Modified Roll Flap Soft-Tissue Augmentation at Single-Stage Implant Placement: A Digital-Scan–Verified Case Report
Abstract
1. Introduction
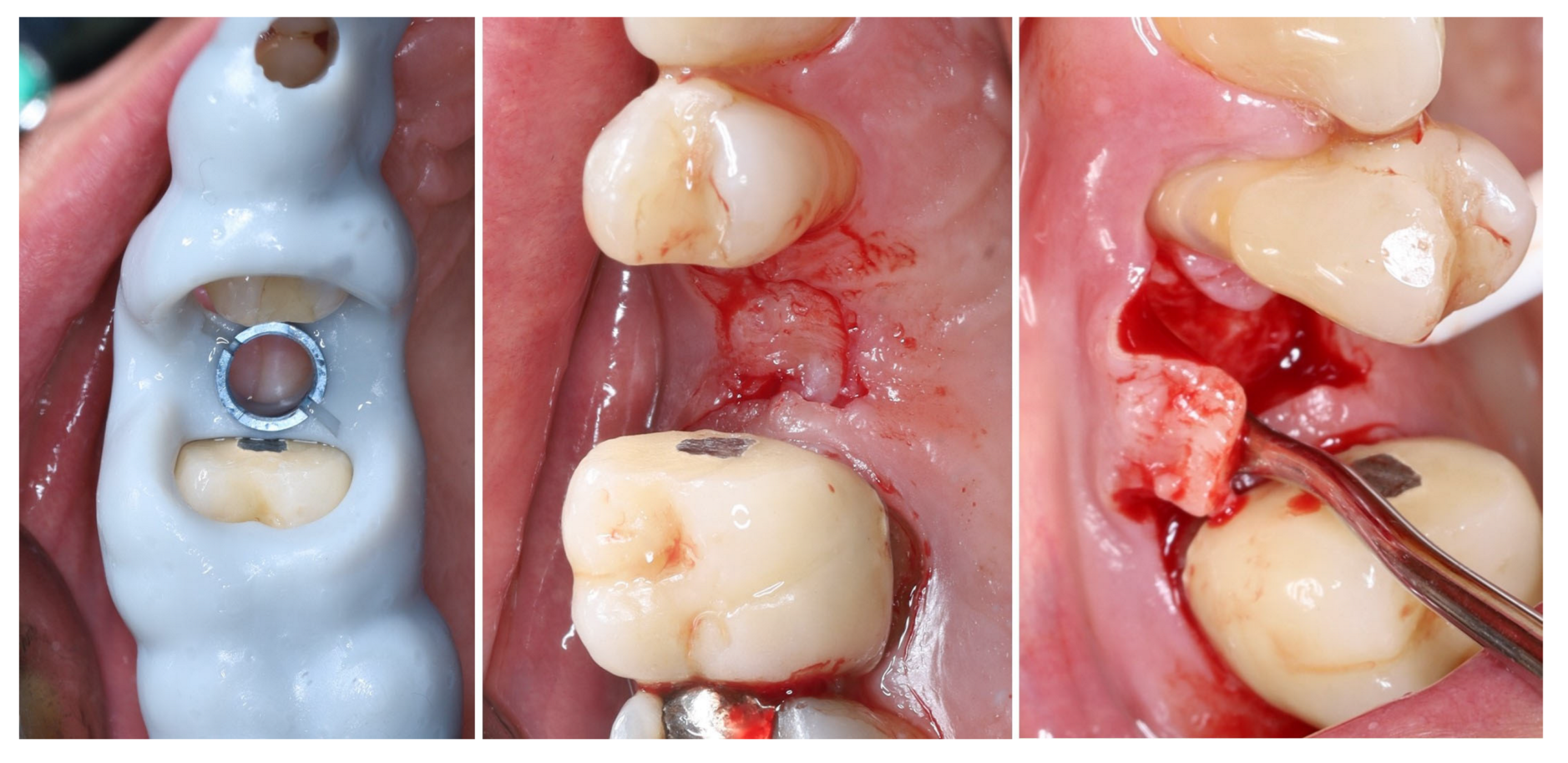
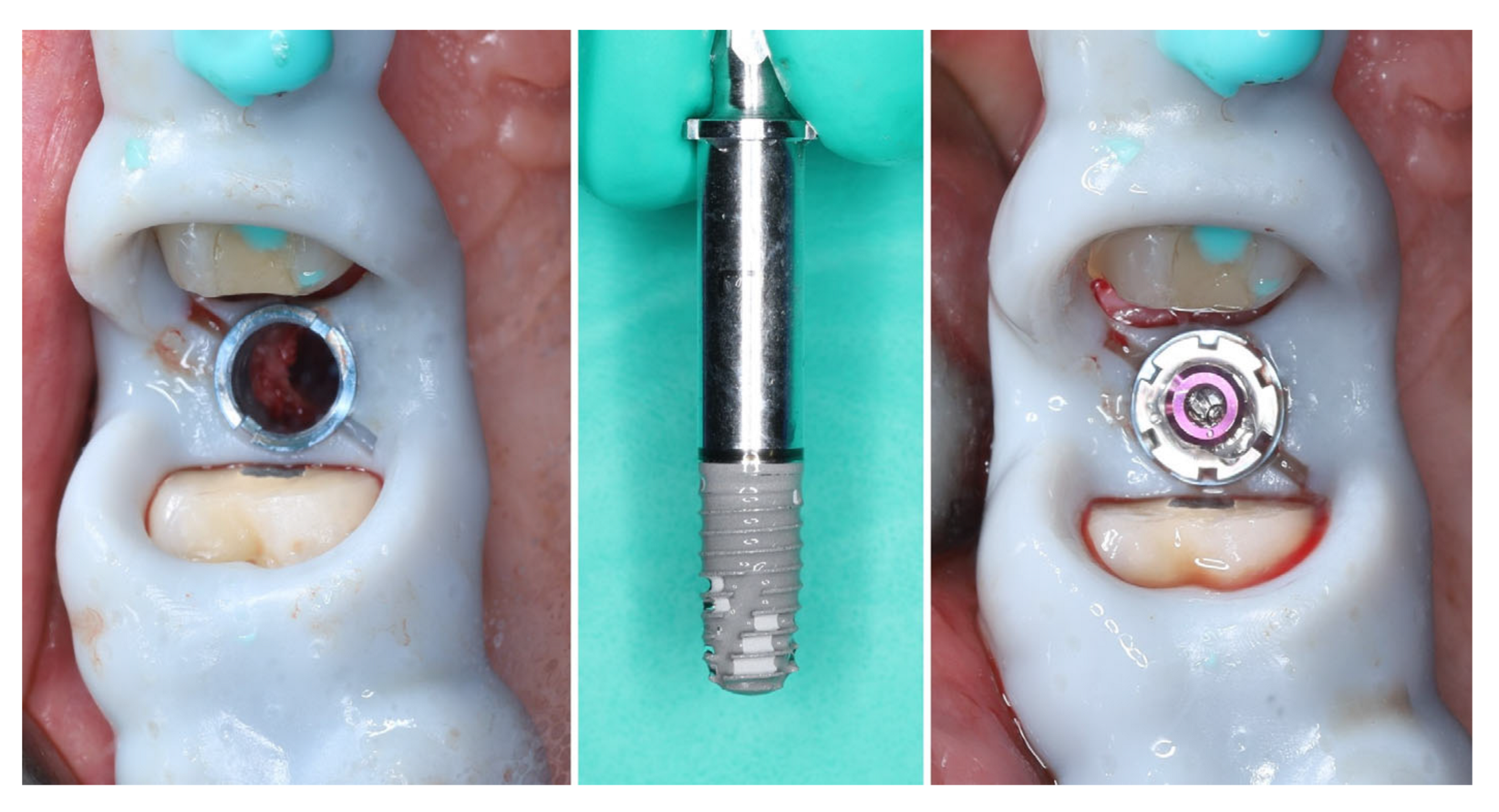
2. Materials and Methods—Clinical Case
- -
- Bucco-lingual width of the crestal keratinized mucosa ≥ 4 mm;
- -
- Mesio-distal width of the crestal mucosa (to the adjacent teeth)—∅ of the implant + min 3 mm;
- -
- Height of the buccal keratinized mucosa ≥ 1 mm;
- -
- Height of the palatal keratinized mucosa ≥ 3 mm;
- -
- Thickness of the crestal mucosa ≥ 2 mm.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S278–S285. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of implant therapy analyzed in a Swedish population: Prevalence of peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.S.; Keys, W.; Richards, D. Long-term (10-year) dental implant survival: A systematic review and sensitivity meta-analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Hämmerle, C.H.F.; Tarnow, D. The etiology of hard- and soft-tissue deficiencies at dental implants: A narrative review. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S267–S277. [Google Scholar] [CrossRef]
- Benic, G.I.; Mokti, M.; Chen, C.J.; Weber, H.P.; Hämmerle, C.H.; Gallucci, G.O. Dimensions of buccal bone and mucosa at immediately placed implants after 7 years: A clinical and cone beam computed tomography study. Clin. Oral Implant. Res. 2012, 23, 560–566. [Google Scholar] [CrossRef]
- Kuchler, U.; Chappuis, V.; Gruber, R.; Lang, N.P.; Salvi, G.E. Immediate implant placement with simultaneous guided bone regeneration in the esthetic zone: 10-year clinical and radiographic outcomes. Clin. Oral Implant. Res. 2016, 27, 253–257. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Benic, G.I.; Hämmerle, C.H. Horizontal bone augmentation by means of guided bone regeneration. Periodontol. 2000 2014, 66, 13–40. [Google Scholar] [CrossRef]
- Berglundh, T.; Persson, L.; Klinge, B. A systematic review of the incidence of biological and technical complications in implant dentistry reported in prospective longitudinal studies of at least 5 years. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 197–212. [Google Scholar] [CrossRef]
- del Amo, F.S.; Lin, G.H.; Monje, A.; Galindo-Moreno, P.; Wang, H. Influence of Soft Tissue Thickness on Peri-Implant Marginal Bone Loss: A Systematic Review and Meta-Analysis. J. Periodontol. 2016, 87, 690–699. [Google Scholar] [CrossRef]
- Albrektsson, T.; Buser, D.; Sennerby, L. Crestal bone loss and oral implants. Clin. Implant Dent. Relat. Res. 2012, 14, 783–791. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is Marginal Bone Loss around Oral Implants the Result of a Provoked Foreign Body Reaction? Clin. Implant Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Linkevicius, T.; Puisys, A.; Svediene, O.; Linkevicius, R.; Linkeviciene, L. Radiological comparison of laser-microtextured and platform-switched implants in thin mucosal biotype. Clin. Oral Implant. Res. 2015, 26, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kaminaka, A.; Nakano, T.; Ono, S.; Kato, T.; Yatani, H. Cone-beam computed tomography evaluation of horizontal and vertical dimensional changes in buccal peri-implant alveolar bone and soft tissue: A 1-year prospective clinical study. Clin. Implant Dent. Relat. Res. 2015, 17 (Suppl. S2), e576–e585. [Google Scholar] [CrossRef]
- Linkevicius, T.; Puisys, A.; Linkeviciene, L.; Peciuliene, V.; Schlee, M. Crestal Bone Stability around Implants with Horizontally Matching Connection after Soft Tissue Thickening: A Prospective Clinical Trial. Clin. Implant Dent. Relat. Res. 2015, 17, 497–508. [Google Scholar] [CrossRef]
- Puisys, A.; Linkevicius, T. The influence of mucosal tissue thickening on crestal bone stability around bone-level implants. A prospective controlled clinical trial. Clin. Oral Implant. Res. 2015, 26, 123–129. [Google Scholar] [CrossRef]
- Fickl, S.; Kröger, A.T.; Dietrich, T.; Kebschull, M. Influence of soft tissue augmentation procedures around dental implants on marginal bone level changes—A systematic review. Clin. Oral Implant. Res. 2021, 32 (Suppl. S21), 108–137. [Google Scholar] [CrossRef]
- Thoma, D.S.; Naenni, N.; Figuero, E.; Hämmerle, C.H.F.; Schwarz, F.; Jung, R.E.; Sanz-Sánchez, I. Effects of soft tissue augmentation procedures on peri-implant health or disease: A systematic review and meta-analysis. Clin. Oral Implant. Res. 2018, 29 (Suppl. S15), 32–49. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J. Periodontol. 2020, 92, 21–44. [Google Scholar] [CrossRef]
- Oh, S.L.; Masri, R.M.; Williams, D.A.; Ji, C.; Romberg, E. Free gingival grafts for implants exhibiting lack of keratinized mucosa: A prospective controlled randomized clinical study. J. Clin. Periodontol. 2017, 44, 195–203. [Google Scholar] [CrossRef]
- Bianchi, A.E.; Sanfilippo, F. Single-tooth replacement by immediate implant and connective tissue graft: A 1–9-year clinical evaluation. Clin. Oral Implant. Res. 2004, 15, 269–277. [Google Scholar] [CrossRef]
- Kotsilkov, K. Modified single roll flap approach for simultaneous implant placement and gingival augmentation. J. IMAB 2017, 23, 1667–1672. [Google Scholar] [CrossRef]
- Covani, U.; Bortolaia, C.; Barone, A.; Sbordone, L. Bucco-Lingual Crestal Bone Changes After Immediate and Delayed Implant Placement. J. Periodontol. 2004, 75, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, M.; Zucchelli, G. Coronally advanced flap: A modified surgical approach for isolated recession-type defects: Three-year results. J. Clin. Periodontol. 2007, 34, 262–268. [Google Scholar] [CrossRef]
- Zucchelli, G.; De Sanctis, M. Treatment of Multiple Recession-Type Defects in Patients with Esthetic Demands. J. Periodontol. 2000, 71, 1506–1514. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araújo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Lombardi, T.; Berton, F.; Salgarello, S.; Barbalonga, E.; Rapani, A.; Piovesana, F.; Gregorio, C.; Barbati, G.; Di Lenarda, R.; Stacchi, C. Factors Influencing Early Marginal Bone Loss around Dental Implants Positioned Subcrestally: A Multicenter Prospective Clinical Study. J. Clin. Med. 2019, 8, 1168. [Google Scholar] [CrossRef]
- Ioannidis, A.; Cathomen, E.; Jung, R.E.; Fehmer, V.; Hüsler, J.; Thoma, D.S. Discoloration of the mucosa caused by different restorative materials—A spectrophotometric in vitro study. Clin. Oral Implant. Res. 2017, 28, 1133–1138. [Google Scholar] [CrossRef]
- Agarwal, S.; Durrani, F.; Pandey, A.; Rashika, M.; Shilpi, S.; Meena, D. Peri-Implant Esthetics in Focus: Comparing Anodized Titanium and Zirconium Dioxide Abutments in a Randomized Clinical Trial. J. Esthet. Restor. Dent. 2025, 37, 2459–2471. [Google Scholar] [CrossRef]
- Peitsinis, P.R.; Blouchou, A.; Chatzopoulos, G.S.; Vouros, I.D. Optimizing Implant Placement Timing and Loading Protocols for Successful Functional and Esthetic Outcomes: A Narrative Literature Review. J. Clin. Med. 2025, 14, 1442. [Google Scholar] [CrossRef]
- Hang, S.; Lee, K. Soft tissue augmentation of dental implants—Before/during/after placement. J. Oral Med. Dent. Res. 2022, 3, 1–13. [Google Scholar]
- Pavón, P.; Fons-Badal, C.; Pérez-Rostoll, N.; Alonso-Pérez-Barquero, J.; Solá-Ruiz, M.F.; Agustín-Panadero, R. The Trouser Technique: A Novel Approach for Peri-Implant Soft Tissue Augmentation. J. Clin. Med. 2025, 14, 4974. [Google Scholar] [CrossRef] [PubMed]
- Mounssif, I.; Bentivogli, V.; Rendón, A.; Mazzotti, C.; De Rubertis, I.; Zucchelli, G.; Stefanini, M. Peri-Implant Soft Tissue Augmentation with Connective Tissue Graft Substitutes. Appl. Sci. 2025, 15, 10178. [Google Scholar] [CrossRef]
- Guerrero Murillo, M.E.; Martínez Gutiérrez, D.; Mayoral García, V.A. Roll flap technique and implant placement: Case report. Clin. Case Rep. 2025, in press. [Google Scholar] [CrossRef]
- Zuiderveld, E.G.; Meijer, H.J.A.; den Hartog, L.; Vissink, A.; Raghoebar, G.M. Effect of connective tissue grafting on peri-implant tissue in single immediate implant sites: A RCT. J. Clin. Periodontol. 2018, 45, 253–264. [Google Scholar] [CrossRef]
- Torra-Moneny, M.; Mauri-Obradors, E.; Egido-Moreno, S.; Valls-Roca-Umbert, J.; Marí-Roig, A.; López-López, J. Association of Connective Tissue Grafts in Immediate Implants: Systematic Review and Meta-Analysis. Dent. J. 2024, 12, 183. [Google Scholar] [CrossRef]
- Galve-Huertas, A.; Decadt, L.; García-González, S.; Hernández-Alfaro, F.; Centenero, S.A.-H. Immediate Implant Placement with Soft Tissue Augmentation Using Acellular Dermal Matrix Versus Connective Tissue Graft: A Systematic Review and Meta-Analysis. Materials 2024, 17, 5285. [Google Scholar] [CrossRef]
- Emecen-Huja, P.; Leblebicioglu, B. Peri-Implant Wound Healing and Clinical Outcomes. Curr. Oral Health Rep. 2024, 11, 215–225. [Google Scholar] [CrossRef]
- Tavelli, L.; Zucchelli, G.; Stefanini, M.; Rasperini, G.; Wang, H.; Barootchi, S. Vertical soft tissue augmentation to treat implant esthetic complications: A prospective clinical and volumetric case series. Clin. Implant Dent. Relat. Res. 2023, 25, 204–214. [Google Scholar] [CrossRef]
- Gulsever, S.; Uckan, S. Enhanced Palatal Wound Healing with Leucocyte- and Platelet-Rich Fibrin After Free Gingival Graft Harvesting: A Prospective Randomized Controlled Clinical Trial. J. Clin. Med. 2025, 14, 1029. [Google Scholar] [CrossRef]
- Banyś, A.; Fiegler-Rudol, J.; Grzech-Leśniak, Z.; Wiench, R.; Matys, J.; Shibli, J.A.; Grzech-Leśniak, K. Clinical Efficacy of Er,Cr:YSGG Laser for Deepithelialization of Free Gingival Grafts in Gingival Recession Treatment: A Randomized, Split-Mouth Clinical Trial. J. Clin. Med. 2025, 14, 5335. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Greenwell, H.; Wang, H.-L. Is a soft tissue graft harvested from the maxillary tuberosity the approach of choice in an isolated site? J. Periodontol. 2019, 90, 821–825. [Google Scholar] [CrossRef]
- Sculean, A.; Gruber, R.; Bosshardt, D.D. Soft tissue wound healing around teeth and dental implants. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S6–S22. [Google Scholar] [CrossRef] [PubMed]
- Beymouri, A.; Yaghobee, S.; Khorsand, A.; Safi, Y. Comparison of morbidity at the donor site and clinical efficacy at the recipient site between two different connective tissue graft harvesting techniques from the palate: A randomized clinical trial. J. Adv. Periodontol. Implant. Dent. 2023, 15, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.M.; Chvartszaid, D.; Kim, S.W.; Portnof, J.E. Soft-tissue grafting solutions. Dent. Clin. N. Am. 2020, 64, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Tseng, E.S.; Tavelli, L.; Wang, H.-L. Palatal pedicle flaps for soft tissue augmentation. Int. J. Periodontics Restor. Dent. 2020, 40, 581–588. [Google Scholar] [CrossRef]
- Abrams, L. Augmentation of the deformed residual edentulous ridge for fixed prosthesis. Compend. Contin. Educ. Dent. 1980, 1, 205–213. [Google Scholar]
- D’Ambrosio, F.; Caggiano, M.; Chiacchio, A.; Acerra, A.; Giordano, F. Palatal Graft Harvesting Site Healing and Pain Management: What Is the Best Choice? An Umbrella Review. Appl. Sci. 2024, 14, 5614. [Google Scholar] [CrossRef]
- Levin, B.P. Dermal apron technique update. Dent. Rev. 2024, 4, 100092. [Google Scholar] [CrossRef]
- Park, S.-H.; Wang, H.-L. Pouch roll technique—Variation of modified roll technique. Int. J. Periodontics Restor. Dent. 2012, 32, e116–e121. [Google Scholar]
- Saquib, S.A.; Bhat, M.Y.S.; Javali, M.A.; Shamsuddin, S.V.; Khader, M.A. Modified Roll Technique for Soft Tissue Augmentation in Prosthetic Rehabilitation: A Case Report. Clin. Pract. 2019, 9, 1110. [Google Scholar] [CrossRef]
- Kanishk, S.; Sunil, K. Modified palatal roll flap for horizontal ridge augmentation. Cureus 2024, 16, e58890. [Google Scholar]
- Barone, R.; Clauser, C.; Prato, G.P. Localized soft tissue ridge augmentation at phase 2 implant surgery: Case report. Int. J. Periodontics Restor. Dent. 1999, 19, 141–145. [Google Scholar]
- Matthews, D.P. Pediculated connective tissue graft for the “blown-out” esthetic site. Compend. Contin. Educ. Dent. 2008, 29, 350–352, 354, 356–357. [Google Scholar] [PubMed]
- Grover, D.; Kaur, G. Soft tissue ridge augmentation using “roll technique”: Case report. IAIM 2014, 1, 80–85. Available online: https://iaimjournal.com/wp-content/uploads/2014/12/13-Soft-tissue-ridge-augmentation.pdf (accessed on 5 September 2025).
- Mali, A.; Kashish, D.; Agrawal, P. Soft tissue ridge augmentation using “roll technique”: Case report. Int. J. Sci. Res. 2016, 5, 21–23. Available online: https://www.worldwidejournals.com/international-journal-of-scientific-research-(IJSR)/recent_issues_pdf/2016/September/September_2016_1492768493__08.pdf (accessed on 5 September 2025).
- Bressan, E.; Zucchelli, G.; Tommasato, G.; Pesce, P.; Canullo, L.; Consensus Meeting Group IAO; Grusovin, M.G. Consensus Report by the Italian Academy of Osseointegration on the Importance of Peri-Implant Soft Tissues. Medicina 2024, 60, 1393. [Google Scholar] [CrossRef]
- Kotsilkov, K. Modified single roll flap approach for simultaneous implant placement and recession coverage. J. IMAB 2021, 27, 3778–3783. [Google Scholar] [CrossRef]
- El Chaar, E.; Oshman, S.; Cicero, G.; Castano, A.; Dinoi, C.; Soltani, L.; Lee, Y. Soft Tissue Closure of Grafted Extraction Sockets in the Anterior Maxilla: A Modified Palatal Pedicle Connective Tissue Flap Technique. Int. J. Periodontics Restor. Dent. 2017, 37, 99–107. [Google Scholar] [CrossRef]
- Tinti, C.; Parma-Benfenati, S. Minimally invasive gingival augmentation around implants. Int. J. Periodontics Restor. Dent. 2012, 32, 187–193. [Google Scholar]
- Stefanini, M.; Barootchi, S.; Sangiorgi, M.; Pispero, A.; Grusovin, M.G.; Mancini, L.; Zucchelli, G.; Tavelli, L. Do soft tissue augmentation techniques provide stable and favorable peri-implant conditions in the medium and long term? A systematic review. Clin. Oral Implant. Res. 2023, 34, 28–42. [Google Scholar] [CrossRef]
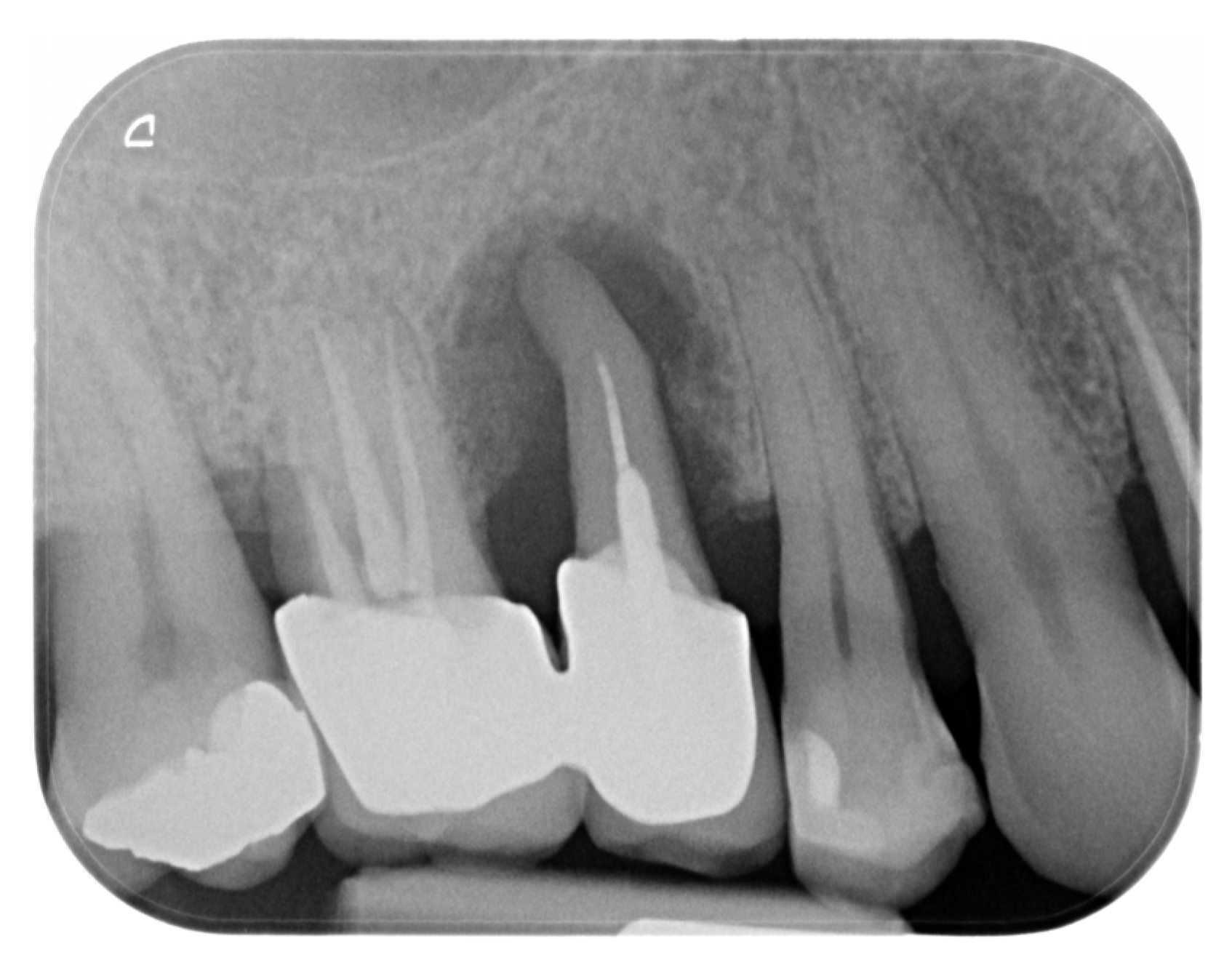
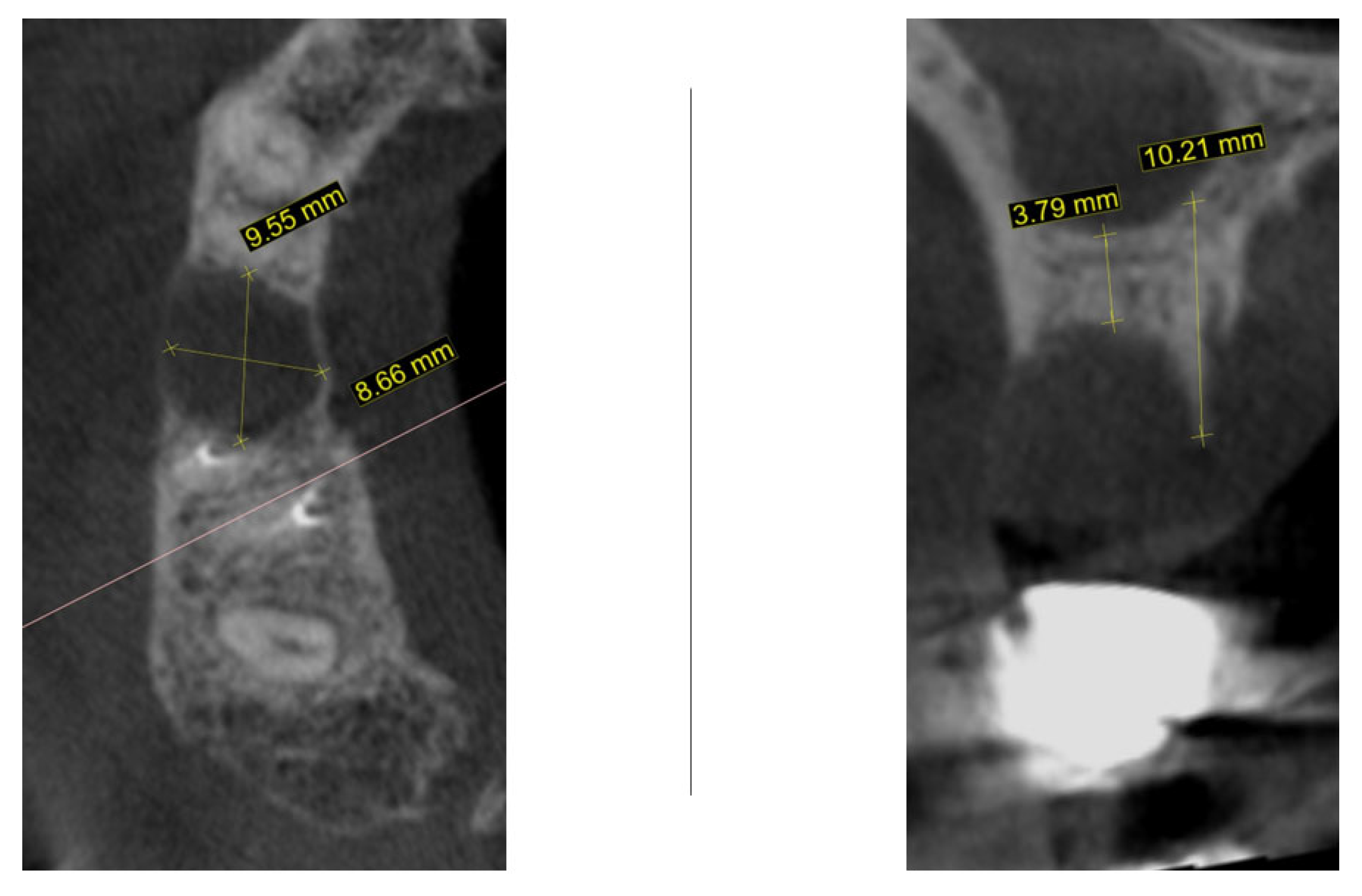
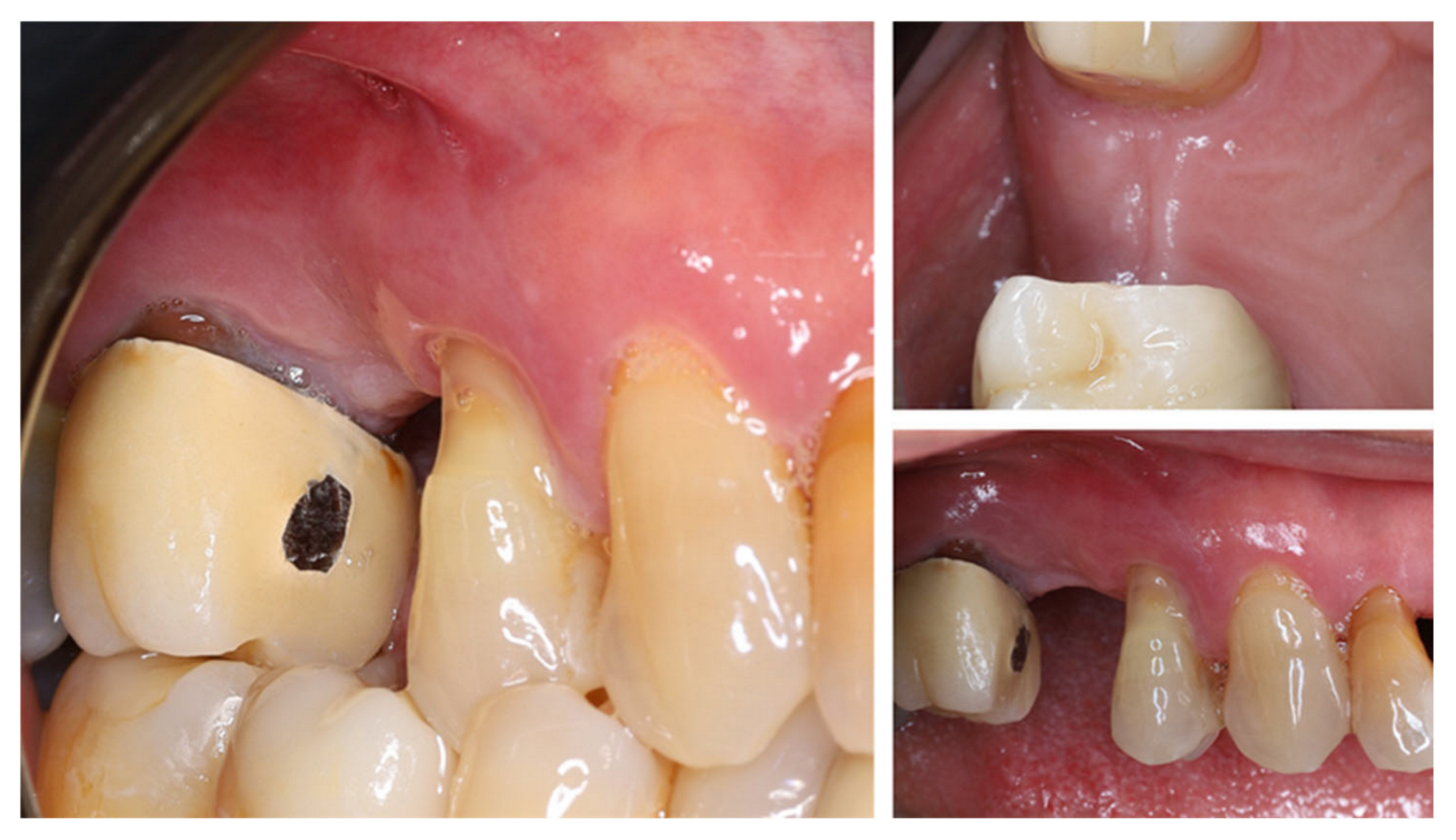
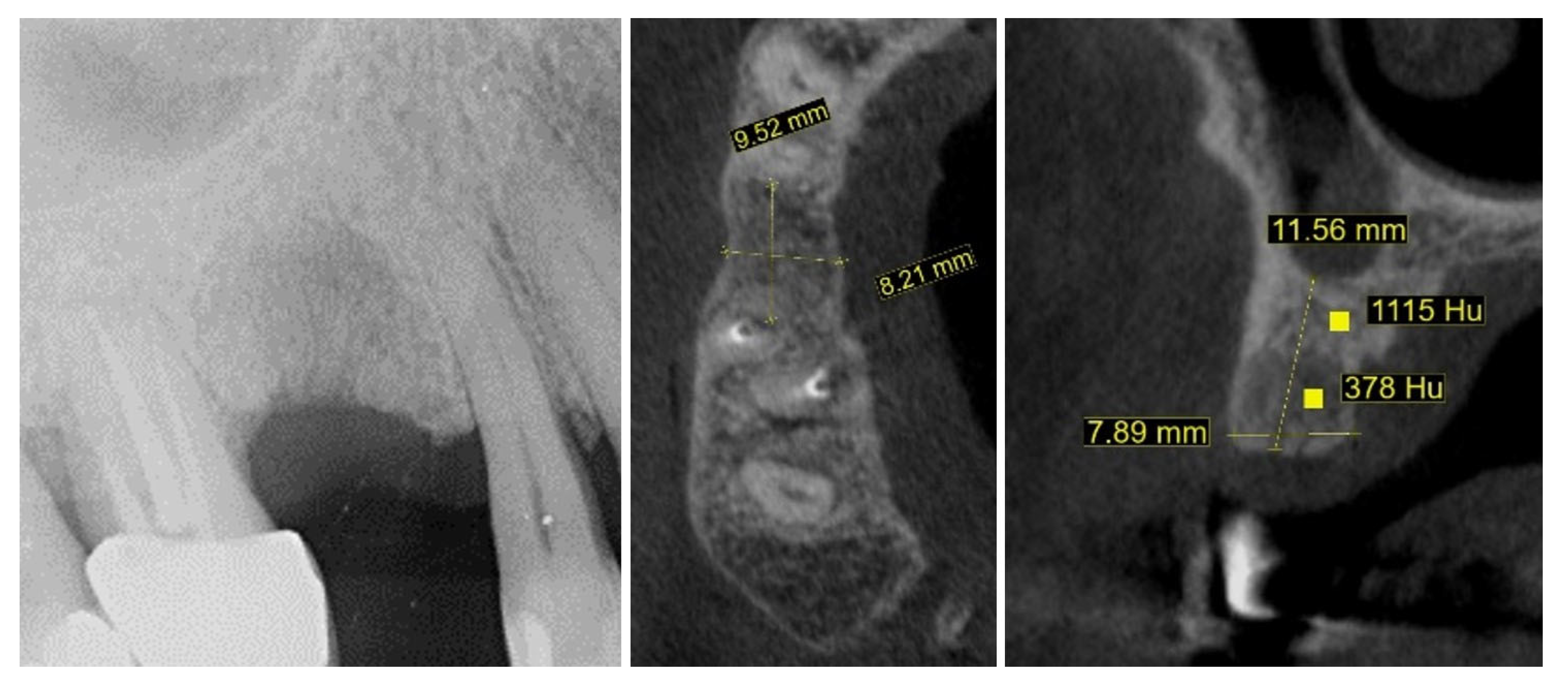
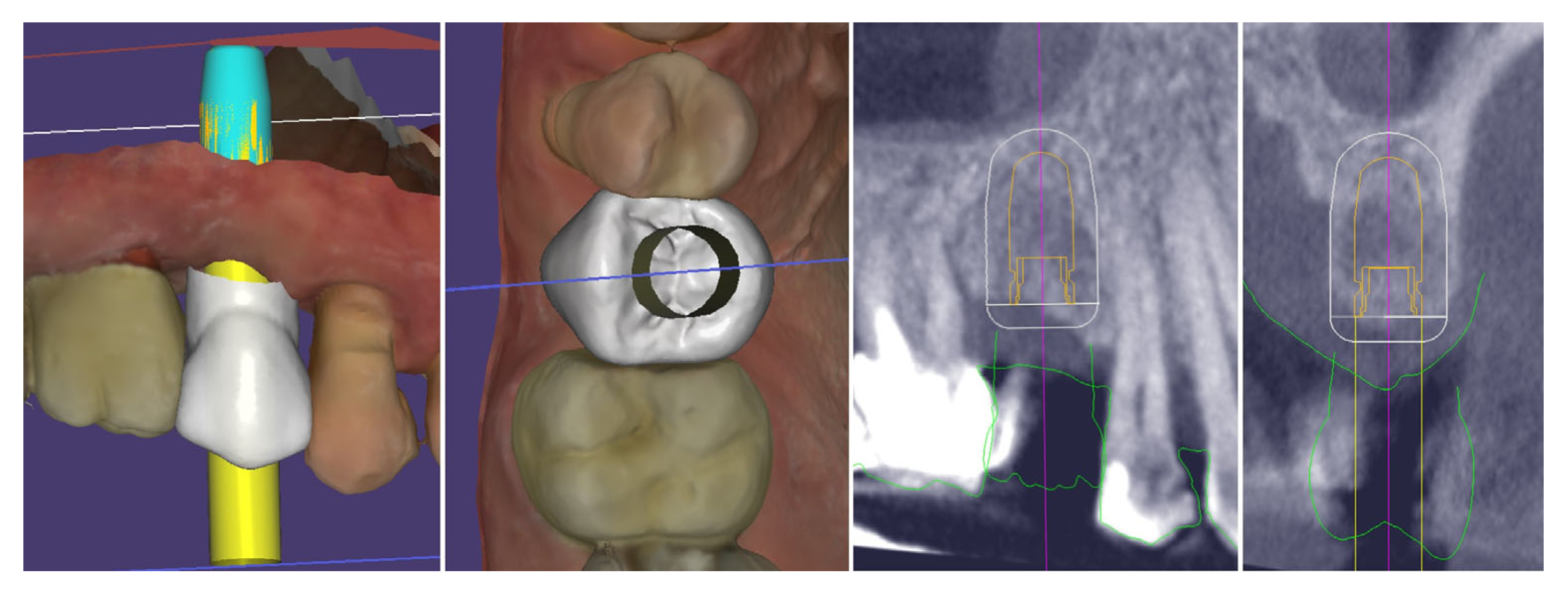
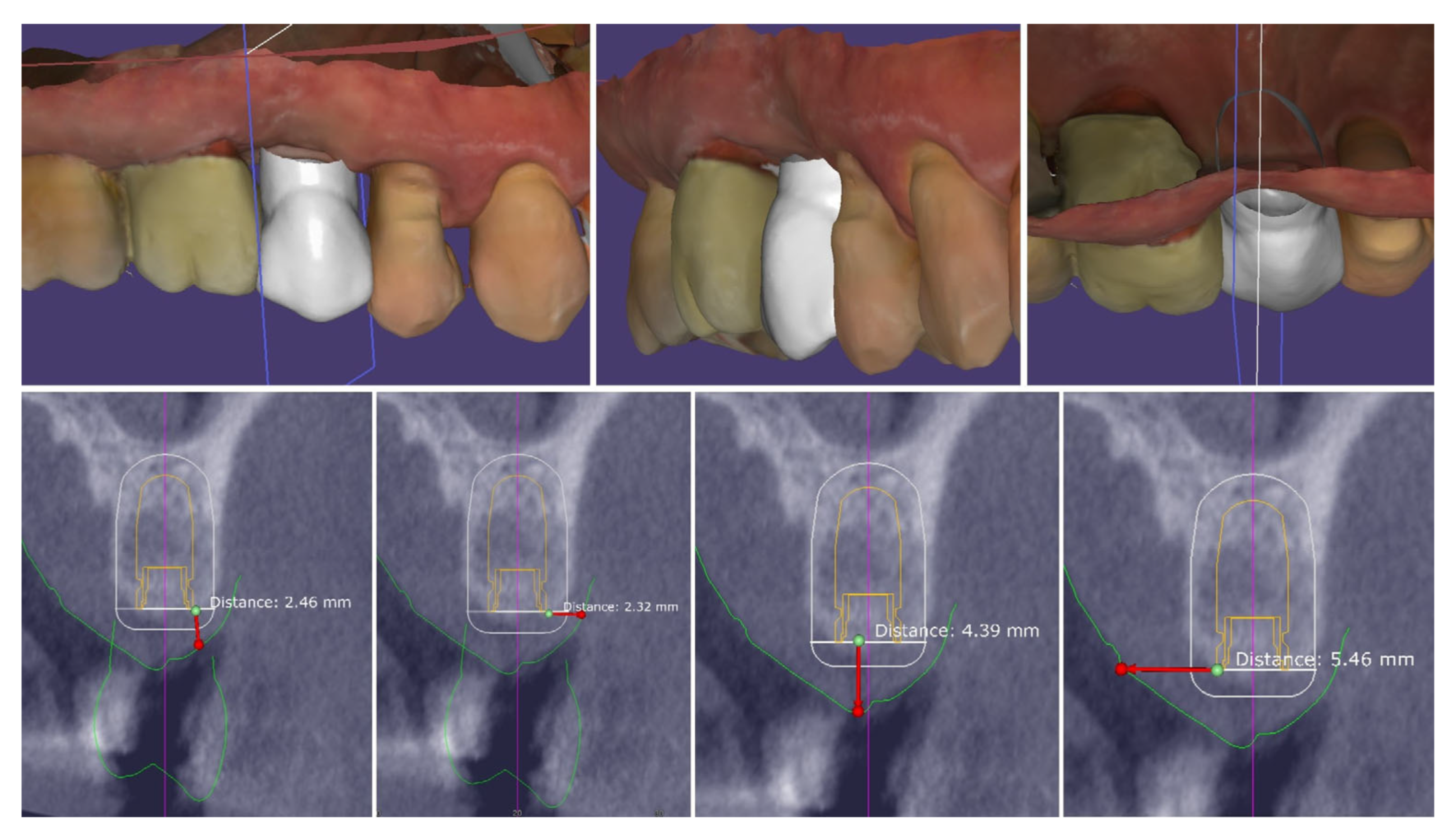
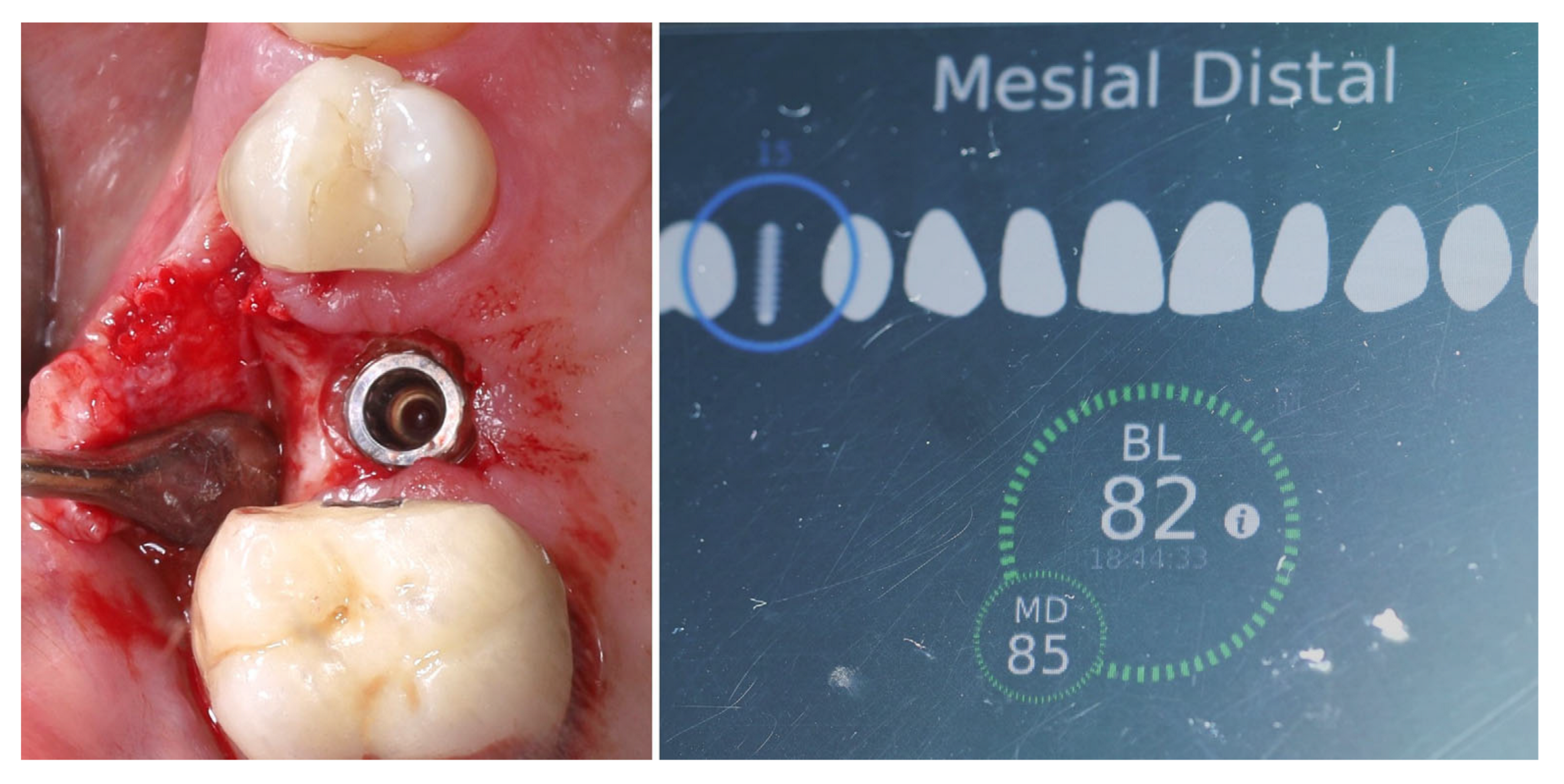
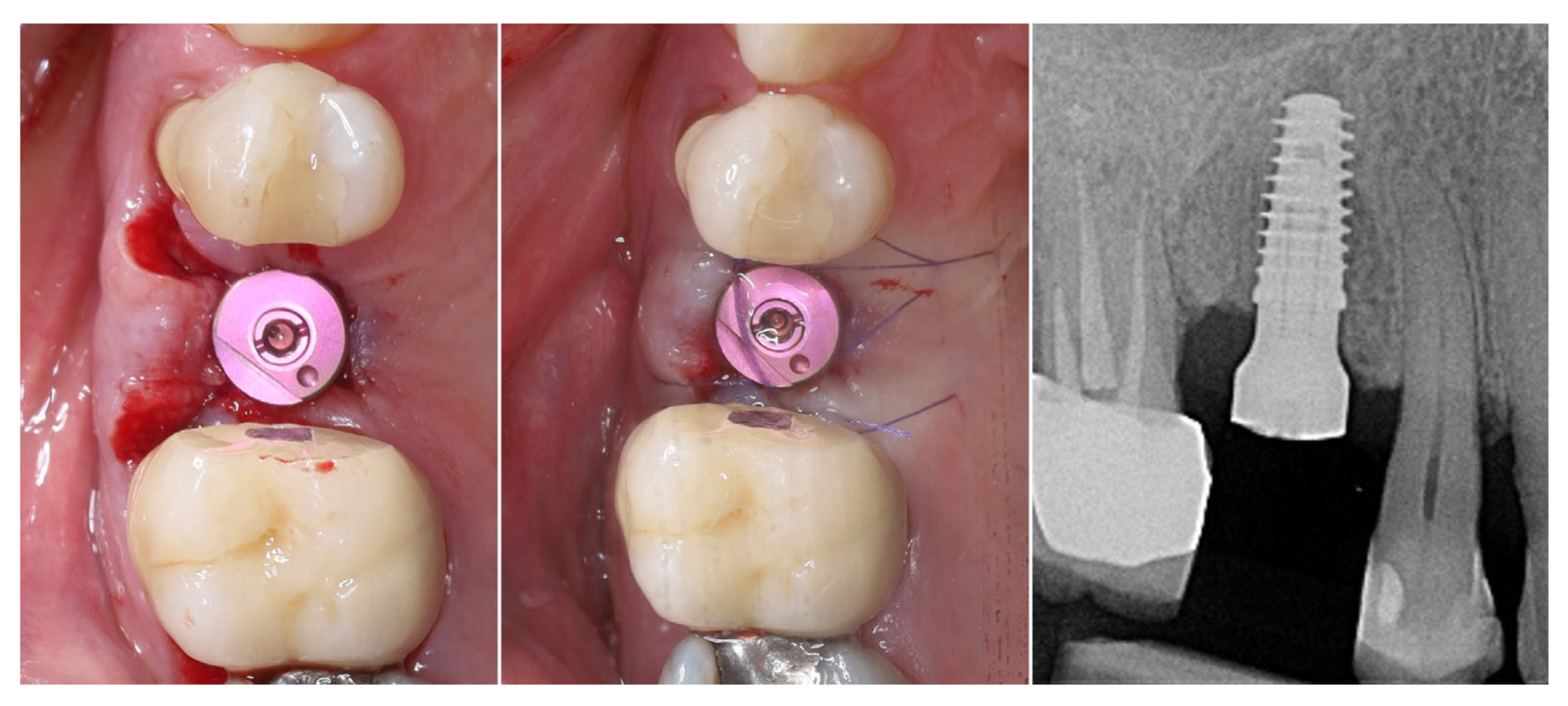
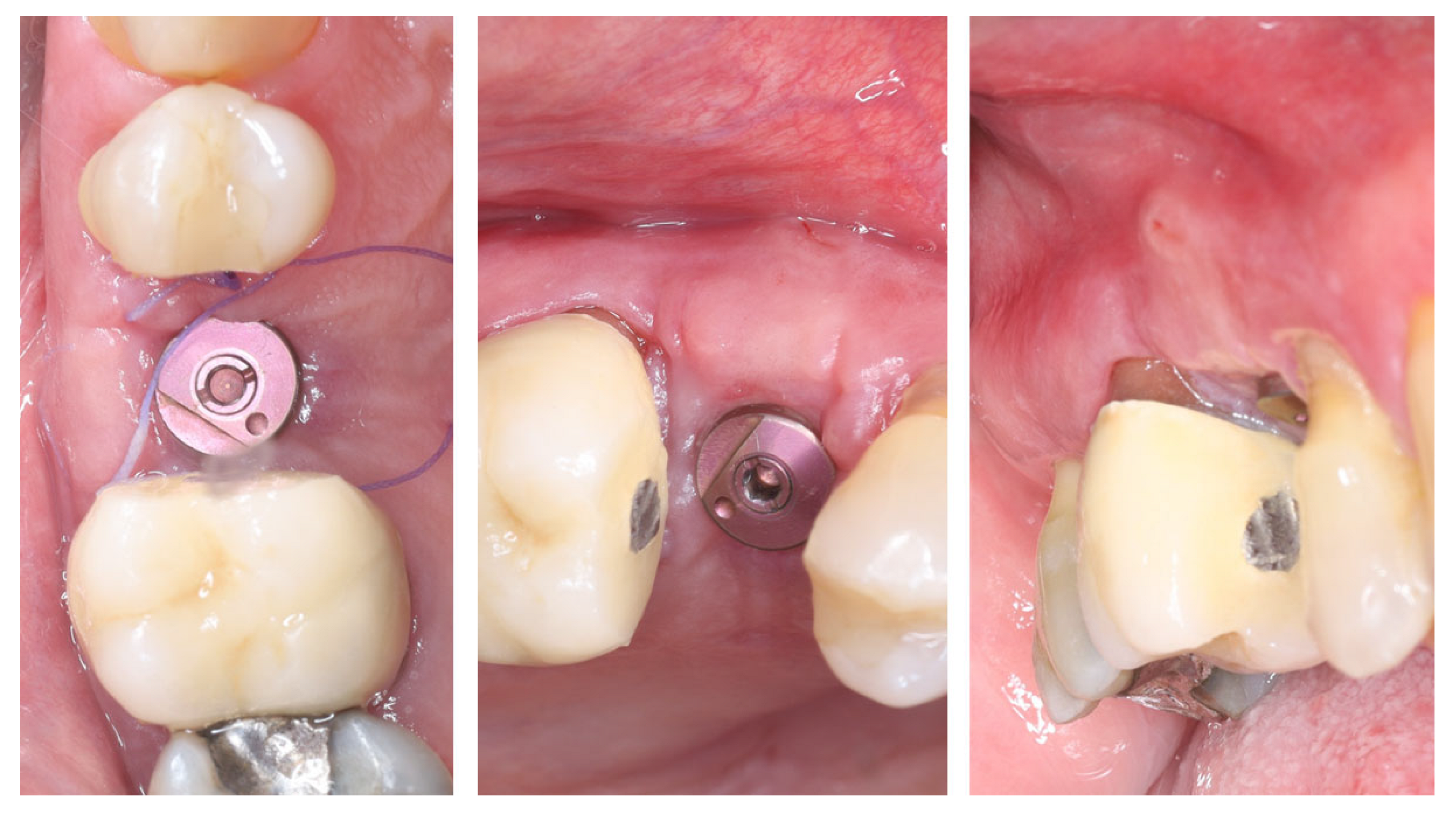
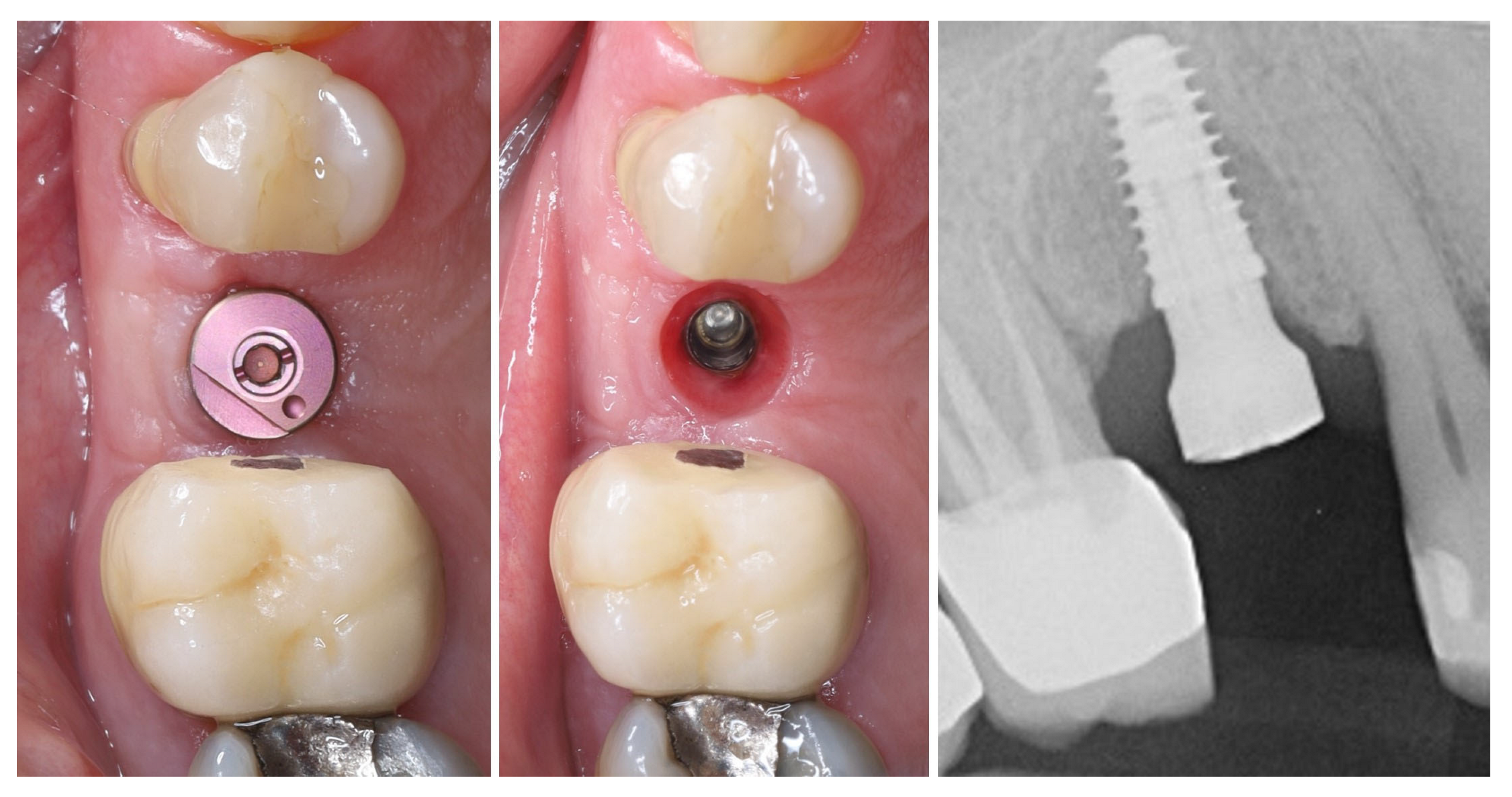
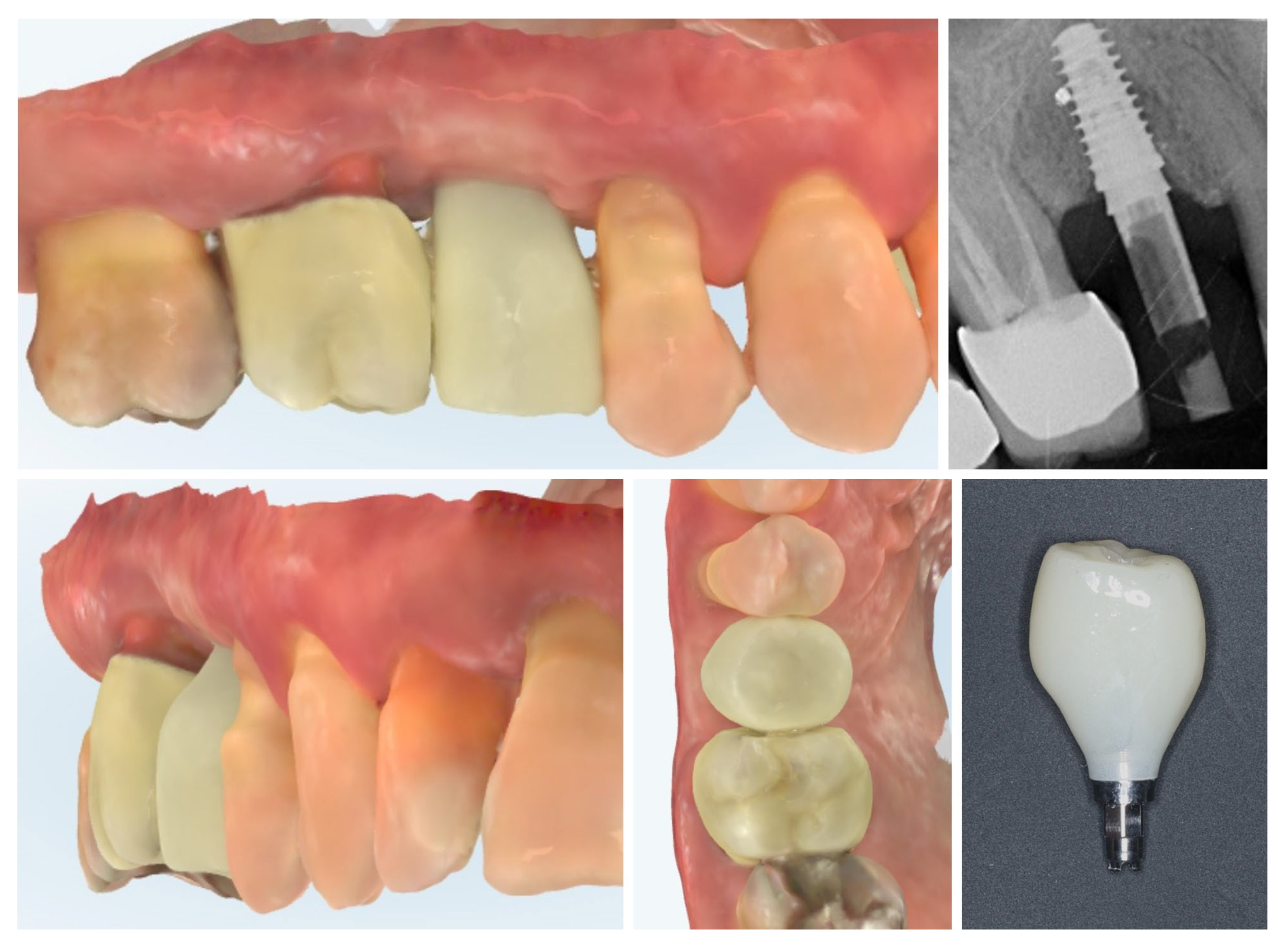



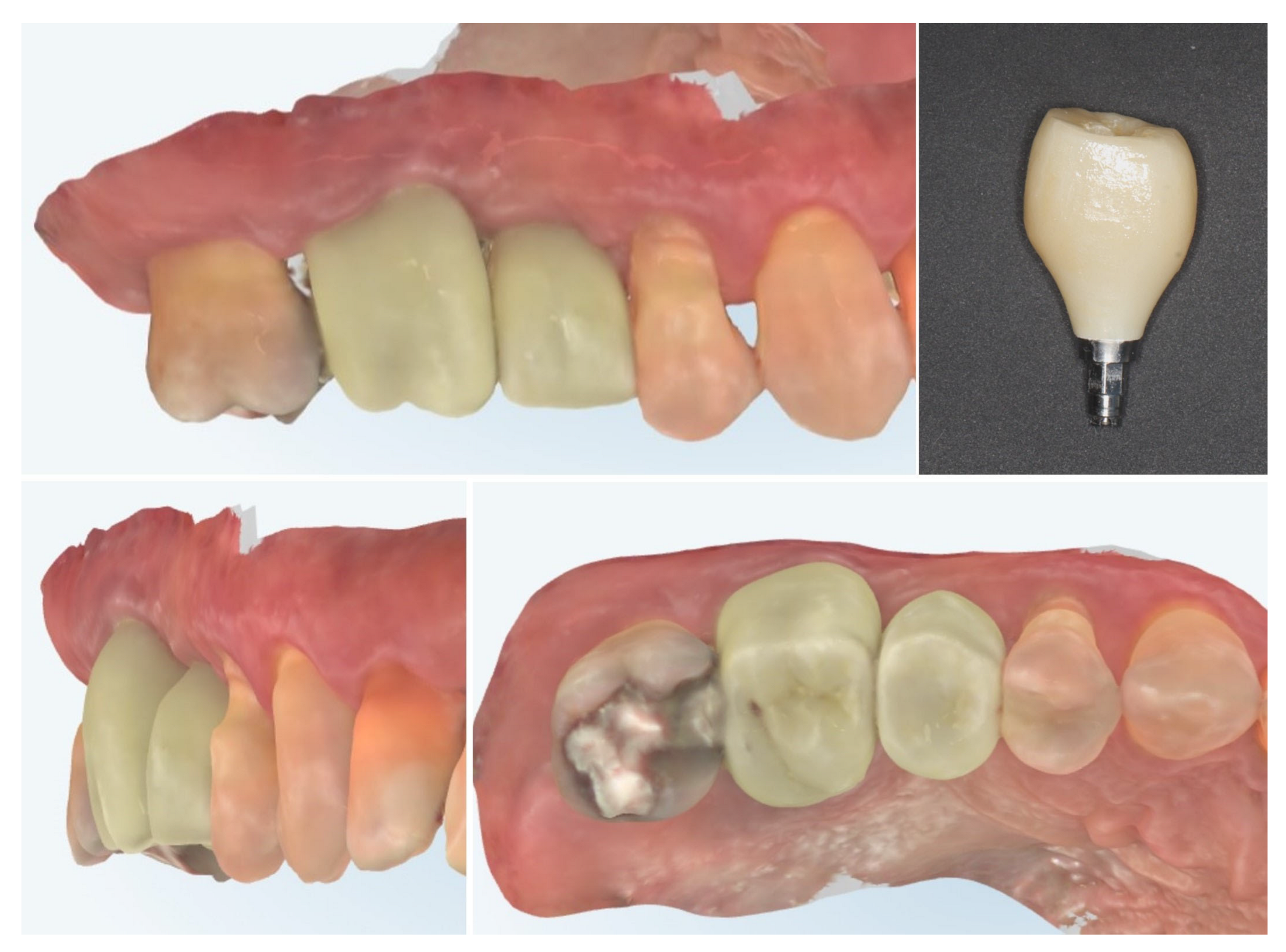
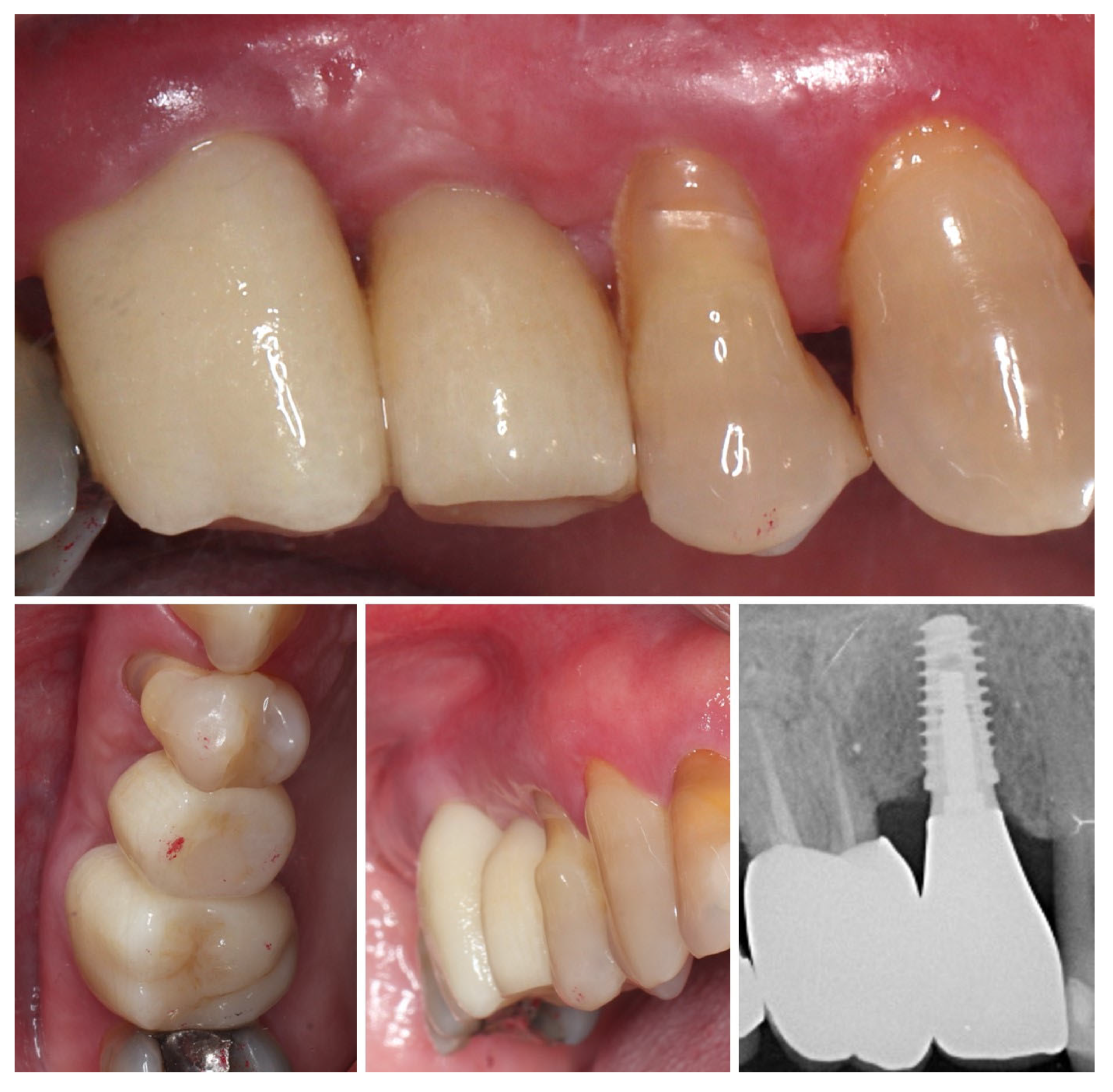
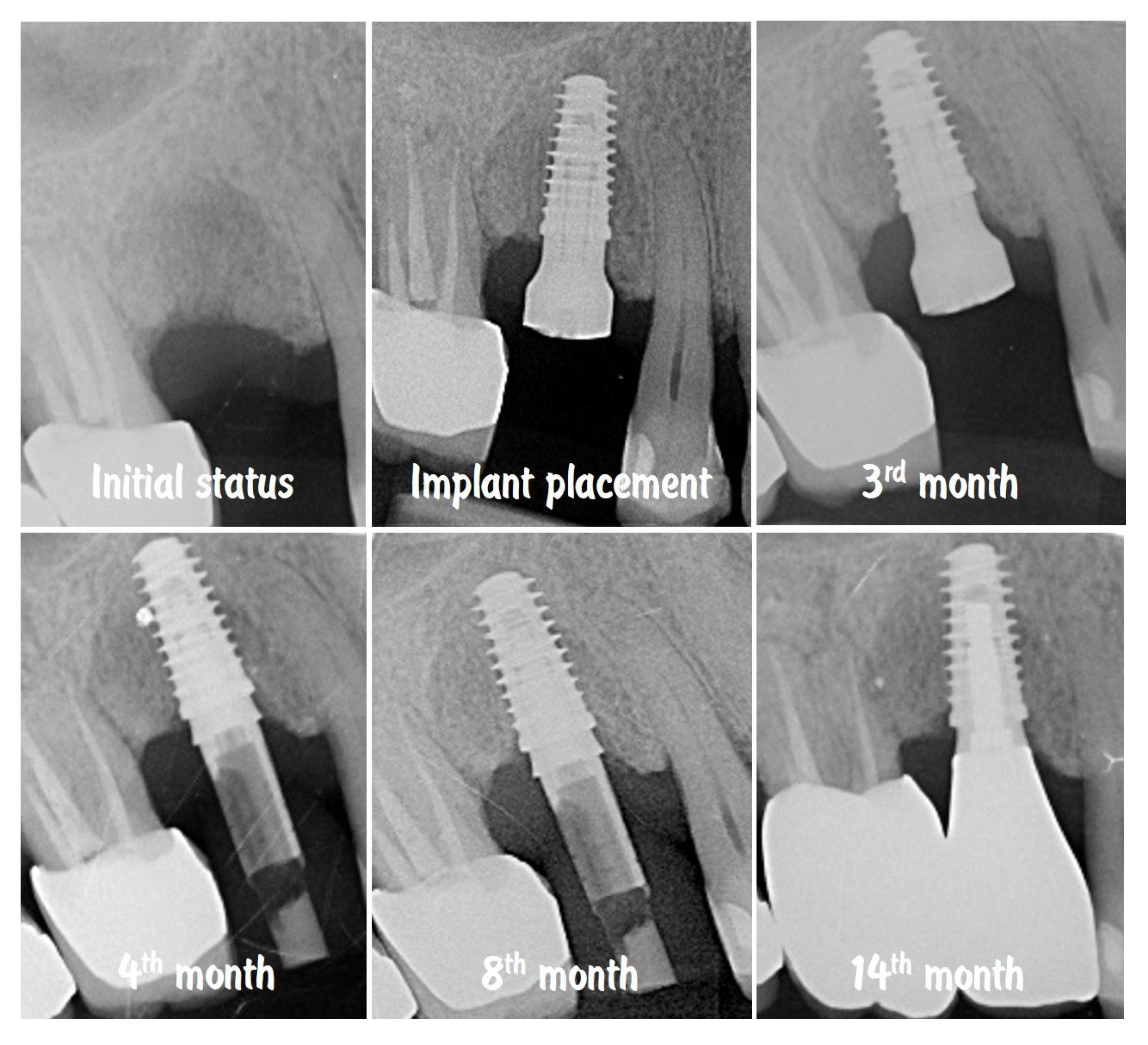
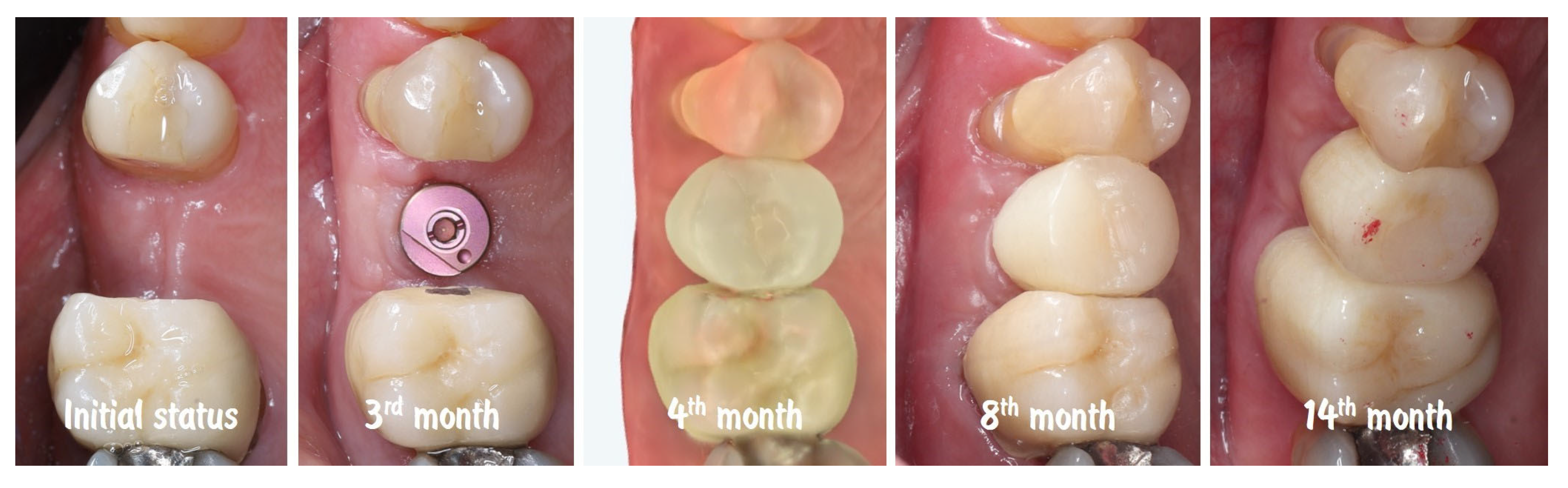


| Timepoint | Horizontal Thickness (mm) | Vertical Thickness (mm) | Clinical/Radiographic Findings | |
|---|---|---|---|---|
| 1 | Baseline (Day 0) | 2.3 | 2.4 | Initial thin buccal tissue |
| 2 | 2 weeks post-op | Uneventful healing, stable mucosal margin | ||
| 3 | 3 months post-op (with provisional) | 3.4 | 3.4 | Increased thickness, stable bone and mucosa |
| 4 | 4 months post-provisional | 3.5 | 3.6 | Stable peri-implant mucosa around provisional crown |
| 5 | 8 months post-op (final crown) | 3.5 | 4.1 | Final crown placed, harmonious soft tissue contours, and stable crestal bone |
| 6 | 14 months post-definitive | Long-term stability of soft tissue and crestal bone confirmed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsilkov, K.; Maynalovska, H.; Pashova-Tasseva, Z. Modified Roll Flap Soft-Tissue Augmentation at Single-Stage Implant Placement: A Digital-Scan–Verified Case Report. Dent. J. 2025, 13, 483. https://doi.org/10.3390/dj13100483
Kotsilkov K, Maynalovska H, Pashova-Tasseva Z. Modified Roll Flap Soft-Tissue Augmentation at Single-Stage Implant Placement: A Digital-Scan–Verified Case Report. Dentistry Journal. 2025; 13(10):483. https://doi.org/10.3390/dj13100483
Chicago/Turabian StyleKotsilkov, Kamen, Hristina Maynalovska, and Zdravka Pashova-Tasseva. 2025. "Modified Roll Flap Soft-Tissue Augmentation at Single-Stage Implant Placement: A Digital-Scan–Verified Case Report" Dentistry Journal 13, no. 10: 483. https://doi.org/10.3390/dj13100483
APA StyleKotsilkov, K., Maynalovska, H., & Pashova-Tasseva, Z. (2025). Modified Roll Flap Soft-Tissue Augmentation at Single-Stage Implant Placement: A Digital-Scan–Verified Case Report. Dentistry Journal, 13(10), 483. https://doi.org/10.3390/dj13100483







