Potential Antimicrobial Use of Cannabidiol in Dentistry: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Criteria Used for the Mapping Process
2.3. Eligibility Criteria
2.4. Information Sources and Search
2.5. Selection of Sources of Evidence
2.6. Data Extraction Process and Data Items
2.7. Critical Appraisal of Individual Sources of Evidence
2.8. Strategy for Data Synthesis
3. Results
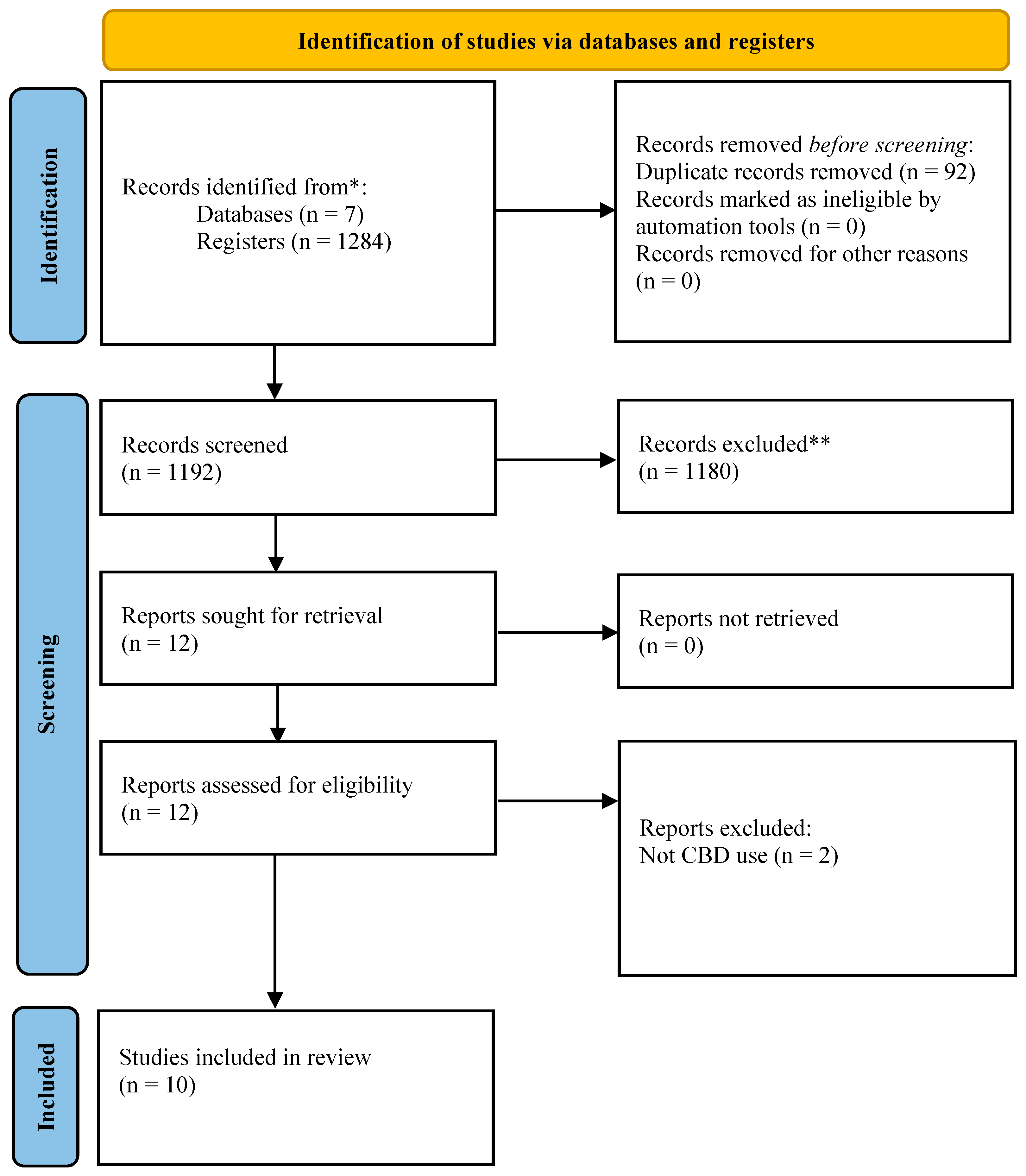
3.1. Results-Selection of Sources of Evidence
3.2. Characteristics of Sources of Evidence
3.3. Critical Appraisal Within Sources of Evidence
3.4. Synthesis of Results and Summary of Evidence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CBD | Cannabidiol |
| CBDA | Cannabidiolic acid |
| CFUs | Colony-forming units |
| CHX | Chlorhexidine |
| CLI | Clindamycin |
| OFX | Ofloxacin |
| MEM | Meropenem |
| TOB | Tobramycin |
| DCT | Direct contact test |
| PBS | Phosphate-buffered saline |
| IH | Inhibition halo |
| DLBM | Dilution in Luria–Bertani medium |
| DMSO | Dimethyl sulfoxide |
| MIC | Minimum inhibitory concentration |
| MO | Microorganism |
| PMMA | Polymethylmethacrylate |
References
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. In Microbiota of the Human Body; Schwiertz, A., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2016; Volume 902, pp. 45–60. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Tahmasebi, E.; Yazdanian, A.; Rezvani, M.B.; Seifalian, A.; Yazdanian, M.; Tebyanian, H. Oral microbial biofilms: An update. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2005–2019. [Google Scholar] [CrossRef]
- Rowińska, I.; Szyperska-Ślaska, A.; Zariczny, P.; Pasławski, R.; Kramkowski, K.; Kowalczyk, P. The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity. Materials 2021, 14, 1444. [Google Scholar] [CrossRef]
- Struzycka, I. The oral microbiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef]
- Marsh, P.D.; Head, D.A.; Devine, D.A. Ecological approaches to oral biofilms: Control without killing. Caries Res. 2015, 49 (Suppl. S1), 46–54. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 28. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, M.; Tian, J.; Lei, L.; Huang, R. Novel antimicrobial agents targeting the Streptococcus mutans biofilms discovery through computer technology. Front. Cell. Infect. Microbiol. 2022, 12, 1065235. [Google Scholar] [CrossRef]
- Sreenivasan, P.K.; Haraszthy, V.I.; Zambon, J.J. Antimicrobial efficacy of 0.05% cetylpyridinium chloride mouthrinses. Lett. Appl. Microbiol. 2013, 56, 14–20. [Google Scholar] [CrossRef]
- Sethi, K.S.; Karde, P.A.; Joshi, C.P. Comparative evaluation of sutures coated with triclosan and chlorhexidine for oral biofilm inhibition potential and antimicrobial activity against periodontal pathogens: An in vitro study. Indian J. Dent. Res. 2016, 27, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Quintas, V.; Prada-López, I.; Prados-Frutos, J.C.; Tomás, I. In situ antimicrobial activity on oral biofilm: Essential oils vs. 0.2 % chlorhexidine. Clin. Oral Investig. 2015, 19, 97–107. [Google Scholar] [CrossRef]
- Aljaffary, M.; Jang, H.; Alomeir, N.; Zeng, Y.; Alkhars, N.; Vasani, S.; Almulhim, A.; Wu, T.T.; Quataert, S.; Bruno, J.; et al. Effects of Nystatin oral rinse on oral Candida species and Streptococcus mutans among healthy adults. Clin. Oral. Investig. 2023, 27, 3557–3568. [Google Scholar] [CrossRef] [PubMed]
- Alkhars, N.; Gaca, A.; Zeng, Y.; Al-Jallad, N.; Rustchenko, E.; Wu, T.T.; Eliav, E.; Xiao, J. Antifungal Susceptibility of Oral Candida Isolates from Mother-Infant Dyads to Nystatin, Fluconazole, and Caspofungin. J. Fungi 2023, 9, 580. [Google Scholar] [CrossRef]
- Poppolo Deus, F.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Hepsø, H.U.; Bjørnland, T.; Skoglund, L.A. Side-effects and patient acceptance of 0.2% versus 0.1% chlorhexidine used as post-operative prophylactic mouthwash. Int. J. Oral Maxillofac. Surg. 1988, 17, 17–20. [Google Scholar] [CrossRef] [PubMed]
- García-Cuesta, C.; Sarrion-Pérez, M.G.; Bagán, J.V. Current treatment of oral candidiasis: A literature review. J. Clin. Exp. Dent. 2014, 6, e576–e582. [Google Scholar] [CrossRef]
- Nordin, R.; Roslan, M.A.; Fathilah, A.R.; Ngui, R.; Musa, S. Evaluation of in vitro antifungal effects of synthetic and herbal mouth rinses on oral Candida albicans and Candida glabrata. Trop. Biomed. 2022, 39, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Flores, M.A.; Quintero-Cabello, K.P.; Palafox-Rivera, P.; Silva-Espinoza, B.A.; Cruz-Valenzuela, M.R.; Ortega-Ramirez, L.A.; Gonzalez-Aguilar, G.A.; Ayala-Zavala, J.F. Plant-Derived Substances with Antibacterial, Antioxidant, and Flavoring Potential to Formulate Oral Health Care Products. Biomedicines 2021, 9, 1669. [Google Scholar] [CrossRef]
- Gupta, A.K.; Talukder, M. Cannabinoids for skin diseases and hair regrowth. J. Cosmet. Dermatol. 2021, 20, 2703–2711. [Google Scholar] [CrossRef]
- Mahmud, M.S.; Hossain, M.S.; Ahmed, A.T.M.F.; Islam, M.Z.; Sarker, M.E.; Islam, M.R. Antimicrobial and Antiviral (SARS-CoV-2) Potential of Cannabinoids and Cannabis sativa: A Comprehensive Review. Molecules 2021, 26, 7216. [Google Scholar] [CrossRef]
- David, C.; Elizalde-Hernández, A.; Barboza, A.S.; Cardoso, G.C.; Santos, M.B.F.; Moraes, R.R. Cannabidiol in Dentistry: A Scoping Review. Dent. J. 2022, 10, 193. [Google Scholar] [CrossRef]
- Mederos, M.; Francia, A. Cannabinoid medicine in the orofacial region: Status and prospects. Odontol. Sanmarquina 2023, 26, e26154. [Google Scholar] [CrossRef]
- Barak, T.; Sharon, E.; Steinberg, D.; Feldman, M.; Sionov, R.V.; Shalish, M. Anti-Bacterial Effect of Cannabidiol against the Cariogenic Streptococcus mutans Bacterium: An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 15878. [Google Scholar] [CrossRef]
- Avraham, M.; Steinberg, D.; Barak, T.; Shalish, M.; Feldman, M.; Sionov, R.V. Improved Anti-Biofilm Effect against the Oral Cariogenic Streptococcus mutans by Combined Triclosan/CBD Treatment. Biomedicines 2023, 11, 521. [Google Scholar] [CrossRef]
- Torabi, J.; Luis, H.P.S.; Mkrtchyan, G.; Alavijeh, S.D.; Dezfoli, S.; Hurlbutt, M. Antimicrobial Effects of Cannabidiol (CBD)-infused Lozenges against Streptococcus mutans in Oral Health. Braz. Dent. J. 2024, 35, e24-5988. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Peters, M.D.; Godfrey, C.M.; Khalil, H.; McInerney, P.; Parker, D.; Soares, C.B. Guidance for conducting systematic scoping reviews. Int. J. Evid.-Based Healthc. 2015, 13, 141–146. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.H.; Sauro, S.; Lima, A.F.; Loguercio, A.D.; Della Bona, A.; Mazzoni, A.; Collares, F.M.; Staxrud, F.; Ferracane, J.; Tsoi, J.; et al. RoBDEMAT: A risk of bias tool and guideline to support reporting of pre-clinical dental materials research and assessment of systematic reviews. J. Dent. 2022, 127, 104350. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Orive, G.; Desimone, M.F. Design of a New 3D Gelatin-Alginate Scaffold Loaded with Cannabis sativa Oil. Polymers 2022, 14, 4506. [Google Scholar] [CrossRef]
- David, C.; de Souza, J.F.; Silva, A.F.; Grazioli, G.; Barboza, A.S.; Lund, R.G.; Fajardo, A.R.; Moraes, R.R. Cannabidiol-loaded microparticles embedded in a porous hydrogel matrix for biomedical applications. J. Mater. Sci. Mater. Med. 2024, 35, 14. [Google Scholar] [CrossRef]
- Gu, Z.; Singh, S.; Niyogi, R.G.; Lamont, G.J.; Wang, H.; Lamont, R.J.; Scott, D.A. Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front. Immunol. 2019, 10, 2288. [Google Scholar] [CrossRef] [PubMed]
- Jirasek, P.; Jusku, A.; Frankova, J.; Urbankova, M.; Diabelko, D.; Ruzicka, F.; Papouskova, B.; Chytilova, K.; Vrba, J.; Havlasek, J.; et al. Phytocannabinoids and gingival inflammation: Preclinical findings and a placebo-controlled double-blind randomized clinical trial with cannabidiol. J. Periodontal Res. 2024, 59, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Martinenghi, L.D.; Jønsson, R.; Lund, T.; Jenssen, H. Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. Biomolecules 2020, 10, 900. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Lamont, G.J.; Sekula, M.; Hong, H.; Sloan, L.; Scott, D.A. The transcriptomic response to cannabidiol of Treponema denticola, a phytocannabinoid-resistant periodontal pathogen. J. Clin. Periodontol. 2024, 51, 222–232. [Google Scholar] [CrossRef]
- Vasudevan, K.; Stahl, V. Cannabinoids infused mouthwash products are as effective as chlorhexidine on inhibition of total-culturable bacterial content in dental plaque samples. J. Cannabis Res. 2020, 2, 20. [Google Scholar] [CrossRef]
- Santos, A.L.O.; Santiago, M.B.; Silva, N.B.S.; Souza, S.L.; Almeida, J.M.D.; Martins, C.H.G. The antibacterial and antibiofilm role of cannabidiol against periodontopathogenic bacteria. J. Appl. Microbiol. 2024, 136, lxae316. [Google Scholar] [CrossRef]
- Garzón, H.S.; Loaiza-Oliva, M.; Martínez-Pabón, M.C.; Puerta-Suárez, J.; Téllez Corral, M.A.; Bueno-Silva, B.; Suárez, D.R.; Díaz-Báez, D.; Suárez, L.J. Antibiofilm and Immune-Modulatory Activity of Cannabidiol and Cannabigerol in Oral Environments—In Vitro Study. Antibiotics 2024, 13, 342. [Google Scholar] [CrossRef]
- Feldman, M.; Gati, I.; Sionov, R.V.; Sahar-Helft, S.; Friedman, M.; Steinberg, D. Potential Combinatory Effect of Cannabidiol and Triclosan Incorporated into Sustained Release Delivery System against Oral Candidiasis. Pharmaceutics 2022, 14, 1624. [Google Scholar] [CrossRef]
- Aqawi, M.; Sionov, R.V.; Gallily, R.; Friedman, M.; Steinberg, D. Anti-Bacterial Properties of Cannabigerol Toward Streptococcus mutans. Front. Microbiol. 2021, 12, 656471. [Google Scholar] [CrossRef]
- Niyangoda, D.; Aung, M.L.; Qader, M.; Tesfaye, W.; Bushell, M.; Chiong, F.; Tsai, D.; Ahmad, D.; Samarawickrema, I.; Sinnollareddy, M.; et al. Cannabinoids as Antibacterial Agents: A Systematic and Critical Review of In Vitro Efficacy Against Streptococcus and Staphylococcus. Antibiotics 2024, 13, 1023. [Google Scholar] [CrossRef]
- Sionov, R.V.; Steinberg, D. Anti-Microbial Activity of Phytocannabinoids and Endocannabinoids in the Light of Their Physiological and Pathophysiological Roles. Biomedicines 2022, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Clinical Laboratory Standard Institute (CLSI). Methods for Determining Bactericidal Activity of Antimicrobial Agents; Approved Guideline; CLSI Document M26-A; Clinical Laboratory Standard Institute: Wayne, PA, USA, 1999; Reaffirmed 2015. [Google Scholar]
- Stahl, V.; Vasudevan, K. Comparison of Efficacy of Cannabinoids versus Commercial Oral Care Products in Reducing Bacterial Content from Dental Plaque: A Preliminary Observation. Cureus 2020, 12, e6809. [Google Scholar] [CrossRef]
- Manikrao, D.L.N.; Bhalchim, D.S.; Tiwari, D.H.; Pendyala, D.S.K.; Kondreddy, D.K.; Chandra, D.J.; Landge, N.; Rahul, V.C.; Tiwari, R.V. Efficacy in reducing bacterial content in oral cavity by cannabinoids in oral care products-a comparative study. Eur. J. Mol. Clin. Med. 2021, 7, 3028–3034. [Google Scholar]
- Newman, T.; Krishnan, L.P.; Lee, J.; Adami, G.R. Microbiomic differences at cancer-prone oral mucosa sites with marijuana usage. Sci. Rep. 2019, 9, 12697. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Steinberg, D.; Jabbour, A. The impact of medical cannabis consumption on the oral flora and saliva. PLoS ONE 2021, 16, e0247044. [Google Scholar] [CrossRef] [PubMed]
- Bahraminia, M.; Cui, S.; Zhang, Z.; Semlali, A.; Le Roux, É.; Giroux, K.A.; Lajoie, C.; Béland, F.; Rouabhia, M. Effect of cannabidiol (CBD), a cannabis plant derivative, against Candida albicans growth and biofilm formation. Can. J. Microbiol. 2025, 71, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.; Lavorgna, M.; Nugnes, R.; Orlo, E.; Isidori, M. Comparative assessment of antimicrobial, antiradical and cytotoxic activities of cannabidiol and its propyl analogue cannabidivarin. Sci. Rep. 2021, 11, 22494. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Matewele, P.; Awamaria, B.; Kraev, I.; Warde, P.; Mastroianni, G.; Nunn, A.V.; Guy, G.W.; Bell, J.D.; Inal, J.M.; et al. Cannabidiol is a Novel Modulator of Bacterial Membrane Vesicles. Front. Cell. Infect. Microbiol. 2019, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Basavaraju, M.; Sisnity, V.S.; Palaparthy, R.; Addanki, P.K. Quorum quenching: Signal jamming in dental plaque biofilms. J. Dent. Sci. 2016, 11, 349–352. [Google Scholar] [CrossRef]
- Kajjari, S.; Vanishree, B.K.; Janardhanan, S.; Patil, V.H.; Uppin, C.; Hugar, S.M. Antimicrobial Efficacy of Mangifera indica, Mentha arvensis, and Chlorhexidine Mouthwashes on Streptococcus mutans and Candida albicans in Children: A Comparative In Vivo Study. Int. J. Clin. Pediatr. Dent. 2024, 17 (Suppl. S1), S78–S83. [Google Scholar] [CrossRef]
- Jităreanu, A.; Trifan, A.; Vieriu, M.; Caba, I.-C.; Mârțu, I.; Agoroaei, L. Current Trends in Toxicity Assessment of Herbal Medicines: A Narrative Review. Processes 2023, 11, 83. [Google Scholar] [CrossRef]
- Sobieraj, J.; Strzelecka, K.; Sobczak, M.; Oledzka, E. How Biodegradable Polymers Can be Effective Drug Delivery Systems for Cannabinoids? Prospectives and Challenges. Int. J. Nanomed. 2024, 19, 4607–4649. [Google Scholar] [CrossRef] [PubMed]
- Söpper, U.; Hoffmann, A.; Daniels, R. Mucoadhesion and Mucopenetration of Cannabidiol (CBD)-Loaded Mesoporous Carrier Systems for Buccal Drug Delivery. Sci. Pharm. 2021, 89, 35. [Google Scholar] [CrossRef]
- Monou, P.K.; Mamaligka, A.M.; Tzimtzimis, E.K.; Tzetzis, D.; Vergkizi-Nikolakaki, S.; Vizirianakis, I.S.; Andriotis, E.G.; Eleftheriadis, G.K.; Fatouros, D.G. Fabrication and Preliminary In Vitro Evaluation of 3D-Printed Alginate Films with Cannabidiol (CBD) and Cannabigerol (CBG) Nanoparticles for Potential Wound-Healing Applications. Pharmaceutics 2022, 14, 1637. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, J.; Sánchez-Martín, M.J.; Valiente, M. Efficient controlled release of cannabinoids loaded in γ-CD-MOFs and DPPC liposomes as novel delivery systems in oral health. Microchim. Acta 2023, 190, 125. [Google Scholar] [CrossRef]
- Tahsin, K.; Xu, W.; Watson, D.; Rizkalla, A.; Charpentier, P. Antimicrobial Denture Material Synthesized from Poly(methyl methacrylate) Enriched with Cannabidiol Isolates. Molecules 2025, 30, 943. [Google Scholar] [CrossRef]
- Karygianni, L.; Al-Ahmad, A.; Argyropoulou, A.; Hellwig, E.; Anderson, A.C.; Skaltsounis, A.L. Natural Antimicrobials and Oral Microorganisms: A Systematic Review on Herbal Interventions for the Eradication of Multispecies Oral Biofilms. Front. Microbiol. 2016, 6, 1529. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Daliri, E.B.; Kim, N.; Kim, J.R.; Yoo, D.; Oh, D.H. Microbial Etiology and Prevention of Dental Caries: Exploiting Natural Products to Inhibit Cariogenic Biofilms. Pathogens 2020, 9, 569. [Google Scholar] [CrossRef]
- Jardim, J.J.; Alves, L.S.; Maltz, M. The history and global market of oral home-care products. Braz. Oral. Res. 2009, 23 (Suppl. S1), 17–22. [Google Scholar] [CrossRef]
- Morrison, C.; Gruenewald, P.J.; Freisthler, B.; Ponicki, W.R.; Remer, L.G. The economic geography of medical cannabis dispensaries in California. Int. J. Drug Policy 2014, 25, 508–515. [Google Scholar] [CrossRef]
- Myran, D.T.; Friesen, E.L.; Dickson, S.; Konikoff, L.; Arora, G.; Tanuseputro, P. Access to legal cannabis market in Canada over the four years following non-medical cannabis legalisation. Drug. Alcohol Rev. 2023, 42, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J. Canada’s Recreational Cannabis Legalization and Medical Cannabis Patient Activity, 2017–2022. Am. J. Public Health 2024, 114 (Suppl. S8), S673–S680. [Google Scholar] [CrossRef] [PubMed]
- Zaami, S.; Di Luca, A.; Di Luca, N.M.; Montanari Vergallo, G. Medical use of cannabis: Italian and European legislation. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- de Souza, M.R.; Henriques, A.T.; Limberger, R.P. Medical cannabis regulation: An overview of models around the world with emphasis on the Brazilian scenario. J. Cannabis Res. 2022, 4, 33. [Google Scholar] [CrossRef]
- Krcevski-Skvarc, N.; Wells, C.; Häuser, W. Availability and approval of cannabis-based medicines for chronic pain management and palliative/supportive care in Europe: A survey of the status in the chapters of the European Pain Federation. Eur. J. Pain. 2018, 22, 440–454. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
|
|
|
|
|
|
| |
| |
|
| Section | Terms |
|---|---|
| #1: Terms related to cannabis | (Cannabis) OR (Cannabidiol) OR (Medical Marijuana) OR (Marijuana, Medical) OR (Medicinal Cannabis) OR (Cannabis, Medicinal) OR (Marijuana Treatment) OR (Treatment, Marijuana) OR (Medicinal Marijuana) OR (Marijuana, Medicinal) OR (Medical Cannabis) OR (Cannabis, Medical) OR (Marijuana Dispensaries) OR (Dispensaries, Marijuana) |
| #2: Terms related to dentistry | (Dentistry) OR (Mouth) OR (Oral Health) OR (Dental) |
| #3: Terms related to antimicrobials and biofilms | (Antimicrobial) OR (Biofilms) OR (Fungi) OR (Mycoses) OR (Anti-Infective Agents) OR (Anti-Bacterial Agents) OR (Toothpastes) OR (Mouthwashes) |
| #1 AND #2 AND #3: Combined terms | All |
| Article Identification | Study Design | Type of Presentation of CBD | Evaluation Method | Contact Method | Control | MO | Time | Primary Outcome | Funding | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author and Year | Country | Title | Journal and Language | |||||||||
| Antezana 2022 [30] | Argentina | Design of a New 3D Gelatin—Alginate Scaffold Loaded with Cannabis sativa Oil | Polymers English | In vitro | Experimental—oil extract | DCT (the solid and the dilution method) | Direct (IH DLBM) | Group without CBD | S. aureus; E. coli | 12–24 h | Alginate scaffolds with CBD extracts were effective against the growth of S. aureus and E. coli. | CONICET, UBACYT, and PIDAE, Argentina |
| David 2024 [31] | Venezuela | Cannabidiol-loaded microparticles embedded in a porous hydrogel matrix for biomedical applications | J. Mater. Sci. Mater. Med. English | In vitro | Commercial—oil extract | DCT (dilution method) | Indirect (dilution in PBS) | Positive control: CHX Negative control: Distilled water | S. aureus | 504 h | Hydrogel groups with PLGA@CBD particles and PLGA@CBD particles alone were shown to be effective against S. aureus. | CAPES and FAPERGS, Brazil |
| Feldman 2022 [39] | Israel | Potential Combinatory Effect of Cannabidiol and Triclosan Incorporated into Sustained Release Delivery System against Oral Candidiasis | Pharmaceutics English | In vitro/ex vivo model | Commercial—N/S | Metabolic activity, total biomass, biofilm hyphae formation | Direct | Group without CBD and triclosan | C. albicans | 336 h | The sustained-release varnish group with the addition of CBD and triclosan demonstrated greater activity against the growth of C. albicans. | N/S |
| Garzón 2024 [38] | Colombia | Antibiofilm and Immune-Modulatory Activity of Cannabidiol and Cannabigerol in Oral Environments—In Vitro Study | Antibiotics English | In vitro | Commercial—powder | MIC Antibiofilm activity (2,3,5-triphenyltetrazolium chloride- TTC) | Indirect (Dilution in PBS) | Positive control: CHX amphotericin B and fluconazole | S. mutans; C. albicans; multispecies biofilm | MIC: 27 antibiofilm activity: 168 h | MIC for S. mutans with CBD was 20 μM. Multispecies biofilm metabolic activity was reduced by 50.38% with CBD at 125 μg/mL. | The Pontificia Universidad Javeriana, Colombia |
| Gu 2019 [32] | United States | Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens | Front Immunol. English | In vitro | Commercial—powder | CFU counting | Indirect (dilution in solvents) | Group without CBD | F. alocis; P. gingivalis; T. denticola | 40, 80, and 250 h | CBD suppressed the growth of P. gingivalis and F. alocis. | NIDCR, USA |
| Jirasek 2024 [33] | Czech Republic | Phytocannabinoids and gingival inflammation: Preclinical findings and a placebo-controlled double-blind randomized clinical trial with cannabidiol | J. Periodontal Res. English | Clinical/In vitro | Commercial—powder | MIC; CFU counting | Indirect (dilution in gel and toothpaste) | Positive control: CHX Group without CBD | P. gingivalis, S. mutans | 168, 672, and 1344 h | MIC for P. gingivalis and S. mutans with CBD was 1.5 and 16 μg/mL, respectively. CBD decreasing trends in bacterial count. | CB21 Pharma Ltd., CBDepot Ltd., and PharmaCan Ltd., Czech Republic |
| Martinenghi 2020 [34] | Denmark | Isolation, Purification, and Antimicrobial Characterization of Cannabidiolic Acid and Cannabidiol from Cannabis sativa L. | Biomolecules English | In vitro | Experimental—oil extract | MIC | Dilution in methanol | Positive control: CLI, OFX, MEM, and TOB | S. aureus; S. epidermidis, E. coli, and P. aeruginosa | 24 h | CBD demonstrated a potent activity against Gram-positive bacteria with a minimal inhibitory concentration between 1 and 2 μg/mL. | The Danish Council for Independent Research, Denmark |
| Tan 2024 [35] | United States | The transcriptomic response to cannabidiol of Treponema denticola, a phytocannabinoid-resistant periodontal pathogen | J. Clin. Periodontol. English | In vitro | Commercial—powder | CFU counting; RNAseq analysis | Dilution in methanol | Group without CBD | T. denticola | 240 h | Growth of T. denticola was not influenced by exposure to various concentrations of CBD (0–10 μg/mL). | University of Louisville, USA |
| Vasudevan 2020 [36] | Belgium | Cannabinoids infused mouthwash products are as effective as chlorhexidine on inhibition of total-culturable bacterial content in dental plaque samples | J. Cannabis Res. English | Clinical/In vitro | Commercial—powder. | MIC; CFU counting | IH DLBM | Positive group: CHX | NS | 36 h | CBD-infused mouthwash products inhibit bacterial growth. | VLAIO Belgium/CannIBite bvba |
| Santos 2024 [37] | Brazil | The antibacterial and antibiofilm role of cannabidiol against periodontopathogenic bacteria | J Appl Microbiol. English. | In vitro | Experimental—N/S | MIC; CFU counting | Dilution in DMSO | Positive group: CHX Group without CBD | A. naeslundii; P. anaerobius; V. parvula; F. nucleatum; A. actinomycetemcomitans | 24, 36, 48, 72, 96, and 168 h | MIC for CBD in the bacteria tested ranged from 0.39 to 3.12 μg/mL. CBD decreasing trends in bacterial count. | Ministry of Education of the Brazil (MEC) and EMBRAPII |
| Bias in Planning and Allocation | Bias in Sample/Specimen Preparation | Bias in Outcome Assessment | Bias in Data Treatment and Outcome Reporting | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control Group | Randomization of Samples | Sample Size Rationale and Reporting | Standardization of Samples and Materials | Identical Experimental Conditions Across Groups | Identical Experimental Conditions Across groups | Adequate and Standardized Testing Procedures and Outcome | Blinding of the Test Operator | Statistical Analysis | Reporting Study Outcomes | Total Criteria Met (%) | |
| Antezana, 2022 [30] | YES | NO | NO | YES | YES | YES | YES | NR | YES | YES | 70% |
| David, 2024 [31] | YES | NO | NO | YES | YES | YES | YES | NR | YES | YES | 70% |
| Feldman, 2022 [39] | YES | NO | NO | YES | YES | YES | YES | NR | YES | YES | 70% |
| Garzón, 2024 [38] | YES | NO | NO | YES | YES | YES | YES | NR | YES | YES | 70% |
| Gu, 2019 [32] | NO | NO | NO | YES | YES | YES | YES | NR | YES | YES | 60% |
| Jirasek, 2024 [33] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 100% |
| Martinenghi, 2020 [34] | YES | NO | NO | YES | YES | YES | YES | NR | NO | YES | 60% |
| Tan, 2024 [35] | YES | NO | NO | YES | YES | YES | YES | NR | NO | YES | 60% |
| Vasudevan, 2020 [36] | YES | NO | NO | YES | YES | YES | YES | NR | YES | YES | 70% |
| Santos, 2024 [37] | YES | NO | NO | YES | YES | YES | YES | NR | NR | YES | 60% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mederos, M.; Elizalde-Hernández, A.; Francia, A.; Chisini, L.A.; Isolan, C.P.; Moraes, R.R.; Lund, R.G.; David, C. Potential Antimicrobial Use of Cannabidiol in Dentistry: A Scoping Review. Dent. J. 2025, 13, 519. https://doi.org/10.3390/dj13110519
Mederos M, Elizalde-Hernández A, Francia A, Chisini LA, Isolan CP, Moraes RR, Lund RG, David C. Potential Antimicrobial Use of Cannabidiol in Dentistry: A Scoping Review. Dentistry Journal. 2025; 13(11):519. https://doi.org/10.3390/dj13110519
Chicago/Turabian StyleMederos, Matias, Alejandro Elizalde-Hernández, Alejandro Francia, Luiz Alexandre Chisini, Cristina Pereira Isolan, Rafael R. Moraes, Rafael Guerra Lund, and Carla David. 2025. "Potential Antimicrobial Use of Cannabidiol in Dentistry: A Scoping Review" Dentistry Journal 13, no. 11: 519. https://doi.org/10.3390/dj13110519
APA StyleMederos, M., Elizalde-Hernández, A., Francia, A., Chisini, L. A., Isolan, C. P., Moraes, R. R., Lund, R. G., & David, C. (2025). Potential Antimicrobial Use of Cannabidiol in Dentistry: A Scoping Review. Dentistry Journal, 13(11), 519. https://doi.org/10.3390/dj13110519






