Photobiomodulation in Patients Taking Denosumab: Case Report and Literature Review
Abstract
1. Introduction
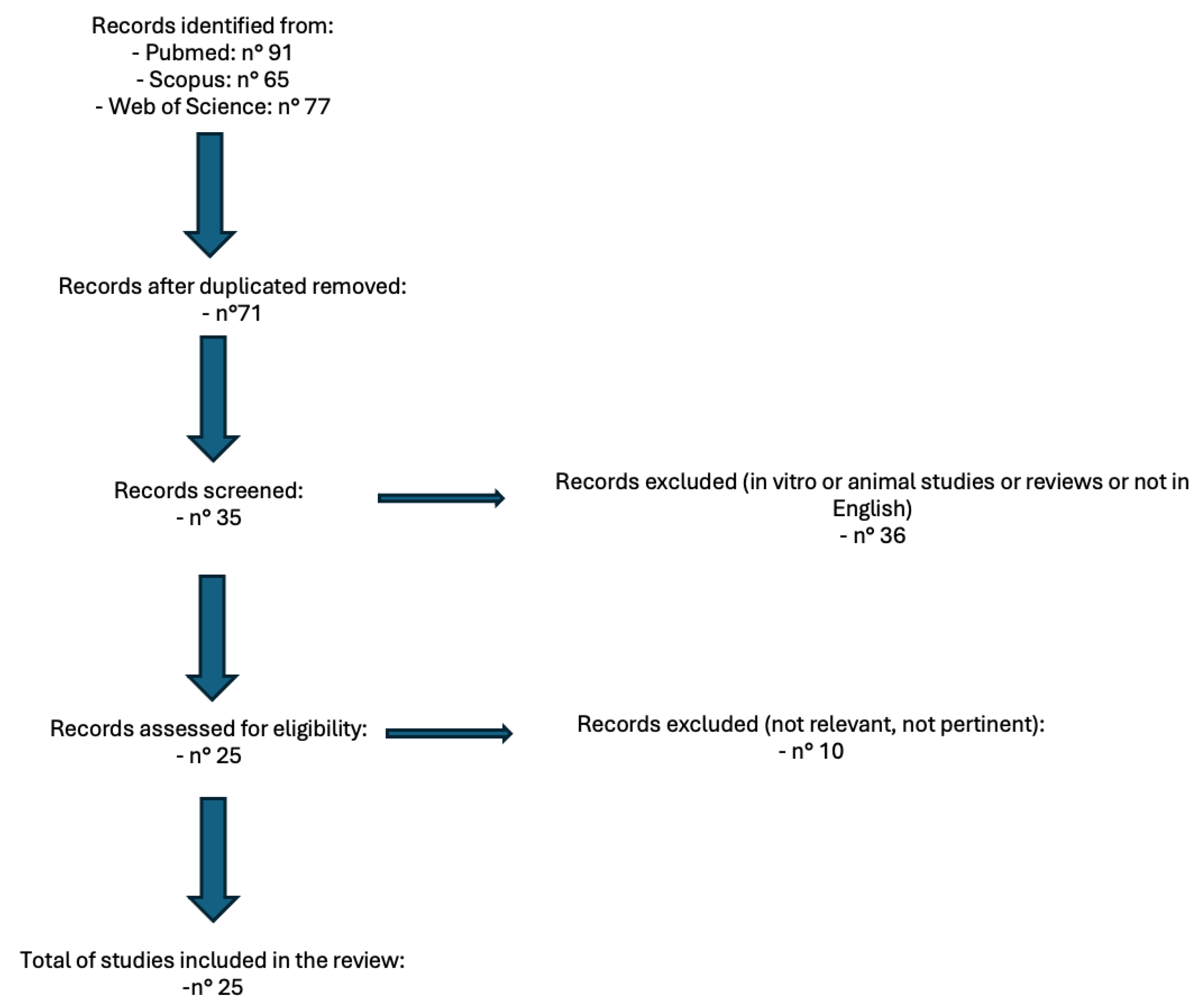
2. Materials and Methods
3. Results
| N° Article | Article | Type of Study | Objectives | Conclusions |
|---|---|---|---|---|
| 1 | Vescovi et al. (2024) [19] | Case series | Evaluate the combined use of piezoelectric surgery, Er:YAG laser, and Nd:YAG laser photobiomodulation for MRONJ treatment. | The combined approach effectively treated MRONJ, improving outcomes and reducing morbidity. |
| 2 | de Freitas et al. (2023) [20] | Case report | Assess phototherapy and Er:YAG laser in managing mandibular osteoradionecrosis. | Phototherapy combined with Er:YAG laser promoted healing and reduced symptoms. |
| 3 | El Mobadder et al. (2023) [21] | Retrospective study | Investigate photobiomodulation with minimal surgical intervention for MRONJ. | Photobiomodulation is effective in enhancing healing with minimal surgery. |
| 4 | Martins et al. (2021) [22] | Case report | Evaluate photobiomodulation and antimicrobial photodynamic therapy for preventing MRONJ. | The preventive approach using phototherapy showed promising results. |
| 5 | Monteiro et al. (2021) [23] | Case report | Analyze LLLT effects on lenvatinib-related osteonecrosis of the jaw. | LLLT was effective in reducing symptoms and aiding recovery. |
| 6 | Almeida et al. (2021) [24] | Case report | Examine photodynamic therapy as an adjunct in MRONJ treatment. | Photodynamic therapy improved treatment outcomes as an adjunct therapy. |
| 7 | Nica et al. (2021) [25] | Case series | Combine photobiomodulation, surgery, and antibiotics for MRONJ management. | The multi-modal approach enhanced recovery and reduced complications. |
| 8 | Torres et al. (2020) [26] | Case report | Study LLLT as an adjuvant in MRONJ treatment. | LLLT effectively reduced pain and supported healing. |
| 9 | Tenore et al. (2020) [27] | Retrospective study | Investigate L-PRF and photobiomodulation in MRONJ management. | The combined therapy enhanced healing and patient outcomes. |
| 10 | Magalhães et al. (2020) [28] | Case report | Evaluate photobiomodulation and antimicrobial photodynamic therapy for osteoradionecrosis prevention. | The therapy demonstrated effectiveness in prevention and symptom control. |
| 11 | Göl et al. (2020) [29] | Comparative study | Compare extracorporeal shock-wave therapy and LLLT for bone healing. | LLLT was more effective in promoting bone healing. |
| 12 | Tartaroti et al. (2020) [30] | Case series | Analyze antimicrobial photodynamic therapy and photobiomodulation for MRONJ prevention. | Combination therapy effectively prevented MRONJ with long-term benefits. |
| 13 | Fornaini et al. (2017) [31] | Case report | Use laser and PRP to treat MRONJ. | Laser and PRP combination improved healing outcomes. |
| 14 | Vescovi et al. (2015) [32] | Case series | Develop a surgical protocol supported by LLLT for high-risk extractions in MRONJ patients. | The protocol reduced complications and promoted healing. |
| 15 | Altay et al. (2014) [33] | Retrospective analysis | Assess LLLT-supported surgical treatment of MRONJ. | LLLT improved surgical outcomes and reduced healing time. |
| 16 | da Guarda et al. (2012) [34] | Case report | Investigate GaAlAs laser photobiomodulation for bisphosphonate-induced osteonecrosis. | Photobiomodulation effectively managed symptoms and improved healing. |
| 17 | Vescovi et al. (2012) [35] | Retrospective analysis | Early surgical laser-assisted management of MRONJ. | Early intervention with laser therapy yielded positive long-term results. |
| 18 | Martins et al. (2012) [36] | Preliminary study | Examine laser phototherapy and PRP for MRONJ in cancer patients. | Combined therapy enhanced healing and reduced symptoms. |
| 19 | Atalay et al. (2011) [37] | Comparative study | Compare laser-assisted and conventional surgery for MRONJ. | Laser-assisted surgery showed superior outcomes. |
| 20 | Luomanen et al. (2012) [38] | Case report | Study Nd:YAG laser biostimulation for MRONJ treatment. | Laser biostimulation reduced symptoms and promoted healing. |
| 21 | Romeo et al. (2011) [39] | Preliminary study | Evaluate pain control in MRONJ with LLLT. | LLLT effectively reduced pain and improved quality of life. |
| 22 | Manfredi et al. (2011) [40] | Case series | Assess MRONJ management in osteoporosis patients. | Multi-modal approach showed effectiveness in managing MRONJ. |
| 23 | Kan et al. (2011) [41] | Case report | Study LLLT for tooth extractions in patients on zoledronate. | LLLT reduced complications and enhanced healing. |
| 24 | Scoletta et al. (2010) [42] | Prospective study | Investigate LLLT effects on bisphosphonate-induced MRONJ. | LLLT improved healing outcomes in preliminary results. |
| 25 | Vescovi et al. (2010) [43] | Case series | Evaluate surgical approaches with Er:YAG laser for MRONJ. | Laser-assisted surgery was effective in managing MRONJ. |
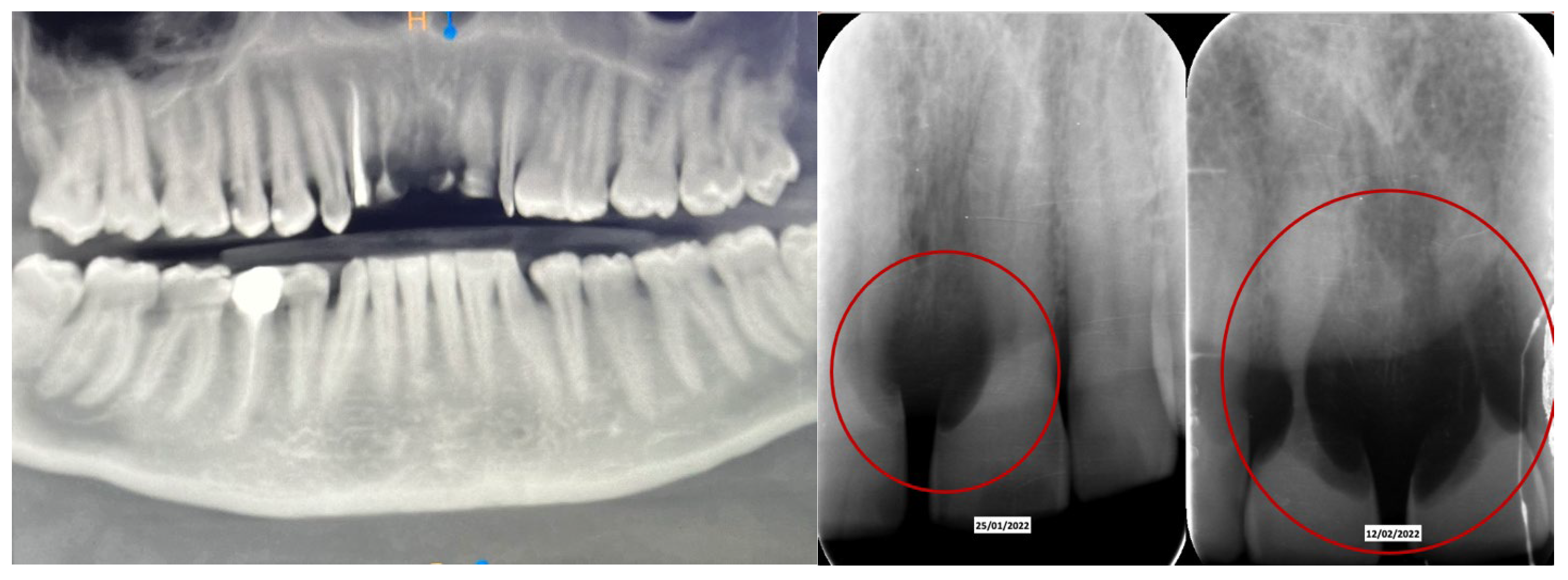
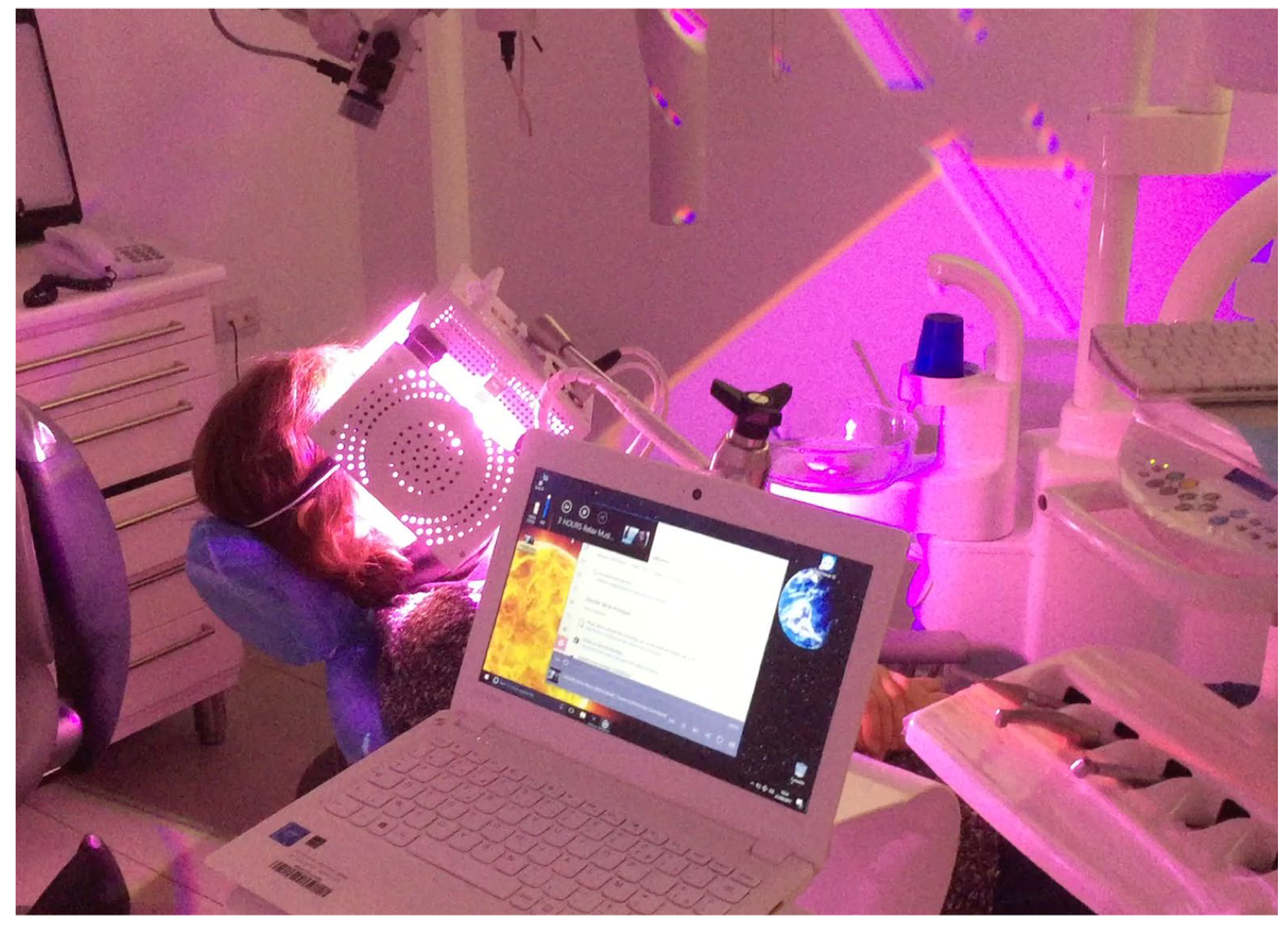
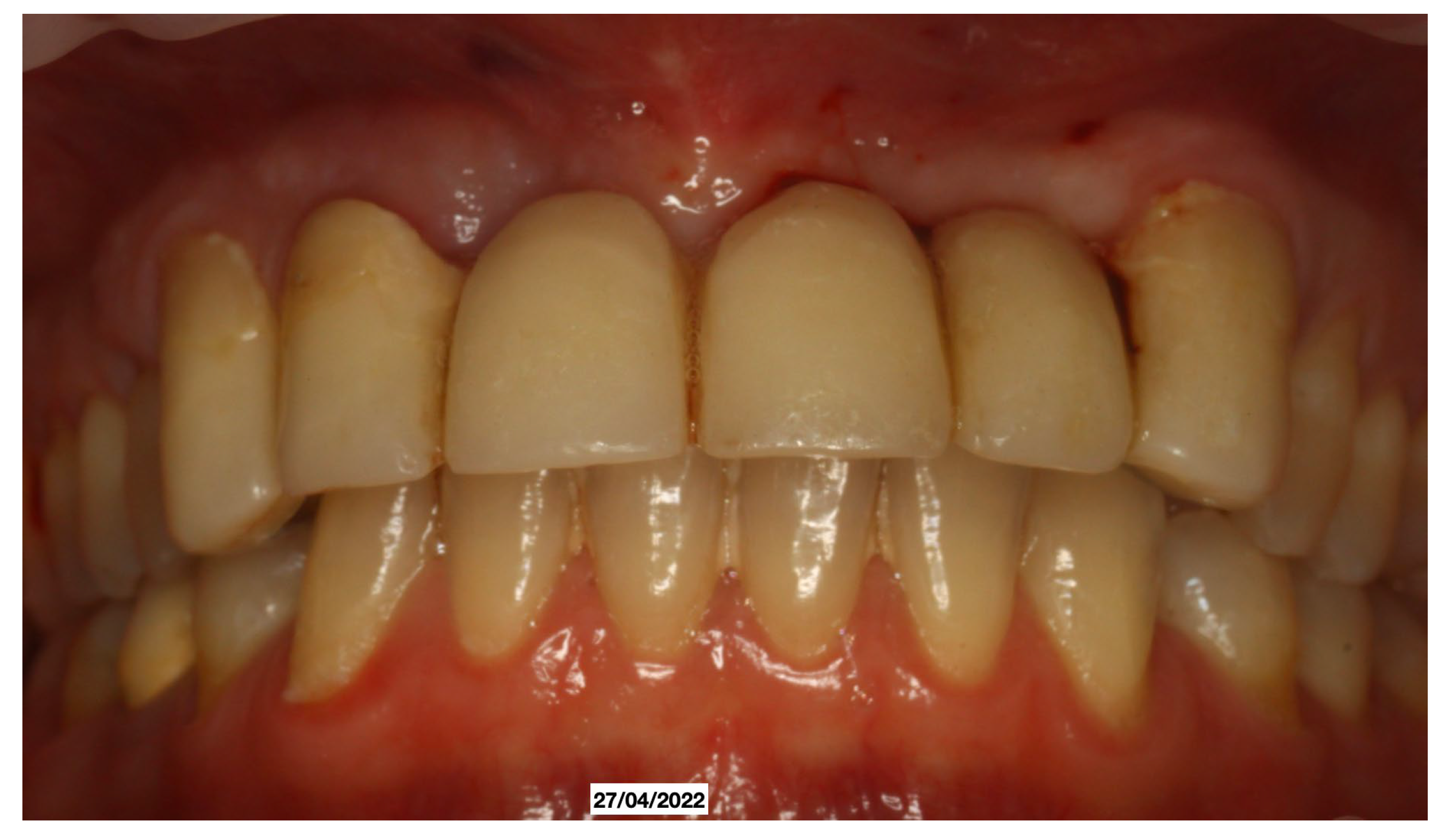
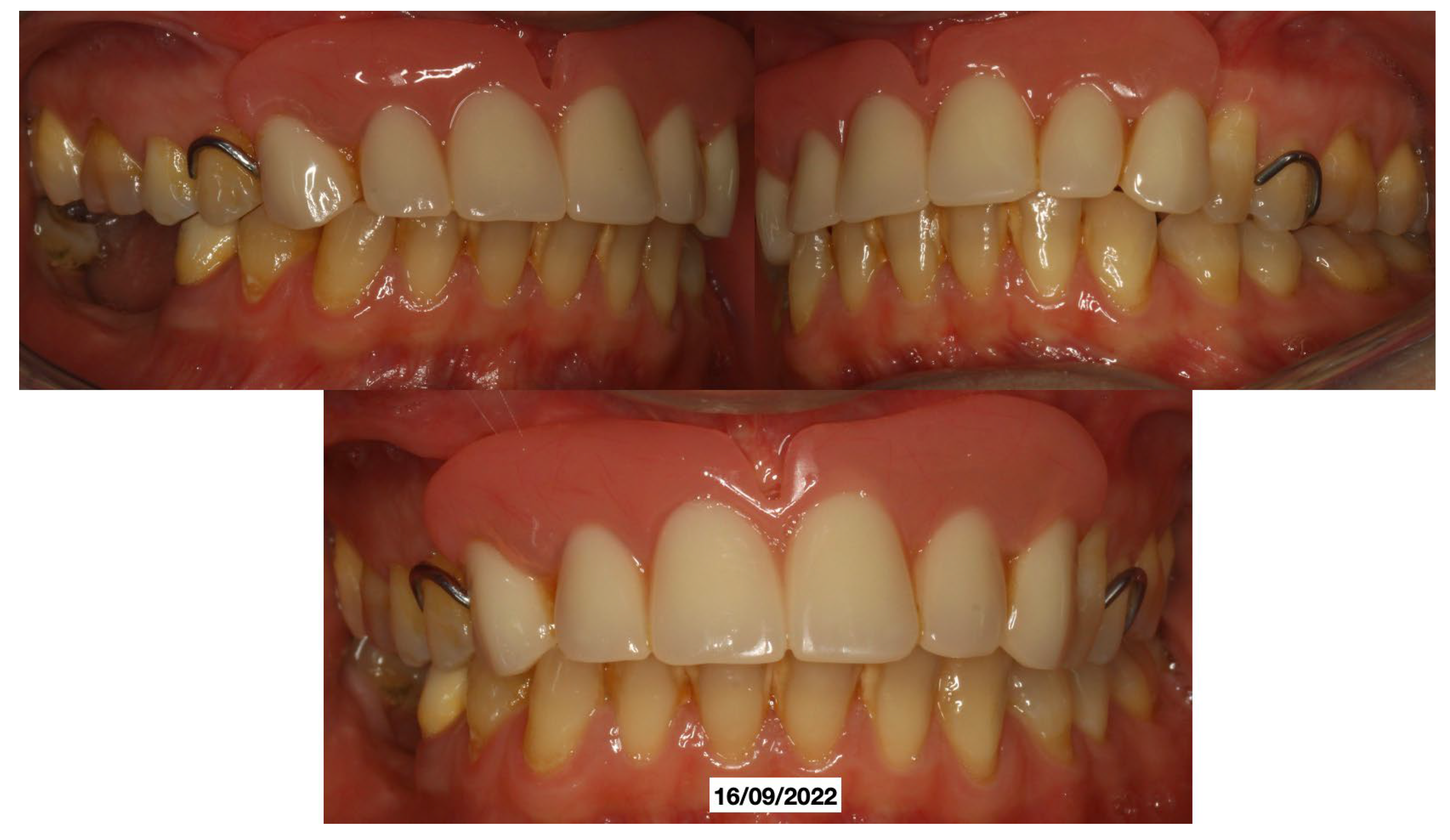
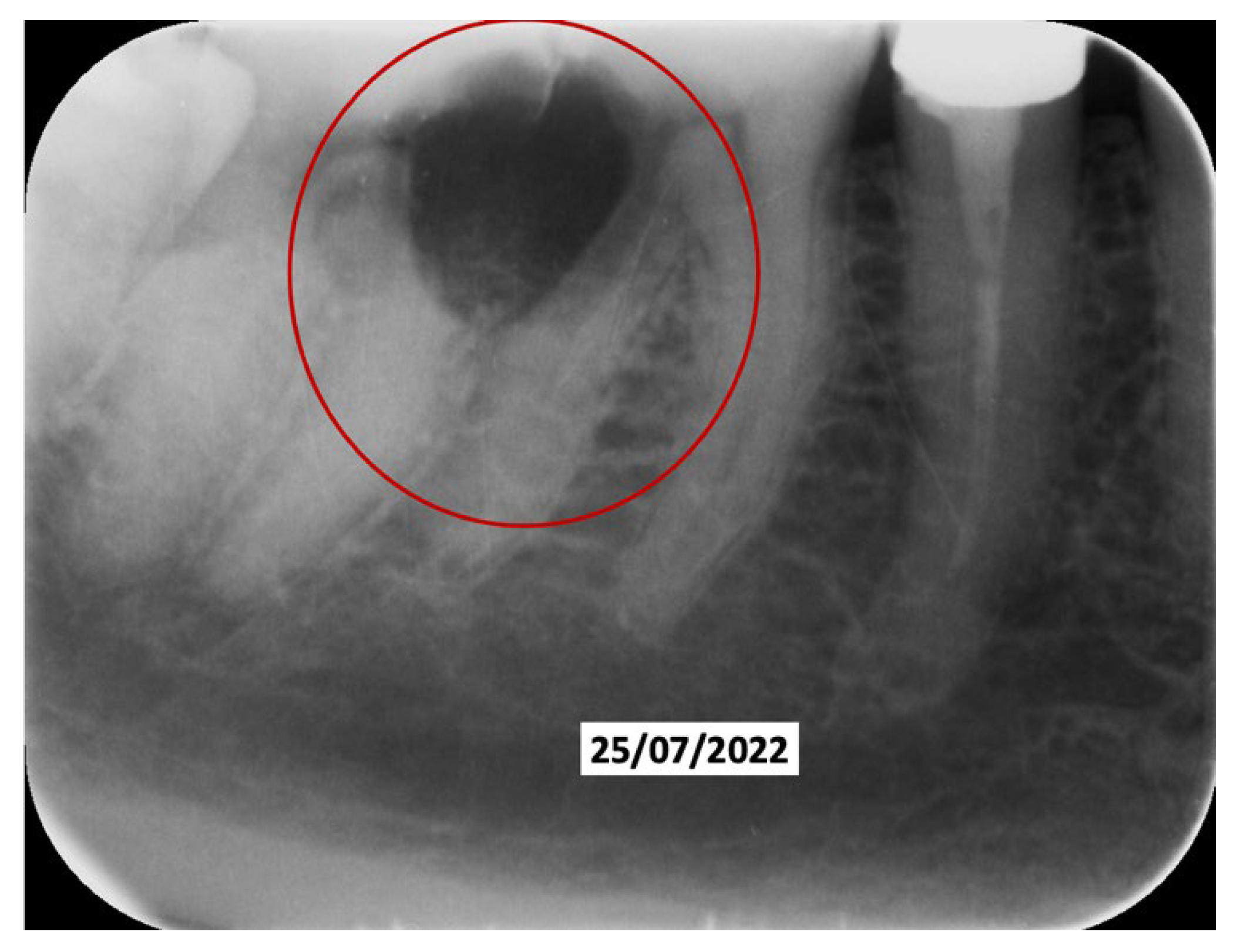
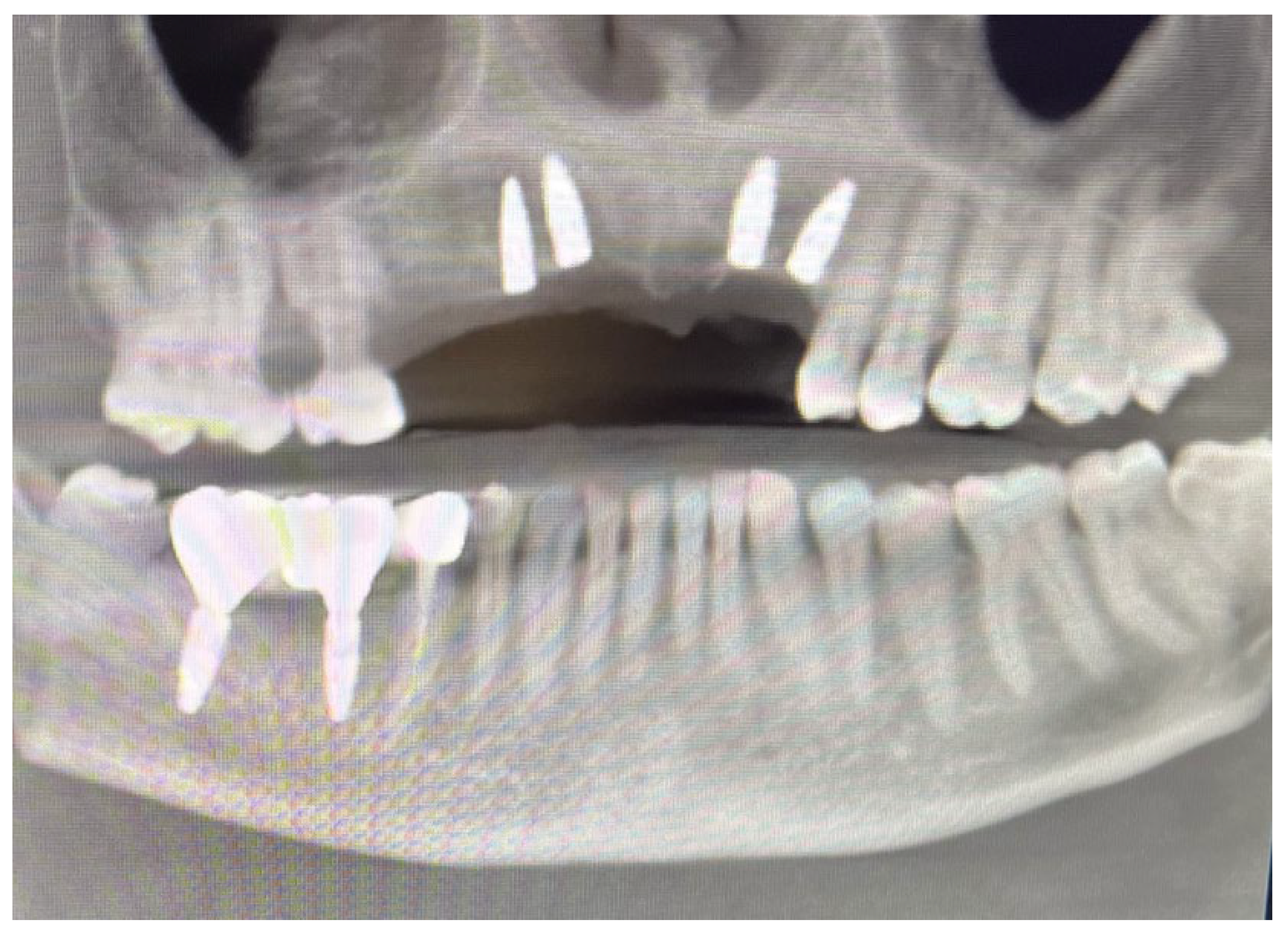
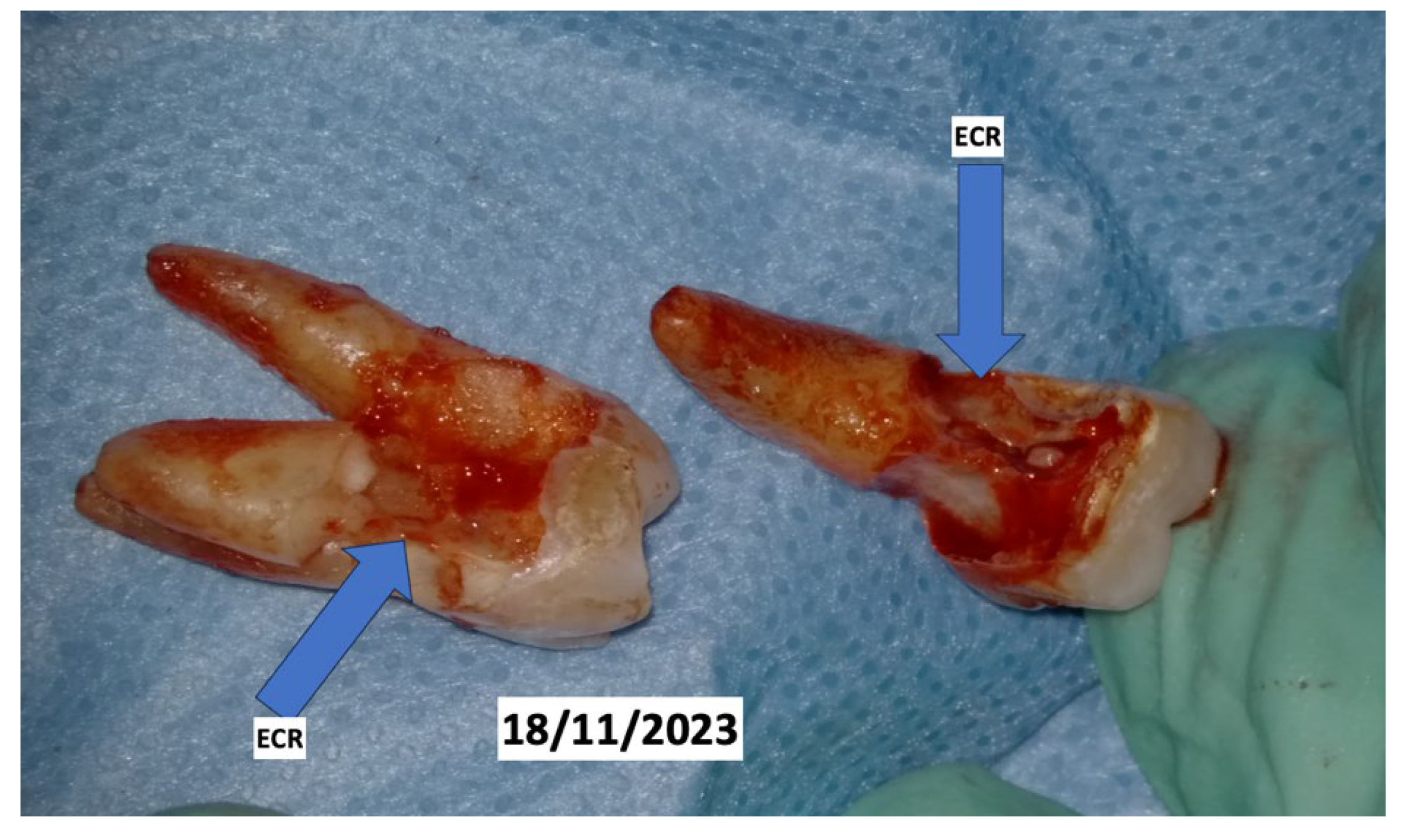
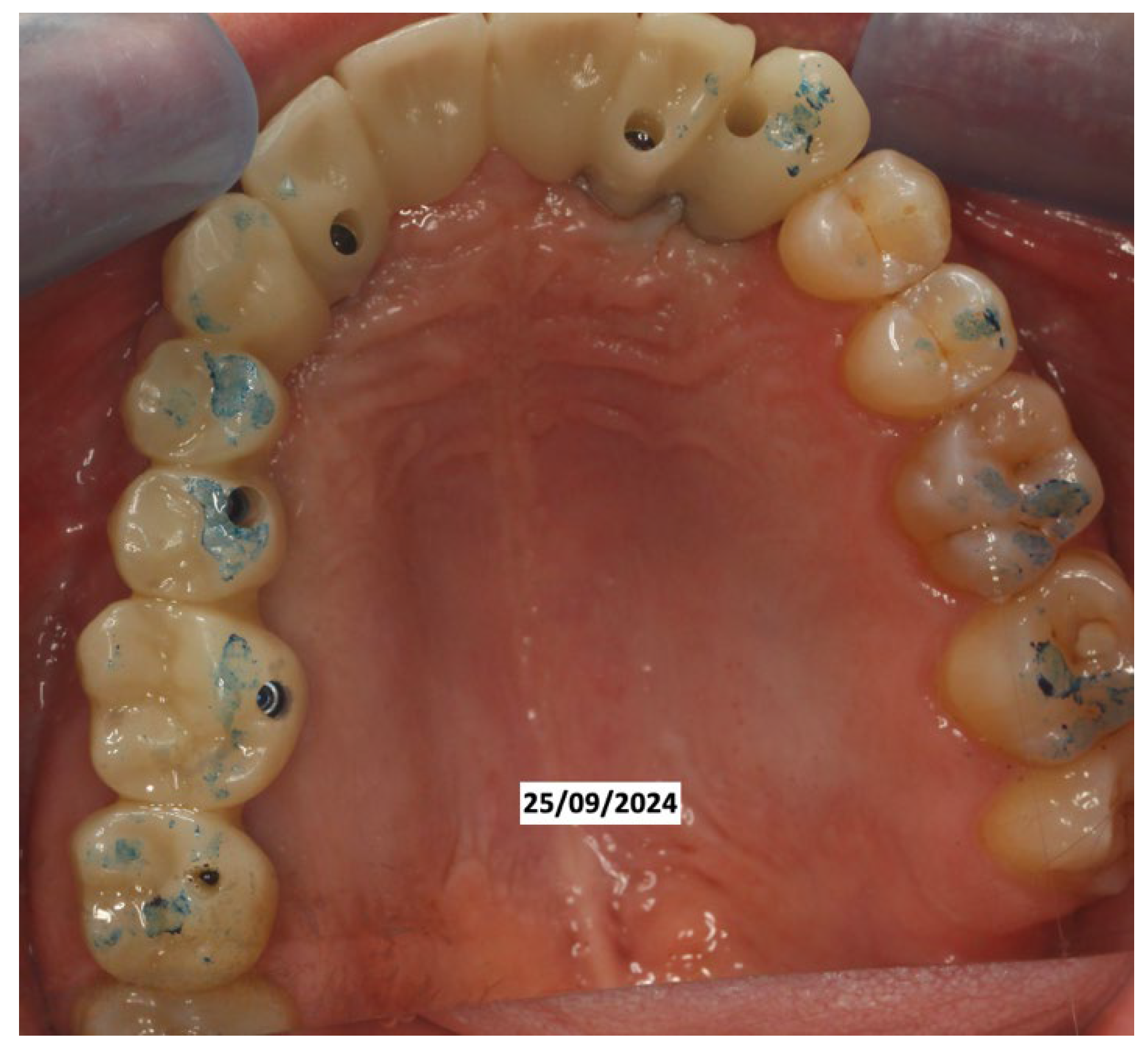
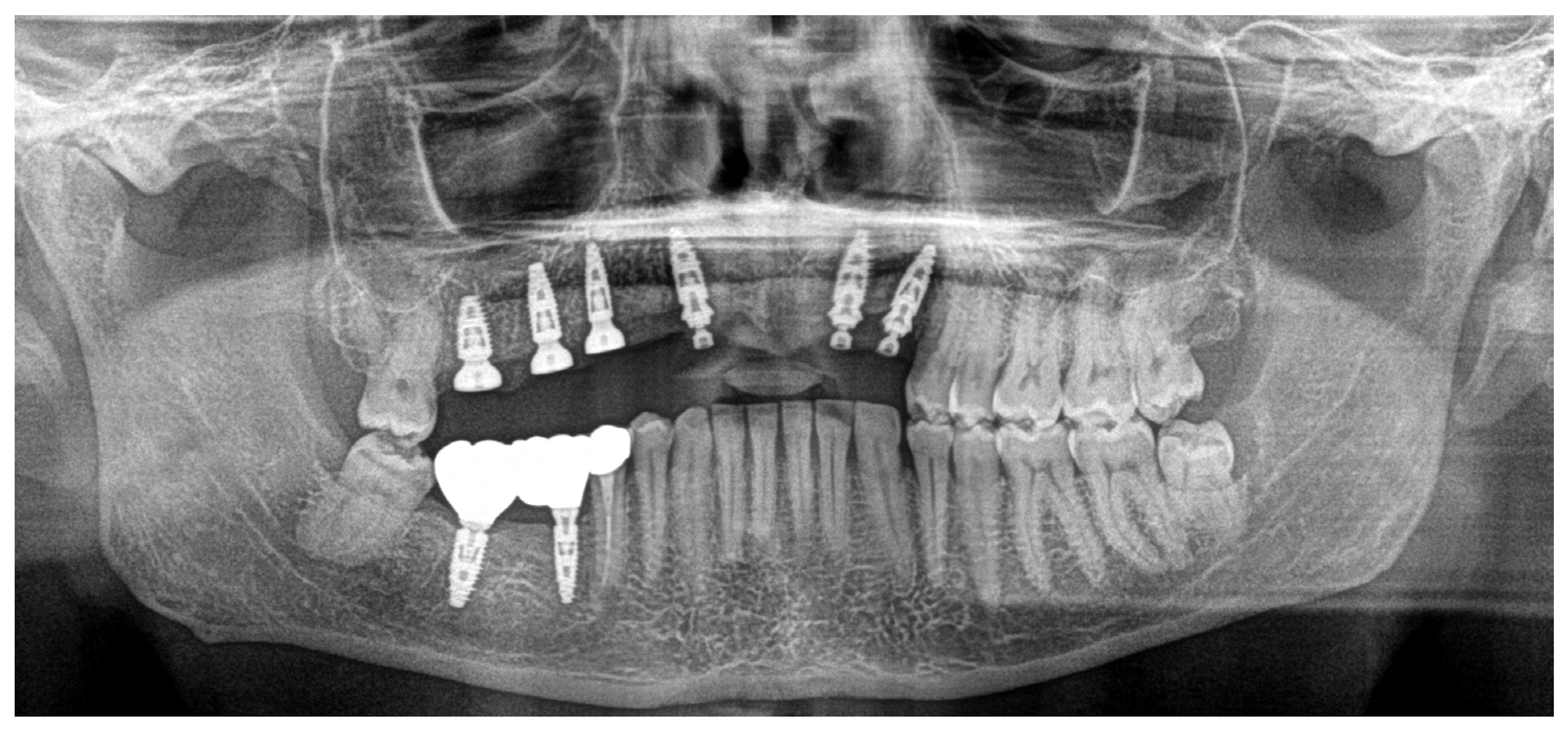
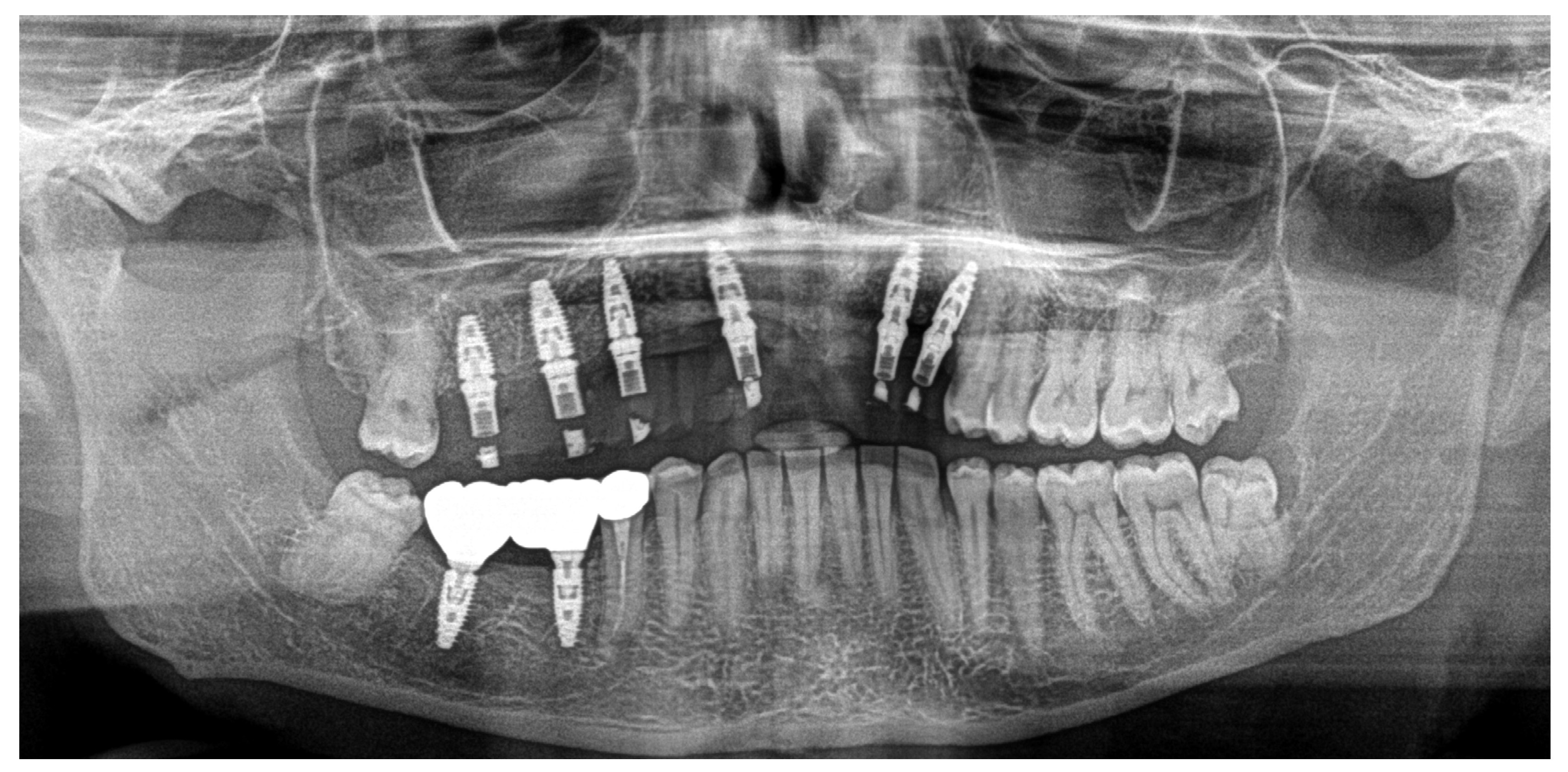
Clinical Case
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aljohani, S.; Gaudin, R.; Weiser, J.; Tröltzsch, M.; Ehrenfeld, M.; Kaeppler, G.; Smeets, R.; Otto, S. Osteonecrosis of the jaw in patients treated with denosumab. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 124, 95–101. [Google Scholar]
- Diab, D.L.; Watts, N.B. The use of denosumab in osteoporosis—An update on efficacy and drug safety. Expert Opin. Drug Saf. 2024, 23, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Rathod, A.; Jaiswal, P.; Bajaj, P.; Kale, B.; Masurkar, D. Implementation of Low-Level Laser Therapy in Dentistry: A Review. Cureus 2022, 14, e28799. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Caccianiga, G.; Perillo, L.; Portelli, M.; Baldoni, M.; Galletti, C.; Gay-Escoda, C. Evaluation of effectiveness of photo-biostimulation in alleviating side effects after dental implant surgery. A randomized clinical trial. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e277–e282. [Google Scholar]
- Caccianiga, G.; Rey, G.; Baldoni, M.; Caccianiga, P.; Baldoni, A.; Ceraulo, S. Periodontal Decontamination Induced by Light and Not by Heat: Comparison between Oxygen High Level Laser Therapy (OHLLT) and LANAP. Appl. Sci. 2021, 11, 4629. [Google Scholar] [CrossRef]
- Liebert, A.; Capon, W.; Pang, V.; Vila, D.; Bicknell, B.; McLachlan, C.; Kiat, H. Photophysical Mechanisms of Photobiomodulation Therapy as Precision Medicine. Biomedicines 2023, 11, 237. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef]
- Maghfour, J.; Ozog, D.M.; Mineroff, J.; Jagdeo, J.; Kohli, I.; Lim, H.W. Photobiomodulation CME part I: Overview and mechanism of action. J. Am. Acad. Dermatol. 2024, 91, 793–802. [Google Scholar] [CrossRef]
- Caccianiga, G.; Rey, G.; Caccianiga, P.; Leonida, A.; Baldoni, M.; Baldoni, A.; Ceraulo, S. Rough Dental Implant Surfaces and Peri-Implantitis: Role of Phase- Contrast Microscopy, Laser Protocols, and Modified Home Oral Hygiene in Maintenance. A 10-Year Retrospective Study. Appl. Sci. 2021, 11, 4985. [Google Scholar] [CrossRef]
- Rani, S.; Dhawan, P.; Kruthiventi, H. Clinical efficacy of photobiomodulation therapy in dental implant stability and crestal bone loss in implants placed in healed sites: A systematic review and meta-analysis of randomized clinical trials. Lasers Med. Sci. 2025, 40, 40. [Google Scholar] [CrossRef]
- AlSayed Hasan, M.M.A.; Sultan, K.; Ajaj, M.; Voborná, I.; Hamadah, O. Low-level laser therapy effectiveness in reducing initial orthodontic archwire placement pain in premolars extraction cases: A single-blind, placebo-controlled, randomized clinical trial. BMC Oral Health 2020, 20, 209. [Google Scholar] [CrossRef] [PubMed]
- Caccianiga, G.; Lo Giudice, A.; Paiusco, A.; Portelli, M.; Militi, A.; Baldoni, M.; Nucera, R. Maxillary Orthodontic Expansion Assisted by Unilateral Alveolar Corticotomy and Low-Level Laser Therapy: A Novel Approach for Correction of a Posterior Unilateral Cross-Bite in Adults. J. Lasers Med. Sci. 2019, 10, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.V.S.; Pinzan, A.; Consolaro, A.; Henriques, J.F.C.; de Freitas, M.R. Systematic Literature Review: Influence of Low-Level Laser on Orthodontic Movement and Pain Control in Humans. Photomed. Laser Surg. 2014, 32, 592–599. [Google Scholar] [CrossRef]
- Soliman, J.; Elsanadi, R.; Messele, F.; Kelly, K.M. The effect of combined red, blue, and near-infrared light-emitting diode (LED) photobiomodulation therapy on speed of wound healing after superficial ablative fractional resurfacing. Lasers Med. Sci. 2024, 39, 94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.Y.; Chen, A.C.; Carroll, J.D.; Hamblin, M.R. Low-level laser therapy (LLLT) for treatment of osteoarthritis. Photomed. Laser Surg. 2022, 40, 14–21. [Google Scholar]
- Rando, R.G.; Buchaim, D.V.; Cola, P.C.; Buchaim, R.L. Effects of Photobiomodulation Using Low-Level Laser Therapy on Alveolar Bone Repair. Photonics 2023, 10, 734. [Google Scholar] [CrossRef]
- Maggioni, M.; Attanasio, T.; Scarpelli, F. I Laser in Odontoiatria; Piccin: Padova, Italy, 2009. [Google Scholar]
- Lu, R.Q.; Zhang, Y.Y.; Zhao, H.Q.; Guo, R.Q.; Jiang, Z.X.; Guo, R. SGK1, a critical regulator of immune modulation and fibrosis and a potential therapeutic target in chronic graft-versus-host disease. Front. Immunol. 2022, 13, 822303. [Google Scholar] [CrossRef]
- Vescovi, P.; De Francesco, P.; Giovannacci, I.; Leão, J.C.; Barone, A. Piezoelectric Surgery, Er: YAG Laser Surgery and Nd: YAG Laser Photobiomodulation: A Combined Approach to Treat Medication-Related Osteonecrosis of the Jaws (MRONJ). Dent. J. 2024, 12, 261. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Freitas, L.C.; Kawamoto, E.L.; Souza, A.M.A.; Kawakami, P.Y.; Gonçalves, A.S.; Azevedo, L.H. Use of Phototherapy and Er-YAG Laser in the Management of Mandible Osteoradionecrosis: A Case Report. J. Lasers Med. Sci. 2023, 14, e58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El Mobadder, M.; Grzech-Lesniak, Z.; El Mobadder, W.; Rifai, M.; Ghandour, M.; Nammour, S. Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention. Dent. J. 2023, 11, 127. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martins, F.; Macedo, D.; Palma, L.F.; Ortega, K.L.; Campos, L. Photobiomodulation and antimicrobial photodynamic therapy for the prevention of osteonecrosis of the jaw in an oncologic patient. Photodiagnosis Photodyn. Ther. 2021, 36, 102587. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Vasconcelos, C.; Pacheco, J.J.; Salazar, F. ; Vasconcelos, C.; Pacheco, J.J.; Salazar, F. Photobiomodulation laser therapy in a Lenvatinib-related osteonecrosis of the jaw: A case report. J. Clin. Exp. Dent. 2021, 13, e626–e629. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Almeida, M.V.D.C.; Moura, A.C.; Santos, L.; Gominho, L.; Cavalcanti, U.D.N.T.; Romeiro, K. Photodynamic Therapy as an adjunct in the Treatment of Medication-Related Osteonecrosis of the Jaw: A Case Report. J. Lasers Med. Sci. 2021, 12, e12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nica, D.F.; Riviș, M.; Roi, C.I.; Todea, C.D.; Duma, V.F.; Sinescu, C. Complementarity of Photo-Biomodulation, Surgical Treatment, and Antibiotherapy for Medication-Related Osteonecrosis of the Jaws (MRONJ). Medicina 2021, 57, 145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Torres, A.A.; de Freitas, B.L.; Carneiro, P.P.; de Sousa, A.L.A.; Arêa Leão Ferraz, M.Â.; de Pinho Mendes, J.; Costa, A.L.F.; Pinto, A.S.B. Medication-Related Osteonecrosis of the Jaw and Low-Level Laser Therapy as Adjuvant Treatment: A Case Report. J. Lasers Med. Sci. 2020, 11, 497–499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using. Leukocyte- and Platelet-Rich. Fibrin (L-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Magalhães, I.A.; Forte, C.P.F.; Viana, T.S.A.; Teófilo, C.R.; Lima Verde, R.M.B.; Magalhães, D.P.; Praxedes Neto, R.A.L.; Lima, R.A.; Dantas, T.S. Photobiomodulation and antimicrobial photodynamic therapy as adjunct in the treatment and prevention of osteoradionecrosis of the jaws: A case report. Photodiagnosis Photodyn. Ther. 2020, 31, 101959. [Google Scholar] [CrossRef] [PubMed]
- Göl, E.B.; Özkan, N.; Bereket, C.; Önger, M.E. Extracorporeal Shock-Wave Therapy or Low-Level Laser Therapy: Which is More Effective in Bone Healing in Bisphosphonate Treatment? J. Craniofac Surg. 2020, 31, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Tartaroti, N.C.; Marques, M.M.; Naclério-Homem, M.D.G.; Migliorati, C.A.; Zindel Deboni, M.C. Antimicrobial photodynamic and photobiomodulation adjuvant therapies for prevention and treatment of medication-related osteonecrosis of the jaws: Case series and long-term follow-up. Photodiagnosis Photodyn. Ther. 2020, 29, 101651. [Google Scholar] [CrossRef] [PubMed]
- Fornaini, C.; Cella, L.; Oppici, A.; Parlatore, A.; Clini, F.; Fontana, M.; Lagori, G.; Merigo, E. Laser and Platelet-Rich. Plasma to treat Medication-Related Osteonecrosis of the Jaws (MRONJ): A case report. Laser Ther. 2017, 26, 223–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vescovi, P.; Giovannacci, I.; Merigo, E.; Meleti, M.; Manfredi, M.; Fornaini, C.; Nammour, S. Tooth extractions in high-risk patients under bisphosphonate therapy and previously affected with osteonecrosis of the jaws: Surgical protocol supported by low-level laser therapy. J. Craniofac Surg. 2015, 26, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Altay, M.A.; Tasar, F.; Tosun, E.; Kan, B. Low-level laser therapy supported surgical treatment of bisphosphonate related osteonecrosis of jaws: A retrospective analysis of 11 cases. Photomed. Laser Surg. 2014, 32, 468–475. [Google Scholar] [CrossRef] [PubMed]
- da Guarda, M.G.; Paraguassú, G.M.; Cerqueira, N.S.; Cury, P.R.; Farias, J.G.; Ramalho, L.M. Laser GaAlAs (λ860 nm) photobiomodulation for the treatment of bisphosphonate-induced osteonecrosis of the jaw. Photomed. Laser Surg. 2012, 30, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; Manfredi, M.; Merigo, E.; Guidotti, R.; Meleti, M.; Pedrazzi, G.; Fornaini, C.; Bonanini, M.; Ferri, T.; Nammour, S. Early surgical laser-assisted management of bisphosphonate-related osteonecrosis of the jaws (BRONJ): A retrospective analysis of 101 treated sites with long-term follow-up. Photomed. Laser Surg. 2012, 30, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.; Martins, M.D.; Lascala, C.A.; Curi, M.M.; Migliorati, C.A.; Tenis, C.A.; Marques, M.M. Association of laser phototherapy with PRP improves healing of bisphosphonate-related osteonecrosis of the jaws in cancer patients: A preliminary study. Oral Oncol. 2012, 48, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Atalay, B.; Yalcin, S.; Emes, Y.; Aktas, I.; Aybar, B.; Issever, H.; Mandel, N.M.; Cetin, O.; Oncu, B. Bisphosphonate-related osteonecrosis: Laser-assisted surgical treatment or conventional surgery? Lasers Med. Sci. 2011, 26, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Luomanen, M.; Alaluusua, S. Treatment of bisphosphonate-induced osteonecrosis of the jaws with Nd: YAG laser biostimulation. Lasers Med. Sci. 2012, 27, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Romeo, U.; Galanakis, A.; Marias, C.; Vecchio, A.D.; Tenore, G.; Palaia, G.; Vescovi, P.; Polimeni, A. Observation of pain control in patients with bisphosphonate-induced osteonecrosis using low level laser therapy: Preliminary results. Photomed. Laser Surg. 2011, 29, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Merigo, E.; Guidotti, R.; Meleti, M.; Vescovi, P. Bisphosphonate-related osteonecrosis of the jaws: A case series of 25 patients affected by osteoporosis. Int. J. Oral. Maxillofac. Surg. 2011, 40, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Kan, B.; Altay, M.A.; Taşar, F.; Akova, M. Low-level laser therapy supported teeth extractions of two patients receiving IV zolendronate. Lasers Med. Sci. 2011, 26, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Scoletta, M.; Arduino, P.G.; Reggio, L.; Dalmasso, P.; Mozzati, M. Effect of low-level laser irradiation on bisphosphonate-induced osteonecrosis of the jaws: Preliminary results of a prospective study. Photomed. Laser Surg. 2010, 28, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Vescovi, P.; Manfredi, M.; Merigo, E.; Meleti, M.; Fornaini, C.; Rocca, J.P.; Nammour, S. Surgical approach with Er: YAG laser on osteonecrosis of the jaws (ONJ) in patients under bisphosphonate therapy (BPT). Lasers Med. Sci. 2010, 25, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, C.F.; Mauriz, J.L.; Freitas Corrêa, D.S.; Moreira, A.J.; Zettler, C.G.; Filippin, L.I.; Marroni, N.P.; González-Gallego, J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg. Med. 2006, 38, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.C.; Arany, P.R.; Huang, Y.Y.; Tomkinson, E.M.; Sharma, S.K.; Kharkwal, G.B.; Saleem, T.; Mooney, D.; Yull, F.E.; Blackwell, T.S.; et al. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS ONE 2011, 6, e22453. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rola, P.; Włodarczak, S.; Lesiak, M.; Doroszko, A.; Włodarczak, A. Changes in Cell Biology under the Influence of Low-Level Laser Therapy. Photonics 2022, 9, 502. [Google Scholar] [CrossRef]
- Mikušková, K.; Vaňuga, P.; Adamicová, K.; Statelová, D.; Janíčková, M.; Malachovský, I.; Siebert, T. Multiple idiopathic external cervical root resorption in patient treated continuously with denosumab: A case report. BMC Oral Health 2022, 22, 129. [Google Scholar] [CrossRef]
| Research Gap | Future Research Ideas |
|---|---|
| Poor statistical analysis to better demonstrate the benefits between photobiomodulation and denosumab | Experimental studies with the aim of establishing the objective parameters to evaluate the benefits of photobiomodulation on patients assuming denosumab |
| Data not sufficient to relate photobiomodulation effects on denosumab action | Experimental studies with the aim of establishing the impact of this therapy on the drug |
| Data not sufficient to relate denosumab action and involvement of odontoclasts, as shown in the clinical case | Experimental studies to better define this correlation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccianiga, G.; Barbarisi, A.; Caccianiga, P.; Lauritano, D.; Ceraulo, S. Photobiomodulation in Patients Taking Denosumab: Case Report and Literature Review. Dent. J. 2025, 13, 128. https://doi.org/10.3390/dj13030128
Caccianiga G, Barbarisi A, Caccianiga P, Lauritano D, Ceraulo S. Photobiomodulation in Patients Taking Denosumab: Case Report and Literature Review. Dentistry Journal. 2025; 13(3):128. https://doi.org/10.3390/dj13030128
Chicago/Turabian StyleCaccianiga, Gianluigi, Antonio Barbarisi, Paolo Caccianiga, Dorina Lauritano, and Saverio Ceraulo. 2025. "Photobiomodulation in Patients Taking Denosumab: Case Report and Literature Review" Dentistry Journal 13, no. 3: 128. https://doi.org/10.3390/dj13030128
APA StyleCaccianiga, G., Barbarisi, A., Caccianiga, P., Lauritano, D., & Ceraulo, S. (2025). Photobiomodulation in Patients Taking Denosumab: Case Report and Literature Review. Dentistry Journal, 13(3), 128. https://doi.org/10.3390/dj13030128









