W.; van Steenberghe, D. Asepsis during periodontal surgery involving oral implants and the usefulness of peri-operative antibiotics: A prospective, randomized, controlled clinical trial. J. Clin. Periodontol. 2008, 35, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Orive, G.; Aguirre, J.J.; Ardanza, B.; Andía, I. 5-year clinical experience with BTI dental implants: Risk factors for implant failure. J. Clin. Periodontol. 2008, 35, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Sasser, H.C.; Fox, P.C.; Paster, B.J.; Bahrani-Mougeot, F.K. Bacteremia associated with toothbrushing and dental extraction. Circulation 2008, 117, 3118–3125. [Google Scholar] [CrossRef]
- Asi, K.S.; Gill, A.S.; Mahajan, S. Postoperative bacteremia in periodontal flap surgery, with and without prophylactic antibiotic administration: A comparative study. J. Indian Soc. Periodontol. 2010, 14, 18–22. [Google Scholar] [CrossRef]
- Esposito, M.; Cannizzaro, G.; Bozzoli, P.; Checchi, L.; Ferri, V.; Landriani, S.; Leone, M.; Todisco, M.; Torchio, C.; Testori, T.; et al. Effectiveness of prophylactic antibiotics at placement of dental implants: A pragmatic multicentre placebo-controlled randomised clinical trial. Eur. J. Oral Implant. 2010, 3, 135–143. [Google Scholar]
- Siddiqi, A.; Morkel, J.A.; Zafar, S. Antibiotic prophylaxis in third molar surgery: A randomized double-blind placebo-controlled clinical trial using split-mouth technique. Int. J. Oral Maxillofac. Surg. 2010, 39, 107–114. [Google Scholar] [CrossRef]
- Chandramohan, P.; Ramesh, B.M.; Laxmi, S.J. Bacteremia during periodontal flap surgery, with and without prophylactic antibiotic administration: A comparative study. Indian J. Dent. Adv. 2011, 643–649. [Google Scholar]
- Maharaj, B.; Coovadia, Y.; Vayej, A.C. A comparative study of amoxicillin, clindamycin and chlorhexidine in the prevention of post-extraction bacteraemia. Cardiovasc. J. Afr. 2012, 23, 491–494. [Google Scholar] [CrossRef]
- DuVall, N.B.; Fisher, T.D.; Hensley, D.; Hancock, R.H.; Vandewalle, K.S. The comparative efficacy of 0.12% chlorhexidine and amoxicillin to reduce the incidence and magnitude of bacteremia during third molar extractions: A prospective, blind, randomized clinical trial. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, 752–763. [Google Scholar] [CrossRef]
- Limeres Posse, J.; Álvarez Fernández, M.; Fernández Feijoo, J.; Medina Henríquez, J.; Lockhart, P.B.; Chu, V.H.; Diz Dios, P. Intravenous amoxicillin/clavulanate for the prevention of bacteraemia following dental procedures: A randomized clinical trial. J. Antimicrob. Chemother. 2016, 71, 2022–2030. [Google Scholar] [CrossRef]
- Bergadà-Pijuan, J.; Frank, M.; Boroumand, S.; Hovaguimian, F.; Mestres, C.A.; Bauernschmitt, R.; Carrel, T.; Stadlinger, B.; Ruschitzka, F.; Zinkernagel, A.S.; et al. Antibiotic prophylaxis before dental procedures to prevent infective endocarditis: A systematic review. Infection 2023, 51, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.J.; Glenny, A.M.; Roberts, G.; Hooper, L.; Worthington, H.V. Antibiotic prophylaxis for preventing bacterial endocarditis following dental procedures. Cochrane Database Syst. Rev. 2022, 5, CD003813. [Google Scholar] [CrossRef]
- Van der Meer, J.T.; Van Wijk, W.; Thompson, J.; Vandenbroucke, J.P.; Valkenburg, H.A.; Michel, M.F. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet 1992, 339, 135–139. [Google Scholar] [CrossRef]
- Karikoski, E.; Sarkola, T.; Blomqvist, M. Dental caries prevalence in children with congenital heart disease—A systematic review. Acta Odontol. Scand. 2021, 79, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Pourmoghaddas, Z.; Meskin, M.; Sabri, M.; Norousali Tehrani, M.H.; Najafi, T. Dental Caries and Gingival Evaluation in Children with Congenital Heart Disease. Int. J. Prev. Med. 2018, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.M.; Mustafa, M.; Hasabalrasol, S.; Elshazali, O.H.; Nasir, E.F.; Ali, R.W.; Berggreen, E.; Skeie, M.S. Presence of plaque, gingivitis and caries in Sudanese children with congenital heart defects. Clin. Oral Investig. 2017, 21, 1299–1307. [Google Scholar] [CrossRef]
- Cantekin, K.; Gumus, H.; Torun, Y.A.; Sahin, H. The evaluation of developmental enamel defects and dental treatment conditions in a group of Turkish children with congenital heart disease. Cardiol. Young 2015, 25, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Cantekin, K.; Yilmaz, Y.; Cantekin, I.; Torun, Y. Comprehensive dental evaluation of children with congenital or acquired heart disease. Cardiol. Young 2013, 23, 705–710. [Google Scholar] [CrossRef]
- Suma, G.; Usha, M.D.; Ambika, G.; Jairanganath. Oral health status of normal children and those affiliated with cardiac diseases. J. Clin. Pediatr. Dent. 2011, 35, 315–318. [Google Scholar] [CrossRef]
- Siahi-Benlarbi, R.; Nies, S.M.; Sziegoleit, A.; Bauer, J.; Schranz, D.; Wetzel, W.E. Caries-, Candida- and Candida antigen/antibody frequency in children after heart transplantation and children with congenital heart disease. Pediatr. Transplant. 2010, 14, 715–721. [Google Scholar] [CrossRef]
- da Fonseca, M.A.; Evans, M.; Teske, D.; Thikkurissy, S.; Amini, H. The impact of oral health on the quality of life of young patients with congenital cardiac disease. Cardiol. Young 2009, 19, 252–256. [Google Scholar] [CrossRef]
- Tasioula, V.; Balmer, R.; Parsons, J. Dental health and treatment in a group of children with congenital heart disease. Pediatr. Dent. 2008, 30, 323–328. [Google Scholar] [PubMed]
- Stecksén-Blicks, C.; Rydberg, A.; Nyman, L.; Asplund, S.; Svanberg, C. Dental caries experience in children with congenital heart disease: A case-control study. Int. J. Paediatr. Dent. 2004, 14, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Noriega, L.A.; Torres, C.C.; Castillo, Y.; Moscoso, S.B.; Mosquera, S.; Díaz-Báez, D.; Chambrone, L. Impact of antibiotic prophylaxis on the incidence, nature, magnitude, and duration of bacteremia associated with dental procedures: A systematic review. J. Am. Dent. Assoc. 2019, 150, 948–959.e944. [Google Scholar] [CrossRef] [PubMed]
- Snanson, D.; Cannon, P.; Wilks, M. Amoxycillin compared with penicillin V for the prophylaxis of dental bacteraemia. J. AntiMicrob. Chemother. 1978, 4, 431–436. [Google Scholar] [CrossRef]
- Roberts, G.J.; Radford, P.; Holt, R. Prophylaxis of dental bacteraemia with oral amoxycillin in children. Br. Dent. J. 1987, 162, 179–182. [Google Scholar] [CrossRef]
- Hall, G.; Hedström, S.Å.; Heimdahl, A.; Nord, C.E. Prophylactic Administration of Penicillins for Endocarditis Does Not Reduce the Incidence of Postextraction Bacteremia. Clin. Infect. Dis. 1993, 17, 188–194. [Google Scholar] [CrossRef]
- Hall, G.; Heimdahl, A.; Nord, C.E. Effects of prophylactic administration of cefaclor on transient bacteremia after dental extraction. Eur. J. Clin. Microbiol. Infect. Dis. 1996, 15, 646–649. [Google Scholar] [CrossRef]
- Wahlmann, U.; Al-Nawas, B.; Jütte, M.; Wagner, W. Clinical and microbiological efficacy of single dose cefuroxime prophylaxis for dental surgical procedures. Int. J. Antimicrob. Agents 1999, 12, 253–256. [Google Scholar] [CrossRef]
- Lockhart, P.B.; DeLong, H.R.; Lipman, R.D.; Abt, E.; Baddour, L.M.; Colvin, M.; Miller, C.S.; Sollecito, T.; O’Brien, K.; Estrich, C.G.; et al. Effect of dental treatment before cardiac valve surgery: Systematic review and meta-analysis. J. Am. Dent. Assoc. 2019, 150, 739–747.e739. [Google Scholar] [CrossRef]
- de Souza, A.F.; Rocha, A.L.; Castro, W.H.; Ferreira, F.M.; Gelape, C.L.; Travassos, D.V.; da Silva, T.A. Dental care before cardiac valve surgery: Is it important to prevent infective endocarditis? IJC Heart Vasc. 2016, 12, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Deppe, H.; Auer-Bahrs, J.; Kolk, A.; Hall, D.; Wagenpfeil, S. Need for dental treatment following cardiac valve surgery: A clinical study. J. Cranio-Maxillo-Facial Surg. 2007, 35, 293–301. [Google Scholar] [CrossRef]
- Wu, G.H.; Manzon, S.; Badovinac, R.; Woo, S.B. Oral health, dental treatment, and cardiac valve surgery outcomes. Spec. Care Dent. 2008, 28, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hakeberg, M.; Dernevik, L.; Gatzinsky, P.; Eklöf, C.; Kennergren, C.; Jontell, M. The significance of oral health and dental treatment for the postoperative outcome of heart valve surgery. Scand. Cardiovasc. J. SCJ 1999, 33, 5–8. [Google Scholar] [CrossRef]
- Nakamura, Y.; Tagusari, O.; Seike, Y.; Ito, Y.; Saito, K.; Miyamoto, R.; Nakano, K.; Shikata, F. Prevalence of periodontitis and optimal timing of dental treatment in patients undergoing heart valve surgery. Interact. Cardiovasc. Thorac. Surg. 2011, 12, 696–700. [Google Scholar] [CrossRef]
- Bratel, J.; Kennergren, C.; Dernevik, L.; Hakeberg, M. Treatment of oral infections prior to heart valve surgery does not improve long-term survival. Swed. Dent. J. 2011, 35, 49–55. [Google Scholar]
- González Navarro, B.; Jané Salas, E.; Estrugo Devesa, A.; López López, J.; Viñas, M. Bacteremia Associated with Oral Surgery: A Review. J. Evid.-Based Dent. Pract. 2017, 17, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Brennan, M.T.; Thornhill, M.; Michalowicz, B.S.; Noll, J.; Bahrani-Mougeot, F.K.; Sasser, H.C. Poor oral hygiene as a risk factor for infective endocarditis–related bacteremia. J. Am. Dent. Assoc. 2009, 140, 1238–1244. [Google Scholar] [CrossRef]
- Bahrani-Mougeot, F.K.; Paster, B.J.; Coleman, S.; Ashar, J.; Barbuto, S.; Lockhart, P.B. Diverse and novel oral bacterial species in blood following dental procedures. J. Clin. Microbiol. 2008, 46, 2129–2132. [Google Scholar] [CrossRef]
- Heimdahl, A.; Hall, G.; Hedberg, M.; Sandberg, H.; Söder, P.O.; Tunér, K.; Nord, C.E. Detection and quantitation by lysis-filtration of bacteremia after different oral surgical procedures. J. Clin. Microbiol. 1990, 28, 2205–2209. [Google Scholar] [CrossRef]
- Rajasuo, A.; Perkki, K.; Nyfors, S.; Jousimies-Somer, H.; Meurman, J.H. Bacteremia following surgical dental extraction with an emphasis on anaerobic strains. J. Dent. Res. 2004, 83, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.J.; Jaffray, E.C.; Spratt, D.A.; Petrie, A.; Greville, C.; Wilson, M.; Lucas, V.S. Duration, prevalence and intensity of bacteraemia after dental extractions in children. Heart 2006, 92, 1274–1277. [Google Scholar] [CrossRef] [PubMed]
- Tomás, I.; Alvarez, M.; Limeres, J.; Tomás, M.; Medina, J.; Otero, J.L.; Diz, P. Effect of a chlorhexidine mouthwash on the risk of postextraction bacteremia. Infect. Control Hosp. Epidemiol. 2007, 28, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Roberts, G.J.; Watts, R.; Longhurst, P.; Gardner, P. Bacteremia of dental origin and antimicrobial sensitivity following oral surgical procedures in children. Pediatr. Dent. 1998, 20, 28–36. [Google Scholar]
- Benítez-Páez, A.; Álvarez, M.; Belda-Ferre, P.; Rubido, S.; Mira, A.; Tomás, I. Detection of transient bacteraemia following dental extractions by 16S rDNA pyrosequencing: A pilot study. PLoS ONE 2013, 8, e57782. [Google Scholar] [CrossRef]
- Maharaj, B.; Coovadia, Y.; Vayej, A.C. An investigation of the frequency of bacteraemia following dental extraction, tooth brushing and chewing. Cardiovasc. J. Afr. 2012, 23, 340–344. [Google Scholar] [CrossRef]
- Peterson, L.J.; Peacock, R. The incidence of bacteremia in pediatric patients following tooth extraction. Circulation 1976, 53, 676–679. [Google Scholar] [CrossRef]
- Rahn, R.; Schneider, S.; Diehl, O.; Schäfer, V.; Shah, P.M. Preventing post-treatment bacteremia: Comparing topical povidone-iodine and chlorhexidine. J. Am. Dent. Assoc. 1995, 126, 1145–1149. [Google Scholar] [CrossRef]
- Sefton, A.M.; Maskell, J.P.; Kerawala, C.; Cannell, H.; Seymour, A.; Sun, Z.M.; Williams, J.D. Comparative efficacy and tolerance of erythromycin and josamycin in the prevention of bacteraemia following dental extraction. J. Antimicrob. Chemother. 1990, 25, 975–984. [Google Scholar] [CrossRef]
- Hall, G.; Nord, C.E.; Heimdahl, A. Elimination of bacteraemia after dental extraction: Comparison of erythromycin and clindamycin for prophylaxis of infective endocarditis. J. Antimicrob. Chemother. 1996, 37, 783–795. [Google Scholar] [CrossRef]
- Tuna, A.; Delilbasi, C.; Arslan, A.; Gurol, Y.; Tazegun Tekkanat, Z. Do antibacterial mouthrinses affect bacteraemia in third molar surgery? A pilot study. Aust. Dent. J. 2012, 57, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Sweet, J.B.; Gill, V.J.; Chusid, M.J.; Elin, R.J. Nitroblue tetrazolium and Limulus assays for bacteremia after dental extraction: Effect of topical antiseptics. J. Am. Dent. Assoc. 1978, 96, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Cannell, H.; Kerawala, C.; Sefton, A.M.; Maskell, J.P.; Seymour, A.; Sun, Z.M.; Williams, J.D. Failure of two macrolide antibiotics to prevent post-extraction bacteraemia. Br. Dent. J. 1991, 171, 170–173. [Google Scholar] [CrossRef]
- Aitken, C.; Cannell, H.; Sefton, A.M.; Kerawala, C.; Seymour, A.; Murphy, M.; Whiley, R.A.; Williams, J.D. Comparative efficacy of oral doses of clindamycin and erythromycin in the prevention of bacteraemia. Br. Dent. J. 1995, 178, 418–422. [Google Scholar] [CrossRef]
- Josefsson, K.; Heimdahl, A.; von Konow, L.; Nord, C.E. Effect of phenoxymethylpenicillin and erythromycin prophylaxis on anaerobic bacteraemia after oral surgery. J. Antimicrob. Chemother. 1985, 16, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Tomás, I.; Pereira, F.; Llucián, R.; Poveda, R.; Diz, P.; Bagán, J.V. Prevalence of bacteraemia following third molar surgery. Oral Dis. 2008, 14, 89–94. [Google Scholar] [CrossRef]
- Piñeiro, A.; Tomás, I.; Blanco, J.; Alvarez, M.; Seoane, J.; Diz, P. Bacteraemia following dental implants’ placement. Clin. Oral Implant. Res. 2010, 21, 913–918. [Google Scholar] [CrossRef]
- Cahill, T.J.; Harrison, J.L.; Jewell, P.; Onakpoya, I.; Chambers, J.B.; Dayer, M.; Lockhart, P.; Roberts, N.; Shanson, D.; Thornhill, M.; et al. Antibiotic prophylaxis for infective endocarditis: A systematic review and meta-analysis. Heart 2017, 103, 937–944. [Google Scholar] [CrossRef]
- Imperiale, T.F.; Horwitz, R.I. Does prophylaxis prevent postdental infective endocarditis? A controlled evaluation of protective efficacy. Am. J. Med. 1990, 88, 131–136. [Google Scholar] [CrossRef]
- Baltch, A.L.; Schaffer, C.; Hammer, M.C.; Sutphen, N.T.; Smith, R.P.; Conroy, J.; Shayegani, M. Bacteremia following dental cleaning in patients with and without penicillin prophylaxis. Am. Heart J. 1982, 104, 1335–1339. [Google Scholar] [CrossRef]
- Coulter, W.A.; Coffey, A.; Saunders, I.D.; Emmerson, A.M. Bacteremia in children following dental extraction. J. Dent. Res. 1990, 69, 1691–1695. [Google Scholar] [CrossRef]
- Head, T.W.; Bentley, K.C.; Millar, E.P.; deVries, J.A. A comparative study of the effectiveness of metronidazole and penicillin V in eliminating anaerobes from postextraction bacteremias. Oral Surg. Oral Med. Oral Pathol. 1984, 58, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Horstkotte, D.; Rosin, H.; Friedrichs, W.; Loogen, F. Contribution for choosing the optimal prophylaxis of bacterial endocarditis. Eur. Heart J. 1987, 8, 379–381. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Dayer, M.J.; Forde, J.M.; Corey, G.R.; Chu, V.H.; Couper, D.J.; Lockhart, P.B. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: Before and after study. BMJ 2011, 342, d2392. [Google Scholar] [CrossRef]
- Desimone, D.C.; Tleyjeh, I.M.; Correa de Sa, D.D.; Anavekar, N.S.; Lahr, B.D.; Sohail, M.R.; Steckelberg, J.M.; Wilson, W.R.; Baddour, L.M. Incidence of infective endocarditis caused by viridans group streptococci before and after publication of the 2007 American Heart Association’s endocarditis prevention guidelines. Circulation 2012, 126, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Albinali, H.; Singh, R.; Al-Qahtani, A.; Al Suwaidi, J. Incidence of infective endocarditis before and after the 2007 endocarditis prevention guidelines: A population-based study from Qatar (2002–2012). Eur. Heart J. 2014, 908. [Google Scholar]
- Zhurakivska, K.; Russo, L.L.; Muzio, L.L.; Caponio, V.C.A.; Laino, L.; Arena, C.; Cirillo, N.; Troiano, G. Antibiotic prophylaxis at the time of dental implant placement: A cost-effectiveness analysis. BMC Health Serv. Res. 2022, 22, 1073. [Google Scholar] [CrossRef]
- Li, M.; Kim, J.B.; Sastry, B.K.S.; Chen, M. Infective endocarditis. Lancet 2024, 404, 377–392. [Google Scholar] [CrossRef]
- Ortiz, C.; López, J.; García, H.; Sevilla, T.; Revilla, A.; Vilacosta, I.; Sarriá, C.; Olmos, C.; Ferrera, C.; García, P.E.; et al. Clinical classification and prognosis of isolated right-sided infective endocarditis. Medicine 2014, 93, e137. [Google Scholar] [CrossRef]
- Raza, S.S.; Sultan, O.W.; Sohail, M.R. Gram-negative bacterial endocarditis in adults: State-of-the-heart. Expert Rev. Anti-Infect. Ther. 2010, 8, 879–885. [Google Scholar] [CrossRef]
- Kern, W.V.; Rieg, S. Burden of bacterial bloodstream infection-a brief update on epidemiology and significance of multidrug-resistant pathogens. Clin. Microbiol. Infect. 2020, 26, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Vikram, H.R. The long and short of vegetations in infective endocarditis. Expert Rev. Anti-Infect. Ther. 2007, 5, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G., Jr.; Bolger, A.F.; Levison, M.E.; Ferrieri, P.; Gerber, M.A.; Tani, L.Y.; Gewitz, M.H.; et al. Infective endocarditis: Diagnosis, antimicrobial therapy, and management of complications: A statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: Endorsed by the Infectious Diseases Society of America. Circulation 2005, 111, e394–e434. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Knoll, M.D.; Stamler, J.; Neaton, J.D.; Dyer, A.R.; Garside, D.B.; Wilson, P.W. Major Risk Factors as Antecedents of Fatal and Nonfatal Coronary Heart Disease Events. JAMA 2003, 290, 891–897. [Google Scholar] [CrossRef]
- Chamat-Hedemand, S.; Dahl, A.; Østergaard, L.; Arpi, M.; Fosbøl, E.; Boel, J.; Oestergaard, L.B.; Lauridsen, T.K.; Gislason, G.; Torp-Pedersen, C.; et al. Prevalence of Infective Endocarditis in Streptococcal Bloodstream Infections Is Dependent on Streptococcal Species. Circulation 2020, 142, 720–730. [Google Scholar] [CrossRef]
- Green, J.G.; Haisch, L. Infective endocarditis and antibiotic prophylaxis failure following an endodontic procedure. Gen. Dent. 1988, 36, 131–133. [Google Scholar]
- Dioguardi, M.; La Notte, D.; Sovereto, D.; Quarta, C.; Martella, A.; Ballini, A. Influence of Cavity Designs on Fracture Resistance: Analysis of the Role of Different Access Techniques to the Endodontic Cavity in the Onset of Fractures: Narrative Review. Sci. World J. 2024, 2024, 648011. [Google Scholar] [CrossRef]
- Gomes, B.; Berber, V.B.; Chiarelli-Neto, V.M.; Aveiro, E.; Chapola, R.C.; Passini, M.R.Z.; Lopes, E.M.; Chen, T.; Paster, B.J. Microbiota present in combined endodontic-periodontal diseases and its risks for endocarditis. Clin. Oral Investig. 2023, 27, 4757–4771. [Google Scholar] [CrossRef]
- Reis, L.C.; Rôças, I.N.; Siqueira, J.F., Jr.; de Uzeda, M.; Lacerda, V.S.; Domingues, R.; Miranda, K.R.; Saraiva, R.M. Bacteremia after supragingival scaling and dental extraction: Culture and molecular analyses. Oral Dis. 2018, 24, 657–663. [Google Scholar] [CrossRef]
- Dhotre, S.V.; Davane, M.S.; Nagoba, B.S. Periodontitis, bacteremia and infective endocarditis: A review study. Arch. Pediatr. Infect. Dis. 2017, 5, e41067. [Google Scholar] [CrossRef]
- Wang, A.; Gaca, J.G.; Chu, V.H. Management considerations in infective endocarditis: A review. JAMA 2018, 320, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kilian, M.; Chapple, I.; Hannig, M.; Marsh, P.; Meuric, V.; Pedersen, A.; Tonetti, M.; Wade, W.; Zaura, E. The oral microbiome–an update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Carinci, F.; Martinelli, M.; Contaldo, M.; Santoro, R.; Pezzetti, F.; Lauritano, D.; Candotto, V.; Mucchi, D.; Palmieri, A.; Tagliabue, A.; et al. Focus on periodontal disease and development of endocarditis. J. Biol. Regul. Homeost. Agents 2018, 32, 143–147. [Google Scholar]
- Charlotte Höfer, K.; Graf, I.; Adams, A.; Kuhr, K.; Plum, G.; Schwendicke, F.; Brockmeier, K.; Johannes Noack, M. Bacteraemia of oral origin in children—A Systematic review and network meta-analysis. Oral Dis. 2022, 28, 1783–1801. [Google Scholar] [CrossRef]
- Herzberg, M.C. Persistence of infective endocarditis. In Persistent Bacterial Infections; American Society for Microbiology: Washington, DC, USA, 2000; pp. 355–374. [Google Scholar] [CrossRef]
- Bloch, S.; Hager-Mair, F.F.; Andrukhov, O.; Schäffer, C. Oral streptococci: Modulators of health and disease. Front. Cell. Infect. Microbiol. 2024, 14, 1357631. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontology 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Dioguardi, M.; Quarta, C.; Sovereto, D.; Caloro, G.A.; Ballini, A.; Aiuto, R.; Martella, A.; Lo Muzio, L.; Di Cosola, M. Factors and management techniques in odontogenic keratocysts: A systematic review. Eur. J. Med. Res. 2024, 29, 287. [Google Scholar] [CrossRef]
- Jiun, I.; Siddik, S.; Malik, S.N.; Tin-Oo, M.M.; Alam, M.K.; Khan, M. Association between oral hygiene status and halitosis among smokers and nonsmokers. Oral Health Prev. Dent. 2015, 13, 395–405. [Google Scholar]
- Bergström, J. Tobacco smoking and chronic destructive periodontal disease. Odontology 2004, 92, 1–8. [Google Scholar] [CrossRef]
- Delaye, J.; Etienne, J.; Feruglio, G.; Fraile, J.; Glauser, M.; Gruer, L.; Hagler, W.; Krayenbuehl, H.; Kremer, R.; Meeter, K.L. Prophylaxis of infective endocarditis for dental procedures Report of a Working party of the European Society of Cardiology. Eur. Heart J. 1985, 6, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Jamrozik, E.; Selgelid, M.J. Drug-resistant infection: Causes, consequences, and responses. Ethics Drug Resist. Collect. Responsib. Glob. Public Health 2020, 5, 3–18. [Google Scholar]
- Schmalzle, S.A. A classic and fatal case of Streptococcus mutans subacute bacterial endocarditis; A now potentially underappreciated disease. IDCases 2020, 19, e00701. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Amadoro, C.; Colavita, G. Members of the Lactobacillus Genus Complex (LGC) as Opportunistic Pathogens: A Review. Microorganisms 2019, 7, 126. [Google Scholar] [CrossRef]
- Danneels, P.; Hamel, J.F.; Picard, L.; Rezig, S.; Martinet, P.; Lorleac’h, A.; Talarmin, J.P.; Buzelé, R.; Guimard, T.; Le Moal, G.; et al. Impact of Enterococcus faecalis Endocarditis Treatment on Risk of Relapse. Clin. Infect. Dis. 2023, 76, 281–290. [Google Scholar] [CrossRef]
- Kumar, A.; Rai, A. Oral Health Status, Health Behaviour and Treatment Needs of Patients Undergoing Cardiovascular Surgery. Braz. J. Cardiovasc. Surg. 2018, 33, 151–154. [Google Scholar] [CrossRef]
- Bakhsh, A.; Moyes, D.; Mannocci, F.; Proctor, G.; Niazi, S. Links between Nosocomial Endodontic Infections and Bacteremia Associated with Apical Periodontitis and Endodontic Treatment. J. Endod. 2025, 51, 140–149. [Google Scholar] [CrossRef]
- Tomás, I.; Alvarez, M.; Limeres, J.; Potel, C.; Medina, J.; Diz, P. Prevalence, duration and aetiology of bacteraemia following dental extractions. Oral Dis. 2007, 13, 56–62. [Google Scholar] [CrossRef]
- Dioguardi, M.; Dello Russo, C.; Scarano, F.; Esperouz, F.; Ballini, A.; Sovereto, D.; Alovisi, M.; Martella, A.; Lo Muzio, L. Analysis of Endodontic Successes and Failures in the Removal of Fractured Endodontic Instruments during Retreatment: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis. Healthcare 2024, 12, 1390. [Google Scholar] [CrossRef]
- Pardo, A.; Signoriello, A.; Signoretto, C.; Messina, E.; Carelli, M.; Tessari, M.; De Manna, N.D.; Rossetti, C.; Albanese, M.; Lombardo, G.; et al. Detection of Periodontal Pathogens in Oral Samples and Cardiac Specimens in Patients Undergoing Aortic Valve Replacement: A Pilot Study. J. Clin. Med. 2021, 10, 3874. [Google Scholar] [CrossRef]
- Dioguardi, M.; La Notte, D.; Sovereto, D.; Quarta, C.; Ballini, A.; Crincoli, V.; Aiuto, R.; Alovisi, M.; Martella, A.; Lo Muzio, L. Exploring the Impact of Access Cavity Designs on Canal Orifice Localization and Debris Presence: A Scoping Review. Clin. Exp. Dent. Res. 2024, 10, e70013. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Spirito, F.; Iacovelli, G.; Sovereto, D.; Laneve, E.; Caloro, G.A.; Ballini, A.; Martella, A.; Lo Muzio, L. Abfraction Theory: Controversy Analysis, Scoping Review. Curr. Oral Health Rep. 2024, 11, 237–247. [Google Scholar] [CrossRef]
- Wilson, W.; Taubert, K.A.; Gewitz, M.; Lockhart, P.B.; Baddour, L.M.; Levison, M.; Bolger, A.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of infective endocarditis: Guidelines from the American Heart Association: A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007, 116, 1736–1754. [Google Scholar] [CrossRef]
- Dargaud, Y.; Volot, F.; Desage, S.; Pouplard, C.; Chamouard, V.; Lienhart, A. Haemophilia Prophylaxis in the Age of Innovation: Exploring Opportunities for Personalized Treatment. Haemophilia 2025, 1–9. [Google Scholar] [CrossRef]
- Moser, C.; Lerche, C.J.; Thomsen, K.; Hartvig, T.; Schierbeck, J.; Jensen, P.Ø.; Ciofu, O.; Høiby, N. Antibiotic therapy as personalized medicine–general considerations and complicating factors. Apmis 2019, 127, 361–371. [Google Scholar] [CrossRef]
- Faulkner, E.; Mensah, A.; Rodgers, A.M.; McMullan, L.R.; Courtenay, A.J. The role of epigenetic and biological biomarkers in the diagnosis of periodontal disease: A systematic review approach. Diagnostics 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Benincasa, G.; Schiano, C.; Salvatore, M. Differential epigenetic factors in the prediction of cardiovascular risk in diabetic patients. Eur. Heart J.-Cardiovasc. Pharmacother. 2020, 6, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Panagiotakos, D. Real-world data: A brief review of the methods, applications, challenges and opportunities. BMC Med. Res. Methodol. 2022, 22, 287. [Google Scholar] [CrossRef]
- Mukherjee, A.; Abraham, S.; Singh, A.; Balaji, S.; Mukunthan, K. From data to cure: A comprehensive exploration of multi-omics data analysis for targeted therapies. Mol. Biotechnol. 2025, 67, 1269–1289. [Google Scholar] [CrossRef]
- Khorram-Manesh, A.; Dulebenets, M.A.; Goniewicz, K. Implementing public health strategies—The need for educational initiatives: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 5888. [Google Scholar] [CrossRef]
- Zaman, M.S.; Alam, S.G.; Razzaque, M.S. Oral Hygiene and Cardiovascular Health. Hygiene 2025, 5, 14. [Google Scholar] [CrossRef]
- Rewthamrongsris, P.; Burapacheep, J.; Trachoo, V.; Porntaveetus, T. Accuracy of Large Language Models for Infective Endocarditis Prophylaxis in Dental Procedures. Int. Dent. J. 2025, 75, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Oğuzman, R.T. Can we consult large language models for antibiotic prophylaxis? Int. Dent. J. 2024, 74, S19. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).