The Role of Inflammasomes in Chronic Oral Inflammatory Disease and Oral Cancer: A Narrative Review
Abstract
1. Introduction
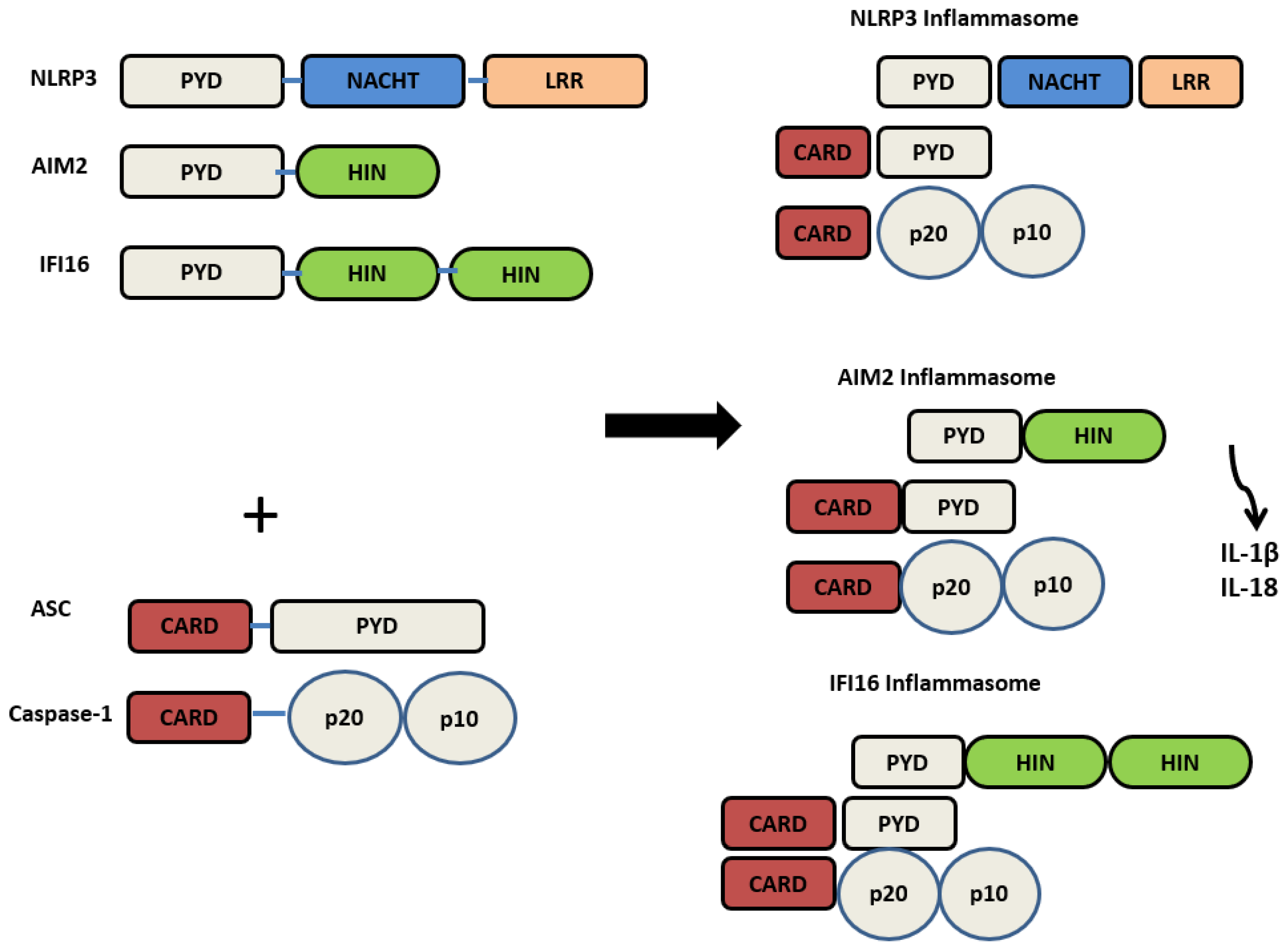
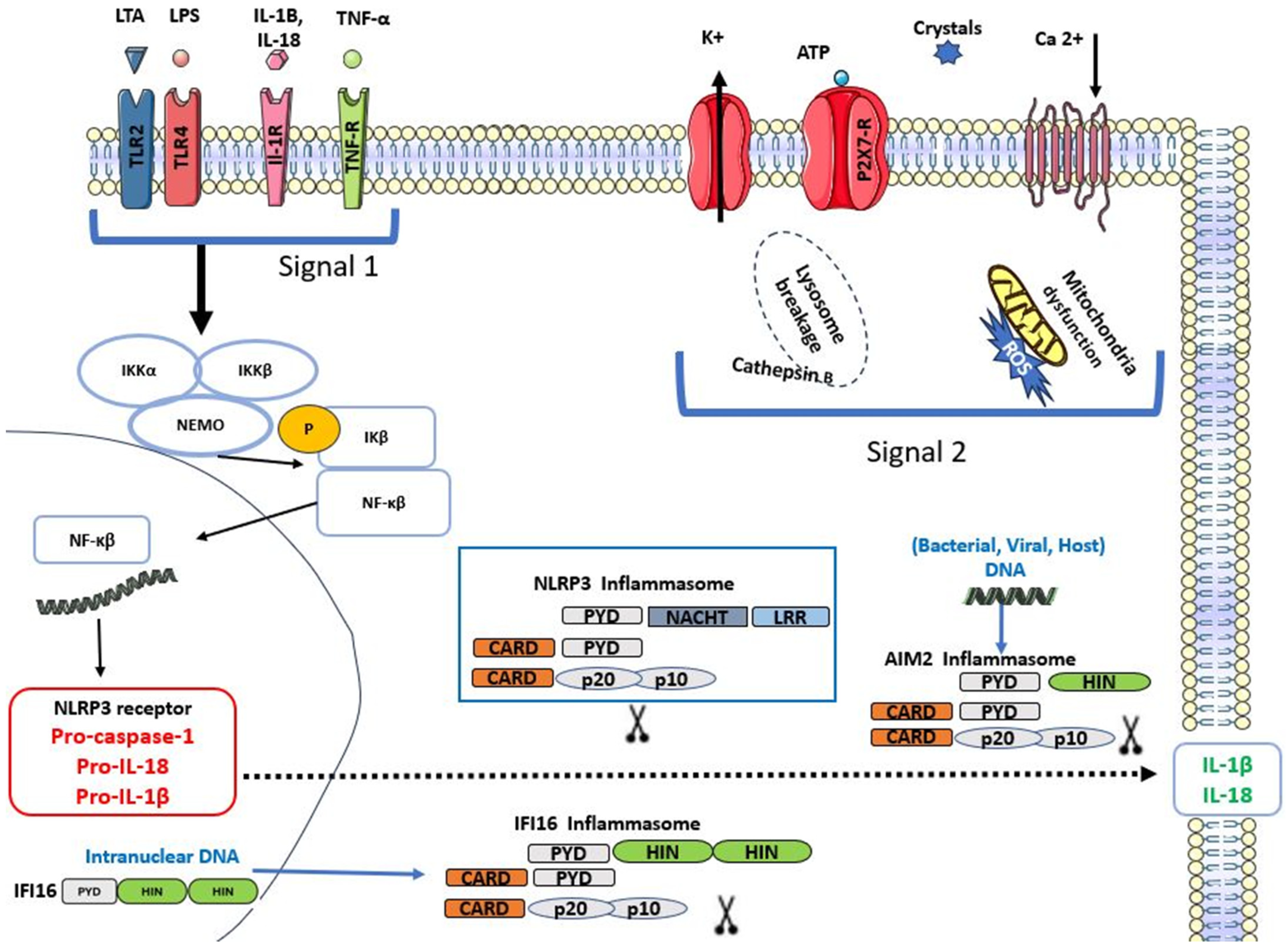
2. Inflammasomes
2.1. Nod-like Receptor Pyrin Domain 3 (NLRP3) Inflammasome
2.2. Absent in Melanoma (AIM2) Inflammasome
2.3. Interferon Inducible Protein 16 (IFI16) Inflammasomes
3. The Role of Inflammasomes in Acute Pulpitis and Potential Therapeutic Targets
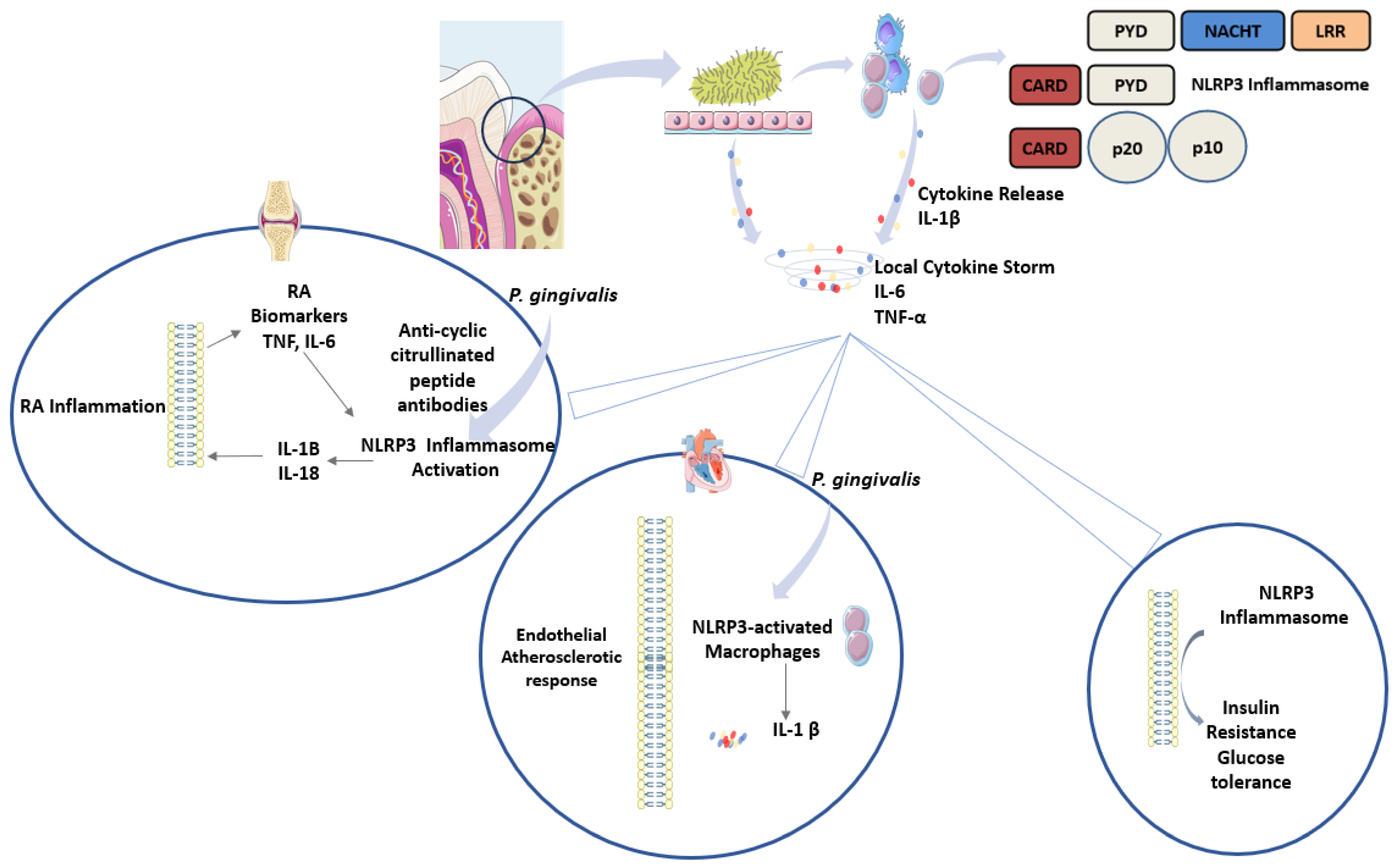
4. Chronic Periodontitis and Systemic Diseases: Is There a Role for Inflammasomes?
5. Chronic Inflammation and Oral Cancer: The Role of Inflammasomes
5.1. Chemical Irritants
5.2. Oral Pathogens
5.3. Oncogenic Viruses
5.4. Immune-Mediated
6. The Role of Inflammasomes (NLRP3, AIM2, IFI16), Nuclear Factor Kappa B (NFκB), Interleukin-1 Beta (IL-1β), in the Pathogenesis of Oral Cancer and Potential Therapeutic Targets
Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMP s and DAMP s: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, K.J.; Dalgleish, A.G. Chronic immune activation and inflammation as the cause of malignancy. Br. J. Cancer 2001, 85, 473–483. [Google Scholar] [CrossRef]
- Koca-Ünsal, R.B.; Şehirli, A.Ö.; Sayıner, S.; Aksoy, U. Relationship of NLRP3 inflammasome with periodontal, endodontic and related systemic diseases. Mol. Biol. Rep. 2022, 49, 11123–11132. [Google Scholar] [CrossRef]
- Wang, H.; Luo, Q.; Feng, X.; Zhang, R.; Li, J.; Chen, F. NLRP3 promotes tumor growth and metastasis in human oral squamous cell carcinoma. BMC Cancer 2018, 18, 500. [Google Scholar] [CrossRef]
- Kondo, Y.; Nagai, K.; Nakahata, S.; Saito, Y.; Ichikawa, T.; Suekane, A.; Taki, T.; Iwakawa, R.; Enari, M.; Taniwaki, M.; et al. Overexpression of the DNA sensor proteins, absent in melanoma 2 and interferon-inducible 16, contributes to tumorigenesis of oral squamous cell carcinoma with p53 inactivation. Cancer Sci. 2012, 103, 782–790. [Google Scholar] [CrossRef]
- Feng, X.; Luo, Q.; Zhang, H.; Wang, H.; Chen, W.; Meng, G.; Chen, F. The role of NLRP3 inflammasome in 5-fluorouracil resistance of oral squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 81. [Google Scholar] [CrossRef]
- Dogra, A.; Hasija, Y. Unraveling prognostic biomarkers in oral squamous cell carcinoma: An approach based on explainable artificial intelligence. Cancer Genet. 2025, 296–297, 163–171. [Google Scholar] [CrossRef]
- Lu, J.; Deng, M.; Lu, L.; Li, J.; Lu, G.; Liao, M.; Li, S.; Han, L. Molecular characteristics of oligomeric protein complexes AIM2 and TM4SF19 and their association with the pathogenesis of oral squamous cell carcinoma: Potential biomarkers. Int. J. Biol. Macromol. 2025, 306, 141816. [Google Scholar] [CrossRef]
- Neha, N.; Abraham, D.; Goyal, A.; Gupta, A.; Sharma, R.; Malhotra, R.K. GCF NLRP3 as a Biomarker for Assessing Endodontic Treatment Outcomes in Symptomatic Irreversible Pulpitis: A Comparative Nonrandomized, Observational Cross-Sectional Study. J. Int. Soc. Prev. Community Dent. 2025, 15, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Chaparro, A.; Valencia, M.I.; Bendek, M.J.; Duncan, H.F.; Segura-Egea, J.J.; Alhucema, C.; Ramírez, V. Proteomic Profiling of Dentinal Fluid for the Identification of Biomarkers in Pulpal Inflammation: An Exploratory Study. Int. Endod. J. 2025, 58, 1890–1901. [Google Scholar] [CrossRef]
- Loo, A.L.S.J.; Cen, R.; Wang, J.; Wu, Z.; Duncan, H.F.; Lee, A.H.C.; Zhang, C. Symptom correlation and spatial distribution of inflammatory mediators in pulpitis—A preliminary study. Int. Endod. J. 2025, 58, 1565–1581. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.P.; Mueller, J.L.; Misaghi, A.; Anderson, S.; Sivagnanam, M.; Kolodner, R.D.; Hoffman, H.M. Initial description of the human NLRP3 promoter. Genes Immun. 2008, 9, 721–726. [Google Scholar] [CrossRef]
- Fernandes-Alnemri, T.; Yu, J.-W.; Datta, P.; Wu, J.; Alnemri, E.S. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature 2009, 458, 509–513. [Google Scholar] [CrossRef]
- Kerur, N.; Veettil, M.V.; Sharma-Walia, N.; Bottero, V.; Sadagopan, S.; Otageri, P.; Chandran, B. IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi Sarcoma-associated herpesvirus infection. Cell Host Microbe 2011, 9, 363–375. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- He, W.-T.; Wan, H.; Hu, L.; Chen, P.; Wang, X.; Huang, Z.; Yang, Z.-H.; Zhong, C.-Q.; Han, J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015, 25, 1285–1298. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Yang, B.; Yu, W.; Xiao, Y.; Yu, D.; Zhang, Q. Cathepsin B links oxidative stress to the activation of NLRP3 inflammasome. Exp. Cell Res. 2018, 362, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Kanneganti, T.-D. NLRP3 inflammasome in cancer and metabolic diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, K.L.; Ray, M.E.; Su, Y.A.; Anzick, S.L.; Johnstone, R.W.; Trapani, J.A.; Meltzer, P.S.; Trent, J.M. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene 1997, 15, 453–457. [Google Scholar] [CrossRef]
- Bürckstümmer, T.; Baumann, C.; Blüml, S.; Dixit, E.; Dürnberger, G.; Jahn, H.; Planyavsky, M.; Bilban, M.; Colinge, J.; Bennett, K.L.; et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat. Immunol. 2009, 10, 266–272. [Google Scholar] [CrossRef]
- Hornung, V.; Ablasser, A.; Charrel-Dennis, M.; Bauernfeind, F.; Horvath, G.; Caffrey, D.R.; Latz, E.; Fitzgerald, K.A. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 2009, 458, 514–518. [Google Scholar] [CrossRef]
- Wang, L.; Sun, L.; Byrd, K.M.; Ko, C.-C.; Zhao, Z.; Fang, J. AIM2 inflammasome’s first decade of discovery: Focus on oral diseases. Front. Immunol. 2020, 11, 1487. [Google Scholar] [CrossRef]
- Lugrin, J.; Martinon, F. The AIM 2 inflammasome: Sensor of pathogens and cellular perturbations. Immunol. Rev. 2018, 281, 99–114. [Google Scholar] [CrossRef]
- Unterholzner, L.; Keating, S.E.; Baran, M.; Horan, K.A.; Jensen, S.B.; Sharma, S.; Sirois, C.M.; Jin, T.; Latz, E.; Xiao, T.S.; et al. IFI16 is an innate immune sensor for intracellular DNA. Nat. Immunol. 2010, 11, 997–1004. [Google Scholar] [CrossRef]
- Jin, T.; Perry, A.; Jiang, J.; Smith, P.; Curry, J.A.; Unterholzner, L.; Jiang, Z.; Horvath, G.; Rathinam, V.A.; Johnstone, R.W.; et al. Structures of the HIN domain: DNA complexes reveal ligand binding and activation mechanisms of the AIM2 inflammasome and IFI16 receptor. Immunity 2012, 36, 561–571. [Google Scholar] [CrossRef]
- Caneparo, V.; Cena, T.; De Andrea, M.; Dell’Oste, V.; Stratta, P.; Quaglia, M.; Tincani, A.; Andreoli, L.; Ceffa, S.; Taraborelli, M.; et al. Anti-IFI16 antibodies and their relation to disease characteristics in systemic lupus erythematosus. Lupus 2013, 22, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Fernández, L.; García-Lozano, J.-R.; Montes-Cano, M.-A.; Conde-Jaldón, M.; Ortego-Centeno, N.; García-Hernández, F.-J.; Espinosa, G.; Graña-Gil, G.; Sánchez-Bursón, J.; Blanco, R.; et al. Variants of the IFI16 gene affecting the levels of expression of mRNA are associated with susceptibility to Behçet disease. J. Rheumatol. 2015, 42, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, M.O.; Cruz, Á.A.; Teixeira, H.M.; Silva, H.d.S.; Gomes-Filho, I.S.; Trindade, S.C.; Soledade, K.R.; Fernandes, J.S.; Santana, C.V.N.; Pinheiro, G.P.; et al. Variants in interferon gamma inducible protein 16 (IFI16) and absent in melanoma 2 (AIM2) genes that modulate inflammatory response are associated with periodontitis. Arch. Oral Biol. 2023, 147, 105640. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Ma, Y.; Xu, Y.; Ogbuehi, A.C.; Liu, X.; Deng, Y.; Xi, J.; Pan, H.; Lin, Q.; Li, B.; et al. The genetic and epigenetic mechanisms involved in irreversible pulp neural inflammation. Dis. Markers 2021, 2021, 1–26. [Google Scholar] [CrossRef]
- Hahn, C.-L.; Liewehr, F.R. Update on the adaptive immune responses of the dental pulp. J. Endod. 2007, 33, 773–781. [Google Scholar] [CrossRef]
- Jang, J.-H.; Shin, H.W.; Lee, J.M.; Lee, H.-W.; Kim, E.-C.; Park, S.H. An overview of pathogen recognition receptors for innate immunity in dental pulp. Mediat. Inflamm. 2015, 2015, 794143. [Google Scholar] [CrossRef]
- Hirao, K.; Yumoto, H.; Takahashi, K.; Mukai, K.; Nakanishi, T.; Matsuo, T. Roles of TLR2, TLR4, NOD2, and NOD1 in pulp fibroblasts. J. Dent. Res. 2009, 88, 762–767. [Google Scholar] [CrossRef]
- El-Sayed, K.M.F.; Klingebiel, P.; Dörfer, C.E. Toll-like receptor expression profile of human dental pulp stem/progenitor cells. J. Endod. 2016, 42, 413–417. [Google Scholar] [CrossRef]
- Keller, J.-F.; Carrouel, F.; Staquet, M.-J.; Kufer, T.A.; Baudouin, C.; Msika, P.; Bleicher, F.; Farges, J.-C. Expression of NOD2 is increased in inflamed human dental pulps and lipoteichoic acid-stimulated odontoblast-like cells. Innate Immun. 2011, 17, 29–34. [Google Scholar] [CrossRef]
- Song, Z.; Lin, Z.; He, F.; Jiang, L.; Qin, W.; Tian, Y.; Wang, R.; Huang, S. NLRP3 is expressed in human dental pulp cells and tissues. J. Endod. 2012, 38, 1592–1597. [Google Scholar] [CrossRef]
- Al Natour, B.; Lundy, F.T.; About, I.; Jeanneau, C.; Dombrowski, Y.; El Karim, I.A. Regulation of caries-induced pulp inflammation by NLRP3 inflammasome: A laboratory-based investigation. Int. Endod. J. 2023, 56, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Lv, H.; Wang, H.; Wang, D.; Sun, S.; Jia, Q.; Wang, P.; Song, B.; Ni, L. Activation of the NLRP3/caspase-1 inflammasome in human dental pulp tissue and human dental pulp fibroblasts. Cell Tissue Res. 2015, 361, 541–555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, P.; Ma, X.; Yin, X.; Li, J.; Wang, H.; Jiang, W.; Jia, Q.; Ni, L. Mechanisms that lead to the regulation of NLRP3 inflammasome expression and activation in human dental pulp fibroblasts. Mol. Immunol. 2015, 66, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Al Natour, B.; Lundy, F.T.; Moynah, P.N.; About, I.; Jeanneau, C.; Irwin, C.; Dombrowski, Y.; El Karim, I.A. Odontoblast cell death induces NLRP3 inflammasome-dependent sterile inflammation and regulates dental pulp cell migration, proliferation and differentiation. Int. Endod. J. 2021, 54, 941–950. [Google Scholar] [CrossRef]
- Pucinelli, C.M.; da Silva, R.A.B.; Nelson-Filho, P.; Lima, R.B.; Lucisano, M.P.; Marchesan, J.T.; da Silva, L.A.B. The effects of NLRP3 inflammasome inhibition or knockout in experimental apical periodontitis induced in mice. Clin. Oral Investig. 2024, 28, 285. [Google Scholar] [CrossRef]
- Huang, S.; Song, Z.; Huang, Q.; Jiang, L.; Chen, L.; Wang, R.; Lin, Z. AIM2 inflammasome is critical for dsDNA-induced IL-1β secretion in human dental pulp cells. Inflammation 2018, 41, 409–417. [Google Scholar] [CrossRef]
- Huang, S.; Song, Z.; Jiang, L.; Chen, L.; Wang, R.; Qin, W.; Liu, P.; Lin, Z. Absent in melanoma 2 (AIM2) expressed in human dental pulp mediates IL-1β secretion in response to cytoplasmic DNA. Inflammation 2015, 38, 566–575. [Google Scholar] [CrossRef]
- Aral, K.; Milward, M.R.; Cooper, P.R. Dysregulation of inflammasomes in human dental pulp cells exposed to Porphyromonas gingivalis and Fusobacterium nucleatum. J. Endod. 2020, 46, 1265–1272. [Google Scholar] [CrossRef]
- Green, J.P.; El-Sharkawy, L.Y.; Roth, S.; Zhu, J.; Cao, J.; Leach, A.G.; Liesz, A.; Freeman, S.; Brough, D. Discovery of an inhibitor of DNA-driven inflammation that preferentially targets the AIM2 inflammasome. iScience 2023, 26, 106758. [Google Scholar] [CrossRef]
- Wang, W.; Yi, X.; Ren, Y.; Xie, Q. Effects of adenosine triphosphate on proliferation and odontoblastic differentiation of human dental pulp cells. J. Endod. 2016, 42, 1483–1489. [Google Scholar] [CrossRef]
- Delima, A.J.; Karatzas, S.; Amar, S.; Graves, D.T. Inflammation and tissue loss caused by periodontal pathogens is reduced by interleukin-1 antagonists. J. Infect. Dis. 2002, 186, 511–516. [Google Scholar] [CrossRef]
- Dutzan, N.; Kajikawa, T.; Abusleme, L.; Greenwell-Wild, T.; Zuazo, C.E.; Ikeuchi, T.; Brenchley, L.; Abe, T.; Hurabielle, C.; Martin, D.; et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 2018, 10, eaat0797. [Google Scholar] [CrossRef]
- Lim, Y.; Totsika, M.; Morrison, M.; Punyadeera, C. Oral Microbiome: A New Biomarker Reservoir for Oral and Oropharyngeal Cancers. Theranostics 2017, 7, 4313–4321. [Google Scholar] [CrossRef] [PubMed]
- Staquet, M.-J.; Carrouel, F.; Keller, J.-F.; Baudouin, C.; Msika, P.; Bleicher, F.; Kufer, T.; Farges, J.-C. Pattern-recognition receptors in pulp defense. Adv. Dent. Res. 2011, 23, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ke, X.; Yan, F.; Lei, L.; Li, H. Necroptosis in the periodontal homeostasis: Signals emanating from dying cells. Oral Dis. 2018, 24, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Ebe, N.; Hara-Yokoyama, M.; Iwasaki, K.; Iseki, S.; Okuhara, S.; Podyma-Inoue, K.; Terasawa, K.; Watanabe, A.; Akizuki, T.; Watanabe, H.; et al. Pocket epithelium in the pathological setting for HMGB1 release. J. Dent. Res. 2011, 90, 235–240. [Google Scholar] [CrossRef]
- Furuse, N.; Takai, H.; Ogata, Y. Effects of Initial Periodontal Therapy on Heat Shock Protein 70 Levels in Gingival Crevicular Fluid from Periodontitis Patients. J. Clin. Med. 2020, 9, 3072. [Google Scholar] [CrossRef]
- Graves, D.T.; Cochran, D. The contribution of interleukin-1 and tumor necrosis factor to periodontal tissue destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Neurath, N.; Kesting, M. Cytokines in gingivitis and periodontitis: From pathogenesis to therapeutic targets. Front. Immunol. 2024, 15, 1435054. [Google Scholar] [CrossRef]
- Engebretson, S.P.; Grbic, J.T.; Singer, R.; Lamster, I.B. GCF IL-1β profiles in periodontal disease. J. Clin. Periodontol. 2002, 29, 48–53. [Google Scholar] [CrossRef]
- Rangbulla, V.; Nirola, A.; Gupta, M.; Batra, P.; Gupta, M. Salivary IgA, interleukin-1β and MMP-8 as salivary biomarkers in chronic periodontitis patients. Chin. J. Dent. Res. 2017, 20, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Arias-Bujanda, N.; Regueira-Iglesias, A.; Blanco-Pintos, T.; Alonso-Sampedro, M.; Relvas, M.; González-Peteiro, M.M.; Balsa-Castro, C.; Tomás, I. Diagnostic accuracy of IL1β in saliva: The development of predictive models for estimating the probability of the occurrence of periodontitis in non-smokers and smokers. J. Clin. Periodontol. 2020, 47, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Isaza-Guzmán, D.M.; Medina-Piedrahíta, V.M.; Gutiérrez-Henao, C.; Tobón-Arroyave, S.I. Salivary levels of NLRP3 inflammasome-related proteins as potential biomarkers of periodontal clinical status. J. Periodontol. 2017, 88, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Na, H.S.; Song, Y.-R.; Shin, S.Y.; Kim, Y.-M.; Chung, J. Activation of NLRP3 and AIM2 inflammasomes by Porphyromonas gingivalis infection. Infect. Immun. 2014, 82, 112–123. [Google Scholar] [CrossRef]
- Cheng, R.; Wu, Z.; Li, M.; Shao, M.; Hu, T. Interleukin-1β is a potential therapeutic target for periodontitis: A narrative review. Int. J. Oral Sci. 2020, 12, 2. [Google Scholar] [CrossRef]
- Wang, J.; Qi, J.; Zhao, H.; He, S.; Zhang, Y.; Wei, S.; Zhao, F. Metagenomic sequencing reveals microbiota and its functional potential associated with periodontal disease. Sci. Rep. 2013, 3, 1843. [Google Scholar] [CrossRef]
- Torrungruang, K.; Jitpakdeebordin, S.; Charatkulangkun, O.; Gleebbua, Y. Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Treponema denticola/Prevotella intermedia Co-Infection Are Associated with Severe Periodontitis in a Thai Population. PLoS ONE 2015, 10, e0136646. [Google Scholar] [CrossRef]
- Byrne, S.; Dashper, S.; Darby, I.; Adams, G.; Hoffmann, B.; Reynolds, E. Progression of chronic periodontitis can be predicted by the levels of Porphyromonas gingivalis and Treponema denticola in subgingival plaque. Oral Microbiol. Immunol. 2009, 24, 469–477. [Google Scholar] [CrossRef]
- Marchesan, J.T.; Girnary, M.S.; Moss, K.; Monaghan, E.T.; Egnatz, G.J.; Jiao, Y.; Zhang, S.; Beck, J.; Swanson, K.V. Role of inflammasomes in the pathogenesis of periodontal disease and therapeutics. Periodontol. 2000 2000, 82, 93–114. [Google Scholar] [CrossRef]
- Bostanci, N.; Emingil, G.; Saygan, B.; Turkoglu, O.; Atilla, G.; A Curtis, M.; Belibasakis, G.N. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin. Exp. Immunol. 2009, 157, 415–422. [Google Scholar] [CrossRef]
- Aral, K.; Berdeli, E.; Cooper, P.R.; Milward, M.R.; Kapila, Y.; Ünal, B.K.; Aral, C.A.; Berdeli, A. Differential expression of inflammasome regulatory transcripts in periodontal disease. J. Periodontol. 2020, 91, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Meier, A.; Guggenheim, B.; Belibasakis, G.N. Regulation of NLRP3 and AIM2 inflammasome gene expression levels in gingival fibroblasts by oral biofilms. Cell. Immunol. 2011, 270, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Taxman, D.J.; Zhang, J.; Champagne, C.; Bergstralh, D.T.; Iocca, H.A.; Lich, J.D.; Ting, J.P.-Y. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and-independent pathways. J. Immunol. 2006, 177, 4252–4256. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Shu, R.; Xie, Y. The expression of NLRP3, NLRP1 and AIM2 in the gingival tissue of periodontitis patients: RT-PCR study and immunohistochemistry. Arch. Oral Biol. 2015, 60, 948–958. [Google Scholar] [CrossRef]
- Ran, S.; Liu, B.; Gu, S.; Sun, Z.; Liang, J. Analysis of the expression of NLRP3 and AIM2 in periapical lesions with apical periodontitis and microbial analysis outside the apical segment of teeth. Arch. Oral Biol. 2017, 78, 39–47. [Google Scholar] [CrossRef]
- Ali Daily, Z.; Al-Ghurabi, B.H.; Al-Qarakhli, A.M.A.; Hussein, H.M. Association Between AIM2 and Pycard Genes Polymorphisms and Susceptibility to Periodontitis with Coronary Heart Disease. Clin. Cosmet. Investig. Dent. 2023, 15, 307–320. [Google Scholar] [CrossRef]
- Chapple, I.L.C.; Genco, R.; on behalf of working group 2 of the joint EFP/AAP workshop*. Diabetes and periodontal diseases: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S106–S112. [Google Scholar] [CrossRef]
- García-Hernández, A.L.; Muñoz-Saavedra, Á.E.; González-Alva, P.; Moreno-Fierros, L.; Llamosas-Hernández, F.E.; Cifuentes-Mendiola, S.E.; Rubio-Infante, N. Upregulation of proteins of the NLRP3 inflammasome in patients with periodontitis and uncontrolled type 2 diabetes. Oral Dis. 2019, 25, 596–608. [Google Scholar] [CrossRef]
- Isola, G.; Polizzi, A.; Santonocito, S.; Alibrandi, A.; Williams, R.C. Periodontitis activates the NLRP3 inflammasome in serum and saliva. J. Periodontol. 2022, 93, 135–145. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Ni, J.; Xie, B.; Liu, Y.; Xuan, D.; Zhang, J. Hyperglucose contributes to periodontitis: Involvement of the NLRP3 pathway by engaging the innate immunity of oral gingival epithelium. J. Periodontol. 2015, 86, 327–335. [Google Scholar] [CrossRef]
- Catano Canizales, Y.G.; Uresti Rivera, E.E.; Garcia Jacobo, R.E.; Portales Perez, D.P.; Yadira, B.; Rodriguez Rivera, J.G.; Gonzalez Amaro, R.; Enciso Moreno, J.A.; Garcia Hernandez, M.H. Increased levels of AIM2 and circulating mitochondrial DNA in type 2 diabetes. Iran. J. Immunol. 2018, 15, 142–155. [Google Scholar] [PubMed]
- Arunachalam, L.T.; Suresh, S.; Lavu, V.; Vedamanickam, S.; Viswanathan, S.; Thirumalai Nathan, R.D. Association of salivary levels of DNA sensing inflammasomes AIM2, IFI16, and cytokine IL18 with periodontitis and diabetes. J. Periodontol. 2024, 95, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; White, S.; Bartold, P.M. Periodontal disease and rheumatoid arthritis: A systematic review. J. Dent. Res. 2013, 92, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Hashimoto, S.; Shimazaki, Y. Functional impairment and periodontitis in rheumatoid arthritis. Int. Dent. J. 2022, 72, 641–647. [Google Scholar] [CrossRef]
- Rosengren, S.; Hoffman, H.; Bugbee, W.; Boyle, D.L. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann. Rheum. Dis. 2005, 64, 708–714. [Google Scholar] [CrossRef]
- Li, Z.; Guo, J.; Bi, L. Role of the NLRP3 inflammasome in autoimmune diseases. Biomed. Pharmacother. 2020, 130, 110542. [Google Scholar] [CrossRef]
- de Torre-Minguela, C.; Mesa del Castillo, P.; Pelegrín, P. The NLRP3 and pyrin inflammasomes: Implications in the pathophysiology of autoinflammatory diseases. Front. Immunol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Corrêa, J.D.; Saraiva, A.M.; Queiroz-Junior, C.M.; Madeira, M.F.M.; Duarte, P.M.; Teixeira, M.M.; Souza, D.G.; da Silva, T.A. Arthritis-induced alveolar bone loss is associated with changes in the composition of oral microbiota. Anaerobe 2016, 39, 91–96. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, D.; Jia, H.; Feng, Q.; Wang, D.; Liang, D.; Wu, X.; Li, J.; Tang, L.; Li, Y.; et al. The oral and gut microbiomes are perturbed in rheumatoid arthritis and partly normalized after treatment. Nat. Med. 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Saccucci, M.; Di Carlo, G.; Lucchetti, R.; Pilloni, A.; Pranno, N.; Luzzi, V.; Valesini, G.; Polimeni, A. Periodontitis and rheumatoid arthritis: The same inflammatory mediators? Mediat. Inflamm. 2019, 2019, 1–8. [Google Scholar] [CrossRef]
- Pellegrini, C.; Martelli, A.; Antonioli, L.; Fornai, M.; Blandizzi, C.; Calderone, V. NLRP3 inflammasome in cardiovascular diseases: Pathophysiological and pharmacological implications. Med. Res. Rev. 2021, 41, 1890–1926. [Google Scholar] [CrossRef] [PubMed]
- Schenkein, H.A.; Papapanou, P.N.; Genco, R.; Sanz, M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontology 2000, 83, 90–106. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Progulske-Fox, A. Invasion of Porphyromonas gingivalis strains into vascular cells and tissue. J. Oral Microbiol. 2015, 7, 28788. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Dhanuthai, K.; Rojanawatsirivej, S.; Thosaporn, W.; Kintarak, S.; Subarnbhesaj, A.; Darling, M.; Kryshtalskyj, E.; Chiang, C.-P.; Shin, H.-I.; Choi, S.-Y.; et al. Oral cancer: A multicenter study. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e23–e29. [Google Scholar] [CrossRef]
- Thompson-Harvey, A.; Yetukuri, M.; Hansen, A.R.; Simpson, M.C.; Boakye, E.A.; Varvares, M.A.; Osazuwa-Peters, N. Rising incidence of late-stage head and neck cancer in the United States. Cancer 2020, 126, 1090–1101. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Van den Brenk, H.; Stone, M.; Kelly, H.; Orton, C.; Sharpington, C. Promotion of growth of tumour cells in acutely inflamed tissues. Br. J. Cancer 1974, 30, 246–260. [Google Scholar] [CrossRef]
- Pupa, S.M.; Ménard, S.; Forti, S.; Tagliabue, E. New insights into the role of extracellular matrix during tumor onset and progression. J. Cell. Physiol. 2002, 192, 259–267. [Google Scholar] [CrossRef]
- Karin, M. Nuclear factor-κB in cancer development and progression. Nature 2006, 441, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2001, 249, 61–74. [Google Scholar] [CrossRef]
- Brigati, C.; Noonan, D.M.; Albini, A.; Benelli, R. Tumors and inflammatory infiltrates: Friends or foes? Clin. Exp. Metastasis 2002, 19, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Choubey, D.; Panchanathan, R. IFI16, an amplifier of DNA-damage response: Role in cellular senescence and aging-associated inflammatory diseases. Ageing Res. Rev. 2016, 28, 27–36. [Google Scholar] [CrossRef]
- Xiao, T.S.; Fitzgerald, K.A. The cGAS-STING pathway for DNA sensing. Mol. Cell 2013, 51, 135–139. [Google Scholar] [CrossRef]
- Ishikawa, H.; Ma, Z.; Barber, G.N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 2009, 461, 788–792. [Google Scholar] [CrossRef]
- El-Omar, E.M.; Carrington, M.; Chow, W.-H.; McColl, K.E.L.; Bream, J.H.; Young, H.A.; Herrera, J.; Lissowska, J.; Yuan, C.-C.; Rothman, N.; et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000, 404, 398–402. [Google Scholar] [CrossRef]
- Haghshenas, M.R.; Hosseini, S.V.; Mahmoudi, M.; Saberi-Firozi, M.; Farjadian, S.; Ghaderi, A. IL-18 serum level and IL-18 promoter gene polymorphism in Iranian patients with gastrointestinal cancers. J. Gastroenterol. Hepatol. 2009, 24, 1119–1122. [Google Scholar] [CrossRef]
- Lin, W.-J.; Jiang, R.-S.; Wu, S.-H.; Chen, F.-J.; Liu, S.-A. Smoking, alcohol, and betel quid and oral cancer: A prospective cohort study. J. Oncol. 2011, 2011, 1–5. [Google Scholar] [CrossRef]
- Wu, C.-S.; Chang, K.-P.; OuYang, C.-N.; Kao, H.-K.; Hsueh, C.; Chen, L.-C.; Cheng, H.-Y.; Liang, Y.; Liou, W.; Liang, C.-L.; et al. ASC contributes to metastasis of oral cavity squamous cell carcinoma. Oncotarget 2016, 7, 50074–50085. [Google Scholar] [CrossRef]
- Lee, C.; Chang, J.S.; Syu, S.; Wong, T.; Chan, J.Y.; Tang, Y.; Yang, Z.; Yang, W.; Chen, C.; Lu, S.; et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J. Cell. Physiol. 2015, 230, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Dang, X.; He, B.; Ning, Q.; Liu, Y.; Guo, J.; Niu, G.; Chen, M. Alantolactone suppresses inflammation, apoptosis and oxidative stress in cigarette smoke-induced human bronchial epithelial cells through activation of Nrf2/HO-1 and inhibition of the NF-κB pathways. Respir. Res. 2020, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Hahad, O.; Kuntic, M.; Keaney, J.F., Jr.; Deanfield, J.E.; Daiber, A. Effects of tobacco cigarettes, e-cigarettes, and waterpipe smoking on endothelial function and clinical outcomes. Eur. Heart J. 2020, 41, 4057–4070. [Google Scholar] [CrossRef] [PubMed]
- Lazos, J.P.; Piemonte, E.D.; Lanfranchi, H.E.; Brunotto, M.N. Characterization of chronic mechanical irritation in oral cancer. Int. J. Dent. 2017, 2017, 6784526. [Google Scholar] [CrossRef]
- Pentenero, M.; Azzi, L.; Lodi, G.; Manfredi, M.; Varoni, E. Chronic mechanical trauma/irritation and oral carcinoma: A systematic review showing low evidence to support an association. Oral Dis. 2022, 28, 2110–2118. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Verma, M.; Panda, M. Role of oral microbiome signatures in diagnosis and prognosis of oral cancer. Technol. Cancer Res. Treat. 2019, 18, 1533033819867354. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Whitmore, S.E.; Lamont, R.J. Oral Bacteria and Cancer. PLoS Pathog. 2014, 10, e1003933. [Google Scholar] [CrossRef]
- Semper, R.P.; Mejías-Luque, R.; Groß, C.; Anderl, F.; Müller, A.; Vieth, M.; Busch, D.H.; Prazeres Da Costa, C.; Ruland, J.; Groß, O.; et al. Helicobacter pylori-induced IL-1β secretion in innate immune cells is regulated by the NLRP3 Inflammasome and requires the cag Pathogenicity Island. J. Immunol. 2014, 193, 3566–3576. [Google Scholar] [CrossRef]
- Mao, S.; Park, Y.; Hasegawa, Y.; Tribble, G.D.; James, C.E.; Handfield, M.; Stavropoulos, M.F.; Yilmaz, Ö.; Lamont, R.J. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell. Microbiol. 2007, 9, 1997–2007. [Google Scholar] [CrossRef]
- Peng, R.; Sun, Y.; Zhou, X.; Liu, S.; Han, Q.; Cheng, L.; Peng, X. Treponema denticola Promotes OSCC Development via the TGF-β Signaling Pathway. J. Dent. Res. 2022, 101, 00220345211067401. [Google Scholar] [CrossRef] [PubMed]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and oral cancer—Current concepts of the etiopathogenesis. Oncol. Rev. 2020, 14, 465. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J. Leptin potentiates Prevotella intermedia lipopolysaccharide-induced production of TNF-alpha in monocyte-derived macrophages. J. Periodontal Implant. Sci. 2010, 40, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Tomic, U.; Nikolic, N.; Carkic, J.; Mihailovic, D.; Jelovac, D.; Milasin, J.; Pucar, A. Streptococcus mitis and Prevotella melaninogenica Influence Gene Expression Changes in Oral Mucosal Lesions in Periodontitis Patients. Pathogens 2023, 12, 1194. [Google Scholar] [CrossRef]
- Schincaglia, G.; Hong, B.; Rosania, A.; Barasz, J.; Thompson, A.; Sobue, T.; Panagakos, F.; Burleson, J.; Dongari-Bagtzoglou, A.; Diaz, P. Clinical, Immune, and Microbiome Traits of Gingivitis and Peri-implant Mucositis. J. Dent. Res. 2017, 96, 47–55. [Google Scholar] [CrossRef]
- Karched, M.; Bhardwaj, R.G.; Qudeimat, M.; Al-Khabbaz, A.; Ellepola, A. Proteomic analysis of the periodontal pathogen Prevotella intermedia secretomes in biofilm and planktonic lifestyles. Sci. Rep. 2022, 12, 5636. [Google Scholar] [CrossRef]
- Patil, C.; Rossa, C., Jr.; Kirkwood, K.L. Actinobacillus actinomycetemcomitans lipopolysaccharide induces interleukin-6 expression through multiple mitogen-activated protein kinase pathways in periodontal ligament fibroblasts. Oral Microbiol. Immunol. 2006, 21, 392–398. [Google Scholar] [CrossRef]
- Patil, C.; Zhu, X.; Rossa, C., Jr.; Kim, Y.J.; Kirkwood, K.L. p38 MAPK regulates IL-1β induced IL-6 expression through mRNA stability in osteoblasts. Immunol. Investig. 2004, 33, 213–233. [Google Scholar] [CrossRef]
- Choi, E.Y.; Choe, S.H.; Hyeon, J.Y.; Park, H.R.; Choi, I.S.; Kim, S.J. Josamycin suppresses Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-1β in murine macrophages. Biomed. Pharmacother. 2018, 105, 498–505. [Google Scholar] [CrossRef]
- Ateia, I.M.; Sutthiboonyapan, P.; Kamarajan, P.; Jin, T.; Godovikova, V.; Kapila, Y.L.; Fenno, J.C. Treponema denticola increases MMP-2 expression and activation in the periodontium via reversible DNA and histone modifications. Cell. Microbiol. 2018, 20, e12815. [Google Scholar] [CrossRef]
- Atanasova, K.R.; Yilmaz, O. Looking in the Porphyromonas gingivalis cabinet of curiosities: The microbium, the host and cancer association. Mol. Oral Microbiol. 2014, 29, 55–66. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.K. Porphyromonas gingivalis in oral squamous cell carcinoma: A review. Microbes Infect. 2022, 24, 104925. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-F.; Chen, L.; Li, Y.-C.; Wu, L.; Yu, G.-T.; Zhang, W.-F.; Sun, Z.-J. NLRP3 inflammasome activation promotes inflammation-induced carcinogenesis in head and neck squamous cell carcinoma. J. Exp. Clin. Cancer Res. 2017, 36, 116. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Shen, X.; Zhou, M.; Tang, B. Periodontal pathogens promote oral squamous cell carcinoma by regulating ATR and NLRP3 inflammasome. Front. Oncol. 2021, 11, 722797. [Google Scholar] [CrossRef] [PubMed]
- Geng, F.; Zhang, Y.; Lu, Z.; Zhang, S.; Pan, Y. Fusobacterium nucleatum caused DNA damage and promoted cell proliferation by the Ku70/p53 pathway in oral cancer cells. DNA Cell Biol. 2020, 39, 144–151. [Google Scholar] [CrossRef]
- Aral, K.; Milward, M.R.; Gupta, D.; Cooper, P.R. Effects of Porphyromonas gingivalis and Fusobacterium nucleatum on inflammasomes and their regulators in H400 cells. Mol. Oral Microbiol. 2020, 35, 158–167. [Google Scholar] [CrossRef]
- Kim, S.M. Human papilloma virus in oral cancer. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 327–336. [Google Scholar] [CrossRef]
- Menezes, F.d.S.; Fernandes, G.A.; Antunes, J.L.F.; Villa, L.L.; Toporcov, T.N. Global incidence trends in head and neck cancer for HPV-related and -unrelated subsites: A systematic review of population-based studies. Oral Oncol. 2021, 115, 105177. [Google Scholar] [CrossRef]
- Chattergoon, M.A.; Latanich, R.; Quinn, J.; Winter, M.E.; Buckheit, R.W.; Blankson, J.N.; Pardoll, D.; Cox, A.L. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 2014, 10, e1004082. [Google Scholar] [CrossRef]
- Maruzuru, Y.; Ichinohe, T.; Sato, R.; Miyake, K.; Okano, T.; Suzuki, T.; Koshiba, T.; Koyanagi, N.; Tsuda, S.; Watanabe, M.; et al. Herpes simplex virus 1 VP22 inhibits AIM2-dependent inflammasome activation to enable efficient viral replication. Cell Host Microbe 2018, 23, 254–265.e7. [Google Scholar] [CrossRef]
- Jakovljevic, A.; Miletic, M.; Nikolic, N.; Beljic-Ivanovic, K.; Andric, M.; Milasin, J. Notch signaling pathway mediates alveolar bone resorption in apical periodontitis. Med. Hypotheses 2019, 124, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Jakovljevic, A.; Nikolic, N.; Carkic, J.; Andric, M.; Miletic, M.; Beljic-Ivanovic, K.; Jovanovic, T.; Milasin, J. Notch-a possible mediator between Epstein-Barr virus infection and bone resorption in apical periodontitis. Acta Odontol. Scand. 2020, 78, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Hausen, H.Z.; Schulte-Holthausen, H.; Klein, G.; Henle, W.; Henle, G.; Clifford, P.; Santesson, L. Epstein-Barr virus in Burkitt’s lymphoma and nasopharyngeal carcinoma: EBV DNA in biopsies of Burkitt tumours and anaplastic carcinomas of the nasopharynx. Nature 1970, 228, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-C.; Wang, L.-J.; Tsang, N.-M.; Ojcius, D.M.; Chen, C.-C.; Ouyang, C.-N.; Hsueh, C.; Liang, Y.; Chang, K.-P.; Chen, C.-C.; et al. Tumour inflammasome-derived IL-1β recruits neutrophils and improves local recurrence-free survival in EBV-induced nasopharyngeal carcinoma. EMBO Mol. Med. 2012, 4, 1276–1293. [Google Scholar] [CrossRef]
- Gregory, S.M.; Davis, B.K.; West, J.A.; Taxman, D.J.; Matsuzawa, S.-I.; Reed, J.C.; Ting, J.P.Y.; Damania, B. Discovery of a viral NLR homolog that inhibits the inflammasome. Science 2011, 331, 330–334. [Google Scholar] [CrossRef]
- He, Q.; Fu, Y.; Tian, D.; Yan, W. The contrasting roles of inflammasomes in cancer. Am. J. Cancer Res. 2018, 8, 566–583. [Google Scholar]
- Wetzel, S.L.; Wollenberg, J. Oral Potentially Malignant Disorders. Dent. Clin. N. Am. 2020, 64, 25–37. [Google Scholar] [CrossRef]
- Pindborg, J.J.; Reichart, P.; Smith, C.; Van der Waal, I. Histological Classification of Cancer and Precancer of the Oral Mucosa. In Histological Typing of Cancer and Precancer of the Oral Mucosa; In Collaboration with LH Sobin and Pathologists in 9 Countries; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Mignogna, M.D.; Fedele, S.; Lo Russo, L.; Lo Muzio, L.; Bucci, E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: Is there any evidence? Oral Oncol. 2004, 40, 120–130. [Google Scholar] [CrossRef]
- Simark-Mattsson, C.; Bergenholtz, G.; Jontell, M.; Eklund, C.; Seymour, G.; Sugerman, P.; Savage, N.; Dahlgren, U. Distribution of interleukin-2, -4, -10, tumour necrosis factor-α and transforming growth factor-β mRNAs in oral lichen planus. Arch. Oral Biol. 1999, 44, 499–507. [Google Scholar] [CrossRef]
- Sohn, K.-C.; Lee, E.J.; Shin, J.-M.; Lim, E.-H.; No, Y.; Lee, J.Y.; Yoon, T.Y.; Lee, Y.H.; Im, M.; Lee, Y.; et al. Regulation of keratinocyte differentiation by O-GlcNAcylation. J. Dermatol. Sci. 2014, 75, 10–15. [Google Scholar] [CrossRef]
- Thi Do, T.; Phoomak, C.; Champattanachai, V.; Silsirivanit, A.; Chaiyarit, P. New evidence of connections between increased O-GlcNAcylation and inflammasome in the oral mucosa of patients with oral lichen planus. Clin. Exp. Immunol. 2018, 192, 129–137. [Google Scholar] [CrossRef]
- Chaiyarit, P.; Kafrawy, A.H.; Miles, D.A.; Zunt, S.L.; Van Dis, M.L.; Gregory, R.L. Oral lichen planus: An immunohistochemical study of heat shock proteins (HSPs) and cytokeratins (CKs) and a unifying hypothesis of pathogenesis. J. Oral Pathol. Med. 1999, 28, 210–215. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 inflammasome: An overview of mechanisms of activation and regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Svolaki, I.P.; Evangelou, K.; Gorgoulis, V.G.; Manoussakis, M.N. Cell-autonomous epithelial activation of AIM2 (absent in melanoma-2) inflammasome by cytoplasmic DNA accumulations in primary Sjögren’s syndrome. J. Autoimmun. 2020, 108, 102381. [Google Scholar] [CrossRef] [PubMed]
- Antiochos, B.; Matyszewski, M.; Sohn, J.; Casciola-Rosen, L.; Rosen, A. IFI16 filament formation in salivary epithelial cells shapes the anti-IFI16 immune response in Sjögren’s syndrome. Jci Insight 2019, 3, 120179. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.-S.; Liu, F.-C.; Wang, C.-H.; Chen, H.-C. Unusual cancer in primary Sjögren syndrome. Can. Fam. Physician 2014, 60, 912–915. [Google Scholar] [PubMed]
- Liang, L.; Tan, X.; Zhou, Q.; Zhu, Y.; Tian, Y.; Yu, H.; Kijlstra, A.; Yang, P. IL-1β triggered by peptidoglycan and lipopolysaccharide through TLR2/4 and ROS-NLRP3 inflammasome-dependent pathways is involved in ocular Behçet’s disease. Investig. Ophthalmol. Vis. Sci. 2013, 54, 402–414. [Google Scholar] [CrossRef]
- Giuroiu, C.L.; Vataman, M.; Melian, G.; Bularda, D.; Lozneanu, L.; Salceanu, M.; Patrascu, A.; Andrian, S.; Melian, A. Detection and assessment of interleukin 6 in irreversible pulp inflamation. Rev. Chim. 2017, 68, 323–327. [Google Scholar] [CrossRef]
- Mazurek-Mochol, M.; Bonsmann, T.; Mochol, M.; Poniewierska-Baran, A.; Pawlik, A. The role of interleukin 6 in periodontitis and its complications. Int. J. Mol. Sci. 2024, 25, 2146. [Google Scholar] [CrossRef]
- Chundru, V.N.S.; Madhavan, R.N.; Chintala, L.; Boyapati, R.; Srikar, M. Evaluation of salivary biomarker interleukin-6 in oral squamous cell carcinoma and oral potentially malignant disorders—A comparative cross-sectional South Indian study. J. Oral Maxillofac. Pathol. 2024, 28, 387–392. [Google Scholar] [CrossRef]
- Xiao, L.; Li, X.; Cao, P.; Fei, W.; Zhou, H.; Tang, N.; Liu, Y. Interleukin-6 mediated inflammasome activation promotes oral squamous cell carcinoma progression via JAK2/STAT3/Sox4/NLRP3 signaling pathway. J. Exp. Clin. Cancer Res. 2022, 41, 166. [Google Scholar] [CrossRef] [PubMed]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J. Endod. 2014, 40, S46–S51. [Google Scholar] [CrossRef] [PubMed]
- Yucel-Lindberg, T.; Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pezzi, M.E.; Cassi, D.; Pertinhez, T.A.; Spisni, A.; Meleti, M. Salivary cytokines as biomarkers for oral squamous cell carcinoma: A systematic review. Int. J. Mol. Sci. 2021, 22, 6795. [Google Scholar] [CrossRef]
- Arora, S.; Cooper, P.R.; Friedlander, L.T.; Rizwan, S.; Seo, B.; Rich, A.M.; Hussaini, H.M. Potential application of immunotherapy for modulation of pulp inflammation: Opportunities for vital pulp treatment. Int. Endod. J. 2021, 54, 1263–1274. [Google Scholar] [CrossRef]
- Brierly, G.; Celentano, A.; Breik, O.; Moslemivayeghan, E.; Patini, R.; McCullough, M.; Yap, T. Tumour necrosis factor alpha (TNF-α) and oral squamous cell carcinoma. Cancers 2023, 15, 1841. [Google Scholar] [CrossRef]
- Kawashima, N.; Okiji, T. Characteristics of inflammatory mediators in dental pulp inflammation and the potential for their control. Front. Dent. Med. 2024, 5, 1426887. [Google Scholar] [CrossRef]
- Alarcón-Sánchez, M.A.; Romero-Castro, N.S.; Becerra-Ruiz, J.S.; Romero-Servin, S.; Heboyan, A. Increased of IL-18 levels are associated with periodontitis: A systematic review and meta-analysis. BMC Oral Health 2024, 24, 981. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, X.; Zhu, N.; Zhao, M.; Hu, Q.; Ni, Y. The balance of serum IL-18/IL-37 levels is disrupted during the development of oral squamous cell carcinoma. Surg. Oncol. 2020, 32, 99–107. [Google Scholar] [CrossRef]
- Kritikou, K.; Greabu, M.; Imre, M.; Miricescu, D.; Totan, A.R.; Burcea, M.; Stanescu-Spinu, I.-I.; Spinu, T. ILs and MMPs levels in inflamed human dental pulp: A systematic review. Molecules 2021, 26, 4129. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic inflammation as a link between periodontitis and carcinogenesis. Mediat. Inflamm. 2019, 2019, 1029857. [Google Scholar] [CrossRef] [PubMed]
- Manek, P.; Singh, V.; Sisodia, G.S.; Tomar, I.; Abraham, D.; Agarwal, P. Role of salivary MMP-9 in OSCC detection and diagnosis: A comprehensive clinical assessment. Bioinformation 2025, 21, 1680–1682. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.; Ahmed, S.S.; Ali, A.; Khan, F.A.; Zulfiqar, G.; Iqbal, J.; Khan, A.A.; Shoaib, M. Salivary immunosuppressive cytokines IL-10 and IL-13 are significantly elevated in oral squamous cell carcinoma patients. Cancer Investig. 2015, 33, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Young Bae, J.; Lee, S.-W.; Shin, Y.-H.; Lee, J.-H.; Won Jahng, J.; Park, K. P2X7 receptor and NLRP3 inflammasome activation in head and neck cancer. Oncotarget 2017, 8, 48972–48982. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Casili, G.; Basilotta, R.; Lanza, M.; Filippone, A.; Raciti, G.; Puliafito, I.; Colarossi, L.; Esposito, E.; Paterniti, I. NLRP3 Inflammasome Inhibitor BAY-117082 Reduces Oral Squamous Cell Carcinoma Progression. Int. J. Mol. Sci. 2021, 22, 11108. [Google Scholar] [CrossRef]
- Hu, B.; Jin, C.; Li, H.-B.; Tong, J.; Ouyang, X.; Cetinbas, N.M.; Zhu, S.; Strowig, T.; Lam, F.C.; Zhao, C.; et al. The DNA-sensing AIM2 inflammasome controls radiation-induced cell death and tissue injury. Science 2016, 354, 765–768. [Google Scholar] [CrossRef]
- Lian, Q.; Xu, J.; Yan, S.; Huang, M.; Ding, H.; Sun, X.; Bi, A.; Ding, J.; Sun, B.; Geng, M. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res. 2017, 27, 784–800. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Multani, S.; Dabholkar, J.; Saranath, D. Whole genome expression profiling in chewing-tobacco-associated oral cancers: A pilot study. Med. Oncol. 2015, 32, 60. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakahata, S.; Kondo, Y.; Izumi, A.; Yamamoto, K.; Ichikawa, T.; Tamura, T.; Noumi, K.; Yamashita, Y.; Morishita, K. Overexpression of absent in melanoma 2 in oral squamous cell carcinoma contributes to tumor progression. Biochem. Biophys. Res. Commun. 2019, 509, 82–88. [Google Scholar] [CrossRef]
- Riva, G.; Pecorari, G.; Biolatti, M.; Pautasso, S.; Cigno, I.L.; Garzaro, M.; Dell’oSte, V.; Landolfo, S. PYHIN genes as potential biomarkers for prognosis of human papillomavirus-positive or-negative head and neck squamous cell carcinomas. Mol. Biol. Rep. 2019, 46, 3333–3347. [Google Scholar] [CrossRef]
- Ponomareva, L.; Liu, H.; Duan, X.; Dickerson, E.; Shen, H.; Panchanathan, R.; Choubey, D. AIM2, an IFN-Inducible Cytosolic DNA Sensor, in the Development of Benign Prostate Hyperplasia and Prostate Cancer. Mol. Cancer Res. 2013, 11, 1193–1202. [Google Scholar] [CrossRef]
- E Wilson, J.; Petrucelli, A.S.; Chen, L.; Koblansky, A.A.; Truax, A.D.; Oyama, Y.; Rogers, A.B.; Brickey, W.J.; Wang, Y.; Schneider, M.; et al. Inflammasome-independent role of AIM2 in suppressing colon tumorigenesis via DNA-PK and Akt. Nat. Med. 2015, 21, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; A Fitzgerald, K.; et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Anti-apoptotic role of the transcription factor NF-κb. Adv. Cell Aging Gerontol. 2001, 5, 269–295. [Google Scholar] [CrossRef]
- Greten, F.R.; Eckmann, L.; Greten, T.F.; Park, J.M.; Li, Z.-W.; Egan, L.J.; Kagnoff, M.F.; Karin, M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004, 118, 285–296. [Google Scholar] [CrossRef]
- Furuta, H.; Osawa, K.; Shin, M.; Ishikawa, A.; Matsuo, K.; Khan, M.; Aoki, K.; Ohya, K.; Okamoto, M.; Tominaga, K.; et al. Selective inhibition of NF-κB suppresses bone invasion by oral squamous cell carcinoma in vivo. Int. J. Cancer 2012, 131, E625–E635. [Google Scholar] [CrossRef]
- Yan, M.; Xu, Q.; Zhang, P.; Zhou, X.-J.; Zhang, Z.-Y.; Chen, W.-T. Correlation of NF-κB signal pathway with tumor metastasis of human head and neck squamous cell carcinoma. BMC Cancer 2010, 10, 437. [Google Scholar] [CrossRef]
- Rao, S.K.; Pavicevic, Z.; Du, Z.; Kim, J.-G.; Fan, M.; Jiao, Y.; Rosebush, M.; Samant, S.; Gu, W.; Pfeffer, L.M.; et al. Pro-inflammatory Genes as Biomarkers and Therapeutic Targets in Oral Squamous Cell Carcinoma. J. Biol. Chem. 2010, 285, 32512–32521. [Google Scholar] [CrossRef]
- Bharti, A.C.; Aggarwal, B.B. Nuclear factor-kappa B and cancer: Its role in prevention and therapy. Biochem. Pharmacol. 2002, 64, 883–888. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Cretney, E.; Kershaw, M.H.; Hayakawa, Y. Cytokines in cancer immunity and immunotherapy. Immunol. Rev. 2004, 202, 275–293. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Bertocchi, F.; Bortolami, M.C.; Regonesi, A.; Tonta, A.; Breviario, F.; Giavazzi, R. Interleukin 1 promotes tumor cell adhesion to cultured human endothelial cells. J. Clin. Investig. 1988, 82, 1466. [Google Scholar] [CrossRef] [PubMed]
- Bani, M.R.; Garofalo, A.; Scanziani, E.; Giavazzi, R. Effect of Interleukin-1-beta on Metastasis Formation in Different Tumor Systems. JNCI J. Natl. Cancer Inst. 1991, 83, 119–123. [Google Scholar] [CrossRef]
- Wu, T.; Hong, Y.; Jia, L.; Wu, J.; Xia, J.; Wang, J.; Hu, Q.; Cheng, B. Modulation of IL-1β reprogrammes the tumor microenvironment to interrupt oral carcinogenesis. Sci. Rep. 2016, 6, 20208. [Google Scholar] [CrossRef]
- Rébé, C.; Ghiringhelli, F. Interleukin-1β and cancer. Cancers 2020, 12, 1791. [Google Scholar] [CrossRef]
- Lopez-Labady, J.; Bologna-Molina, R.; Villarroel-Dorrego, M. Expression of interleukin-1ß and interleukin-8 in oral potentially malignant disorders and carcinomas. Front. Oral Health 2021, 2, 649406. [Google Scholar] [CrossRef]
- Mahale, A.; Routholla, G.; Lavanya, S.; Sharma, P.; Ghosh, B.; Kulkarni, O.P. Pharmacological blockade of HDAC6 attenuates cancer progression by inhibiting IL-1β and modulating immunosuppressive response in OSCC. Int. Immunopharmacol. 2024, 132, 111921. [Google Scholar] [CrossRef]
- Kamatani, T.; Shiogama, S.; Yoshihama, Y.; Kondo, S.; Shirota, T.; Shintani, S. Interleukin-1 beta in unstimulated whole saliva is a potential biomarker for oral squamous cell carcinoma. Cytokine 2013, 64, 497–502. [Google Scholar] [CrossRef]

| Inflammatory Mediator | Chronic Pulpitis | Chronic Periodontitis | Oral Cancer |
|---|---|---|---|
| Interleukin-6 (IL-6) | Biomarker of chronic pulpitis [159]. | Proinflammatory in periodontitis, putative role in systemic sequel in periodontitis [160]. | Elevated in the saliva of patients diagnosed with OSCC [161]. Promotes tumour growth, angiogenesis [162] |
| Interleukin-8 (IL-8) | Neutrophil chemoattractant and biomarker of chronic pulpitis [163]. | Induction of other inflammatory mediators. Increased in GCF in periodontitis [164] | Strong salivary biomarker; associated with poorer survival [165] |
| Tumour necrosis factor-alpha (TNF-α) | An early-stage mediator coordinates inflammatory responses in deeply carious dental pulps and deeper associated periapical infections [166]. | Key and early-stage mediator [63]. Activation of osteoclast in vitro [164]. | Elevated in saliva; TNF-α polymorphisms increase OSCC risk. NF-κB activation; promotes invasion [165,167] |
| Interleukin-18 (IL-18) | Pro-inflammatory mediator and increased in inflamed pulps [168]. | Significant increase in saliva, serum, and GCF in periodontitis, suggested as biomarker for periodontitis [169]. | Elevated levels of serum IL-18, a systemic biomarker for OSCC [170]. |
| Interleukin-1 beta (IL-1β) | The master regulator of cytokines; induces their expression of cytokines such as IL-8 [171]. | A key mediator of inflammation and early-stage mediator [63]. Increase MMP expression and subsequent bone resorption [58]. | Elevated in saliva of patients diagnosed with OSCC; a prognostic biomarker [165]. |
| Matrix metalloproteinase (MMP) | MMP-1, MMP-8, MMP9, and MMP-13 are biomarkers of chronic pulpitis [171]. | MMP-8, MMP-13 have increased levels in chronic periodontitis [58,164] | MMP-9 activated by P. gingivalis inducing cancer invasion and metastasis [172]. Increased levels in saliva of patients with OSCC [173]. |
| Interleukin-10 (IL-10) | Anti-inflammatory; inhibits the release of pro-inflammatory cytokines [171] | Anti-inflammatory effect, suppression of osteoclastic activity [59] | Elevated in saliva/tissue; poor prognosis marker [174]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Natour, B.; Rasheed, I.; El Elkarim, I.A. The Role of Inflammasomes in Chronic Oral Inflammatory Disease and Oral Cancer: A Narrative Review. Dent. J. 2025, 13, 609. https://doi.org/10.3390/dj13120609
Al-Natour B, Rasheed I, El Elkarim IA. The Role of Inflammasomes in Chronic Oral Inflammatory Disease and Oral Cancer: A Narrative Review. Dentistry Journal. 2025; 13(12):609. https://doi.org/10.3390/dj13120609
Chicago/Turabian StyleAl-Natour, Banan, Issam Rasheed, and Ikhlas A. El Elkarim. 2025. "The Role of Inflammasomes in Chronic Oral Inflammatory Disease and Oral Cancer: A Narrative Review" Dentistry Journal 13, no. 12: 609. https://doi.org/10.3390/dj13120609
APA StyleAl-Natour, B., Rasheed, I., & El Elkarim, I. A. (2025). The Role of Inflammasomes in Chronic Oral Inflammatory Disease and Oral Cancer: A Narrative Review. Dentistry Journal, 13(12), 609. https://doi.org/10.3390/dj13120609







