Identifying Key Factors in Papilla Growth Around Implants: Focus on Intraoral Negative Pressure
Abstract
1. Introduction
2. Factors Evaluation and Classification
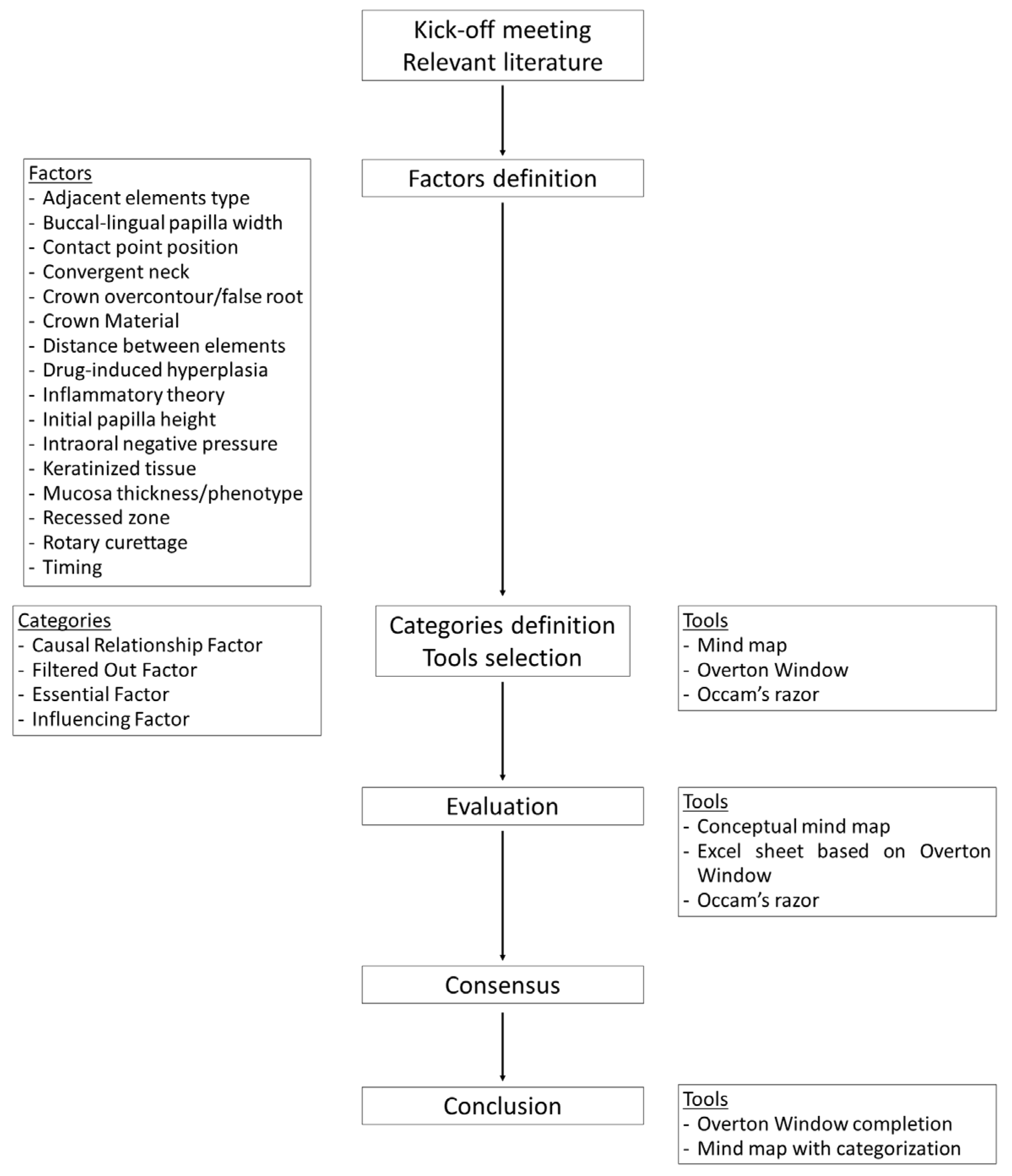
2.1. Study Design and Assessments
2.2. Tools Used for Evaluation
2.3. Methods Applied for Evaluation
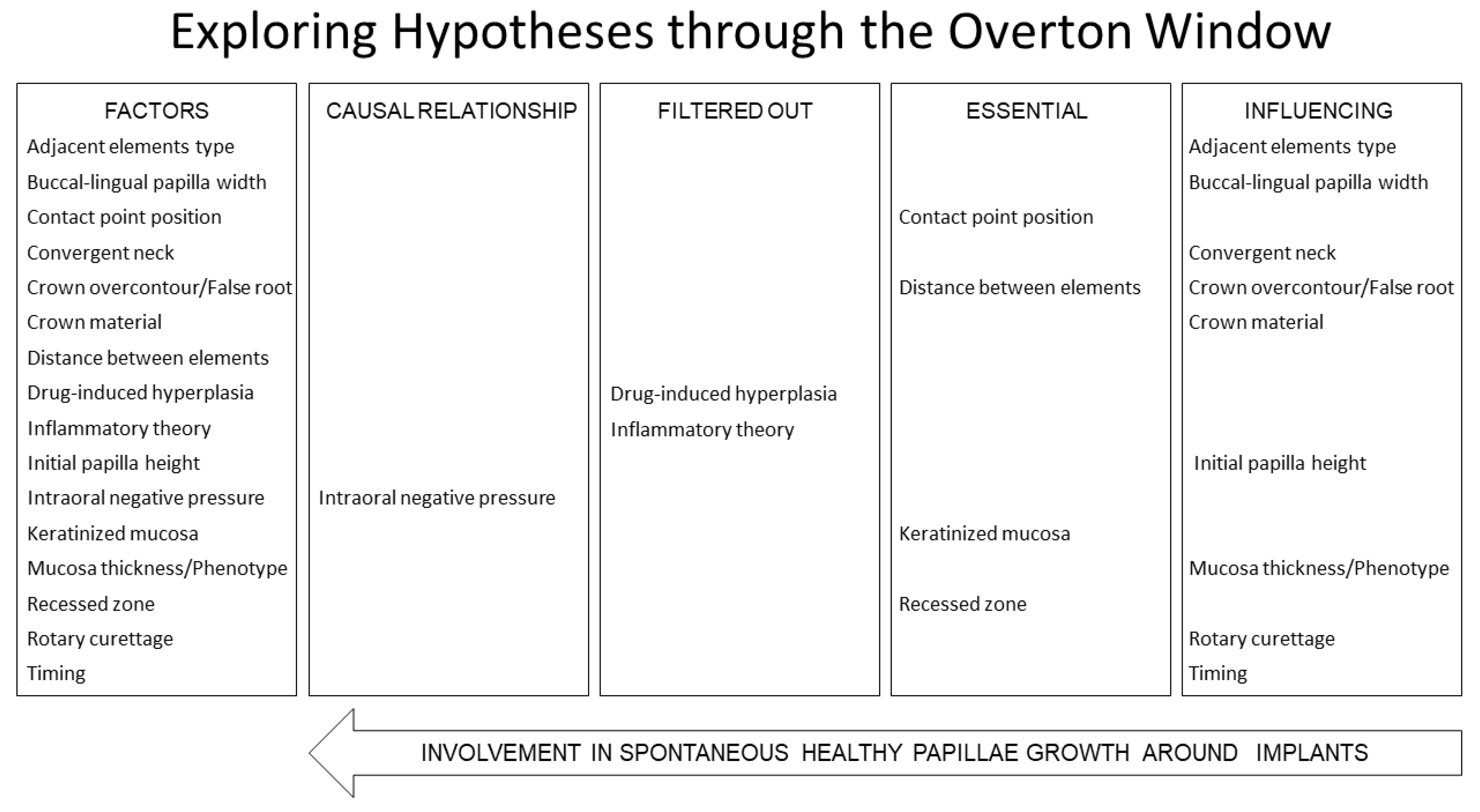
2.4. Categories Applied
- -
- These categories were applied to each factor for consistency with the reported scientific evidence, together with a rational analysis:
- -
- Causal Relationship Factor—Identified when a potential cause-and-effect relationship with papilla growth is observed.
- -
- Filtered Out Factor—Factors that exhibit a cause-and-effect relationship with papilla growth but whose effects are attributable to inflammation or drug influences and, therefore, should not be associated with healthy tissue. This classification may also be applied to factors that are neither essential nor influential.
- -
- Essential Factor—Determined as critical for papilla growth. Papilla growth cannot occur in the absence of this factor, despite no direct cause-and-effect relationship being observed.
- -
- Influencing Factor—Recognized as having an impact on papilla growth, although not essential for it. No direct cause-and-effect relationship is observed with papilla growth.
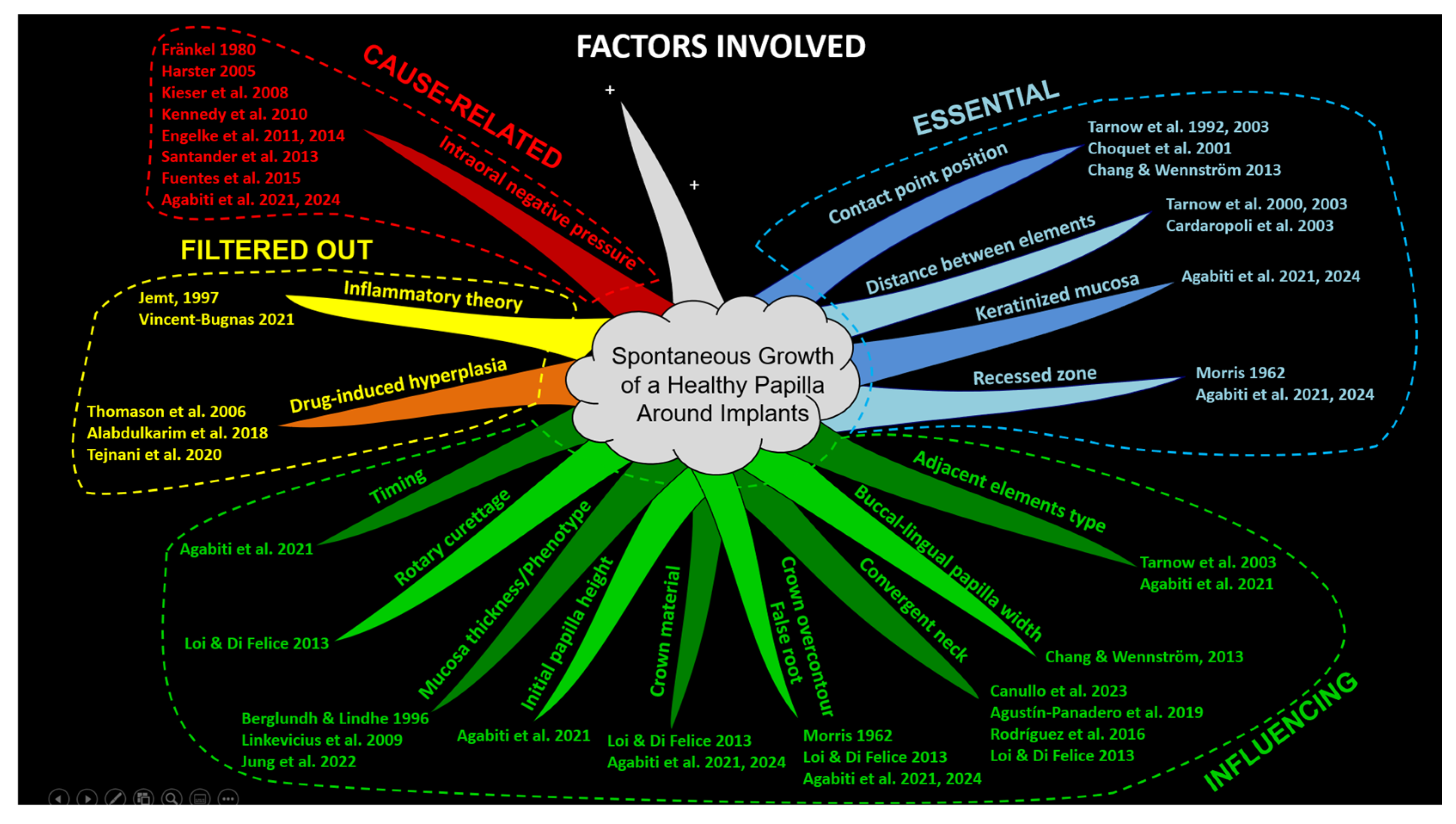
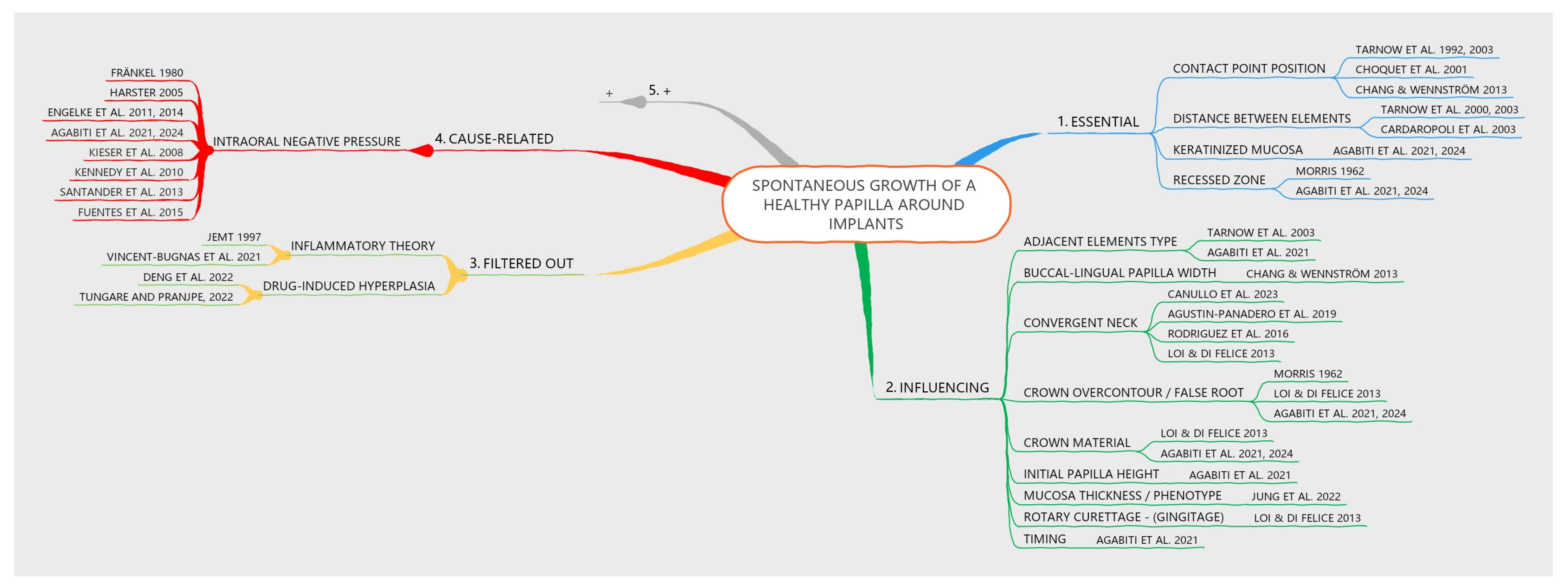
2.5. Factors Evaluated
- -
- -
- Buccal-lingual papilla width: No cause-related relationship was found. The buccal-lingual width was considered to influence papilla growth [10].
- -
- -
- -
- -
- -
- -
- -
- Inflammatory theory: The inflammatory theory presented a causal relationship with papilla growth. However, an inflammatory condition that causes edema and inflammatory infiltrate, leading to tissue growth, cannot be considered healthy [3]. The biofilm theory was invoked primarily in the context of orthodontic treatment, given the noticeable papilla growth despite the absence of significant plaque accumulation [46].
- -
- Initial papilla height: A post hoc analysis of data from a previously published study growth [1] revealed that a lower initial height of the papilla was associated with a greater increase in papilla score (see Table S1). No cause-related relationship was found. This factor was considered to influence papilla growth.
- -
- -
- -
- -
- -
- -
- Timing: No cause-related relationship was found. The timing was considered to influence papilla growth [1].
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agabiti, I.; Alccayhuaman, K.A.A.; Nakajima, Y.; Botticelli, D. An enigmatic soft tissue creeping phenomenon: The spontaneous peri-implant mucosa margin and papilla growth. A retrospective clinical study. Clin. Exp. Dent. Res. 2021, 7, 474–483. [Google Scholar] [CrossRef]
- Agabiti, I.; Alccayhuaman, K.A.A.; Taniguchi, Z.; Kuwano, K.; Botticelli, D. An Enigmatic Soft-Tissue Creeping Phenomenon: The Spontaneous Peri-Implant Mucosa Margin and Papilla Growth, Part Two—A Scientifically Supported Hypothesis Article. Dent. J. 2024, 12, 216. [Google Scholar] [CrossRef]
- Jemt, T. Regeneration of gingival papillae after single-implant treatment. Int. J. Periodontics Restor. Dent. 1997, 17, 326–333. [Google Scholar]
- Harster, P. Tissue modeling: The oral pump. Quintessence Int. 2005, 36, 633–640. [Google Scholar]
- Berglundh, T.; Lindhe, J. Dimension of the periimplant mucosa: Biological width revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral. Maxillofac. Implants. 2009, 24, 712–719. [Google Scholar]
- Jung, R.E.; Becker, K.; Bienz, S.P.; Dahlin, C.; Donos, N.; Hammächer, C.; Iglhaut, G.; Liñares, A.; Ortiz-Vigón, A.; Sanchez, N.; et al. Effect of peri-implant mucosal thickness on esthetic outcomes and the efficacy of soft tissue augmentation procedures: Consensus report of group 2 of the SEPA/DGI/OF workshop. Clin. Oral Implant. Res. 2022, 33 (Suppl. S23), 100–108. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Magner, A.W.; Fletcher, P. The effect of the distance from the contact point to the crest of bone on the presence or absence of the interproximal dental papilla. J. Periodontol. 1992, 63, 995–996. [Google Scholar] [CrossRef]
- Tarnow, D.; Elian, N.; Fletcher, P.; Froum, S.; Magner, A.; Cho, S.; Salama, M.; Salama, H.; Garber, D.A. Vertical Distance from the Crest of Bone to the Height of the Interproximal Papilla Between Adjacent Implants. J. Periodontol. 2003, 74, 1785–1788. [Google Scholar] [CrossRef]
- Chang, M.; Wennström, J.L. Soft tissue topography and dimensions lateral to single implant-supported restorations. A cross-sectional study. Clin. Oral Implant. Res. 2013, 24, 556–562. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Cho, S.C.; Wallace, S.S. The effect of inter-implant distance on the height of inter-implant bone crest. J. Periodontol. 2000, 71, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, G.; Wennström, J.L.; Lekholm, U. Peri-implant bone alterations in relation to inter-unit distances: A 3-year retrospective study. Clin. Oral Implant. Res. 2003, 14, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.L. Artificial crown contours and gingival health. J. Prosthet. Dent. 1962, 12, 1146–1156. [Google Scholar] [CrossRef]
- Prato, G.P.; Rotundo, R.; Cortellini, P.; Tinti, C.; Azzi, R. Interdental papilla management: A review and classification of the therapeutic approaches. Int. J. Periodontics Restor. Dent. 2004, 24, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Martínez, C.; Martínez, J.; McCulloch, C.A. Role of Fibroblast Populations in Periodontal Wound Healing and Tissue Remodeling. Front. Physiol. 2019, 10, 270. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chow, Y.C.; Eber, R.M.; Tsao, Y.P.; Shotwell, J.L.; Wang, H. Factors associated with the appearance of gingival papillae. J. Clin. Periodontol. 2010, 37, 719–727. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, C.A. Origins and functions of cells essential for periodontal repair: The role of fibroblasts in tissue homeostasis. Oral Dis. 1995, 1, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Fadl, A.; Leask, A. Hiding in Plain Sight: Human Gingival Fibroblasts as an Essential, Yet Overlooked, Tool in Regenerative Medicine. Cells 2023, 12, 2021. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, Z.; Guo, K.; Chen, L.; Chen, X.; Zou, D.; Yang, C. Role of periosteum in alveolar bone regeneration comparing with collagen membrane in a buccal dehiscence model of dogs. Sci. Rep. 2023, 13, 2505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, Z.; Fateh, A.; Salem, D.; Intini, G. Periosteum: Biology and applications in craniofacial bone regeneration. J. Dent. Res. 2014, 93, 109–116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saulacic, N.; Lang, N.P.; Corluka, S.; Mendaña, M.P.; Guzón, F.M.M. Vertical Alveolar Ridge Regeneration by Means of Periosteal Activation—A Proof-of-Principle Study. J. Clin. Periodontol. 2024, 51, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, Y.; Han, D. Periosteal Distraction Osteogenesis: An Effective Method for Bone Regeneration. Biomed. Res. Int. 2016, 2016, 2075317. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 32–49. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V.; Jung, R.E.; Schwarz, F.; Groups of the 2nd Osteology Foundation Consensus Meeting. Evidence-based knowledge on the aesthetics and maintenance of peri-implant soft tissues: Osteology Foundation Consensus Report Part 1-Effects of soft tissue augmentation procedures on the maintenance of peri-implant soft tissue health. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 7–10. [Google Scholar] [CrossRef] [PubMed]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J. Periodontol. 2021, 92, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, X.; Li, J.; Yue, Y.; Li, J.; Wang, M.; Wei, N.; Hao, L. Mechanisms of mechanical force in periodontal homeostasis: A review. Front. Immunol. 2024, 15, 1438726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fränkel, R. A Functional Approach to Orofacial Orthopaedics. Br. J. Orthod. 1980, 7, 41–51. [Google Scholar] [CrossRef]
- Engelke, W.; Jung, K.; Knosel, M. Intra-oral compartment pressures: A biofunctional model and experimental measurements under dif-ferent conditions of posture. Clin. Oral. Investig. 2011, 15, 165–176. [Google Scholar] [CrossRef]
- Engelke, W.; Glombek, J.; Psychogios, M.; Schneider, S.; Ellenberger, D.; Santander, P. Displacement of oropharyngeal structures during suction-swallowing cycles. Eur. Arch. Otorhinolaryngol. 2014, 271, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Kieser, J.; Singh, B.; Swain, M.; Ichim, I.; Waddell, J.N.; Kennedy, D.; Foster, K.; Livingstone, V. Measuring intraoral pressure: Adaptation of a dental appliance allows measurement during function. Dysphagia 2007, 23, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.; Kieser, J.; Bolter, C.; Swain, M.; Singh, B.; Waddell, J.N. Tongue pressure patterns during water swallowing. Dysphagia 2010, 25, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Santander, P.; Engelke, W.; Olthoff, A.; Völter, C. Intraoral pressure patterns during swallowing. Eur. Arch. Otorhinolaryngol. 2013, 270, 1019–1025. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Fuentes, R.; Engelke, W.; Flores, T.; Navarro, P.; Borie, E.; Curiqueo, A.; Salamanca, C. Description of intraoral pressures on sub-palatal space in young adult patients with normal occlusion. Int. J. Clin. Exp. Med. 2015, 8, 11208–11213. [Google Scholar] [PubMed] [PubMed Central]
- Buzan, T. The Ultimate Book of Mind Maps: Unlock Your Creativity, Boost Your Memory, Change Your Life; HarperThor-Sons: London, UK, 2006; ISBN 978-0007212910. [Google Scholar]
- Buzan, T. Use Your Head; BBC: London, UK, 1974. [Google Scholar]
- Mackinac Center for Public Policy. An Introduction to the Overton Window of Political Possibilities. Mackinac Center for Public Policy. 2010. Available online: https://www.mackinac.org/OvertonWindow (accessed on 10 June 2024).
- Sober, E. Ockham’s Razors: A User’s Manual; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Choquet, V.; Hermans, M.; Adriaenssens, P.; Daelemans, P.; Tarnow, D.P.; Malevez, C. Clinical and radiographic evaluation of the papilla level adjacent to single-tooth dental implants: A retrospective study in the maxillary anterior region. J. Periodontol. 2001, 72, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Giuliani, A.; Furlani, M.; Menini, M.; Piattelli, A.; Iezzi, G. Influence of abutment macro- and micro-geometry on morphologic and morphometric features of peri-implant connective tissue. Clin. Oral Implant. Res. 2023, 34, 920–933. [Google Scholar] [CrossRef] [PubMed]
- Agustín-Panadero, R.; Martínez-Martínez, N.; Fernandez-Estevan, L.; Faus-López, J.; Solá-Ruíz, M. Influence of Transmucosal Area Morphology on Peri-implant Bone Loss in Tissue-Level Implants. Int. J. Oral Maxillofac. Implant. 2019, 34, 947–952. [Google Scholar] [CrossRef]
- Rodrigues, X.; Navajas, A.; Vela, X.; Fortuño, A.; Jimenez, J.; Nevins, M. Arrangement of Peri-implant Connective Tissue Fibers Around Platform-Switching Implants with Conical Abutments and Its Relationship to the Underlying Bone: A Human Histologic Study. Int. J. Periodontics Restor. Dent. 2016, 36, 533–540. [Google Scholar] [CrossRef]
- Loi, I.; Di Felice, A. Biologically oriented preparation technique (BOPT): A new approach for prosthetic restoration of periodontically healthy teeth. Eur. J. Esthet. Dent. 2013, 8, 10–23. [Google Scholar] [PubMed]
- Deng, Y.; Wu, K.; Kuo, M.Y. Phenytoin induces connective tissue growth factor (CTGF/CCN2) production through NADPH oxidase 4-mediated latent TGFβ1 activation in human gingiva fibroblasts: Suppression by curcumin. J. Periodontal Res. 2022, 57, 1219–1226. [Google Scholar] [CrossRef]
- Tungare, S.; Paranjpe, A.G. Drug-Induced Gingival Overgrowth. 19 September 2022. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Droździk, A.; Droździk, M. Drug-Induced Gingival Overgrowth—Molecular Aspects of Drug Actions. Int. J. Mol. Sci. 2023, 24, 5448. [Google Scholar] [CrossRef]
- Vincent-Bugnas, S.; Borsa, L.; Gruss, A.; Lupi, L. Prioritization of predisposing factors of gingival hyperplasia during orthodontic treatment: The role of amount of biofilm. BMC Oral Health 2021, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, R.; Sochat, P.; Hansing, F.J. Rotary gingival curettage—A technique for tooth preparation and management of the gingival sulcus for impression taking. Int. J. Periodont. Rest. Dent. 1981, 1, 8–33. [Google Scholar]
- Eckert-Möbius, A. Die Bedeutung der Zunge für die Nasen- und Mundatmung. J. Orofac. Orthop. Kieferorthopädie 1953, 14, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lear, C.S.; Flanagan, J.B.; Moorrees, C.F. The frequency of deglutition in man. Arch. Oral Biol. 1965, 10, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pasapula, M.; Wang, Z.; Edwards, K.; Norrish, A. The effectiveness of cupping therapy on low back pain: A systematic review and meta-analysis of randomized control trials. Complement. Ther. Med. 2024, 80, 103013. [Google Scholar] [CrossRef]
- Al-Bedah, A.M.N.; Elsubai, I.S.; Qureshi, N.A.; Aboushanab, T.S.; Ali, G.I.M.; El-Olemy, A.T.; Khalil, A.A.H.; Khalil, M.K.M.; Alqaed, M.S. The medical perspective of cupping therapy: Effects and mechanisms of action. J. Tradit. Complement. Med. 2018, 9, 90–97. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peirce, C.S. Deduction, Induction, and Hypothesis. Pop. Sci. Mon. 1878, 13, 470–482. [Google Scholar]
- Anderson, D.R. The evolution of Peirce’s concept of abduction. Trans. Charles S. Peirce Soc. 1986, 22, 145–164. [Google Scholar]
- Koffka, K. Principles of Gestalt Psychology; Mimesis International: Sesto San Giovanni, Italy, 2020. [Google Scholar]
- Berglundh, T.; Lindhe, J.; Ericsson, I.; Marinello, C.P.; Liljenberg, B.; Thomsen, P. The soft tissue barrier at implants and teeth. Clin. Oral Implants Res. 1991, 2, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Donders, F.C. Under den Mechanismus des Saugens. Pflug. Arch. 1875, 10, 91–94. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botticelli, D.; Agabiti, I.; Yamada, R.; Maniwa, N.; Apaza Alccayhuaman, K.A.; Nakajima, Y. Identifying Key Factors in Papilla Growth Around Implants: Focus on Intraoral Negative Pressure. Dent. J. 2025, 13, 124. https://doi.org/10.3390/dj13030124
Botticelli D, Agabiti I, Yamada R, Maniwa N, Apaza Alccayhuaman KA, Nakajima Y. Identifying Key Factors in Papilla Growth Around Implants: Focus on Intraoral Negative Pressure. Dentistry Journal. 2025; 13(3):124. https://doi.org/10.3390/dj13030124
Chicago/Turabian StyleBotticelli, Daniele, Ivo Agabiti, Rihito Yamada, Nozomi Maniwa, Karol Alí Apaza Alccayhuaman, and Yasushi Nakajima. 2025. "Identifying Key Factors in Papilla Growth Around Implants: Focus on Intraoral Negative Pressure" Dentistry Journal 13, no. 3: 124. https://doi.org/10.3390/dj13030124
APA StyleBotticelli, D., Agabiti, I., Yamada, R., Maniwa, N., Apaza Alccayhuaman, K. A., & Nakajima, Y. (2025). Identifying Key Factors in Papilla Growth Around Implants: Focus on Intraoral Negative Pressure. Dentistry Journal, 13(3), 124. https://doi.org/10.3390/dj13030124






