Densifying the Future: A Critical Review of Osseodensification and Implant Dentistry
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Study Selection
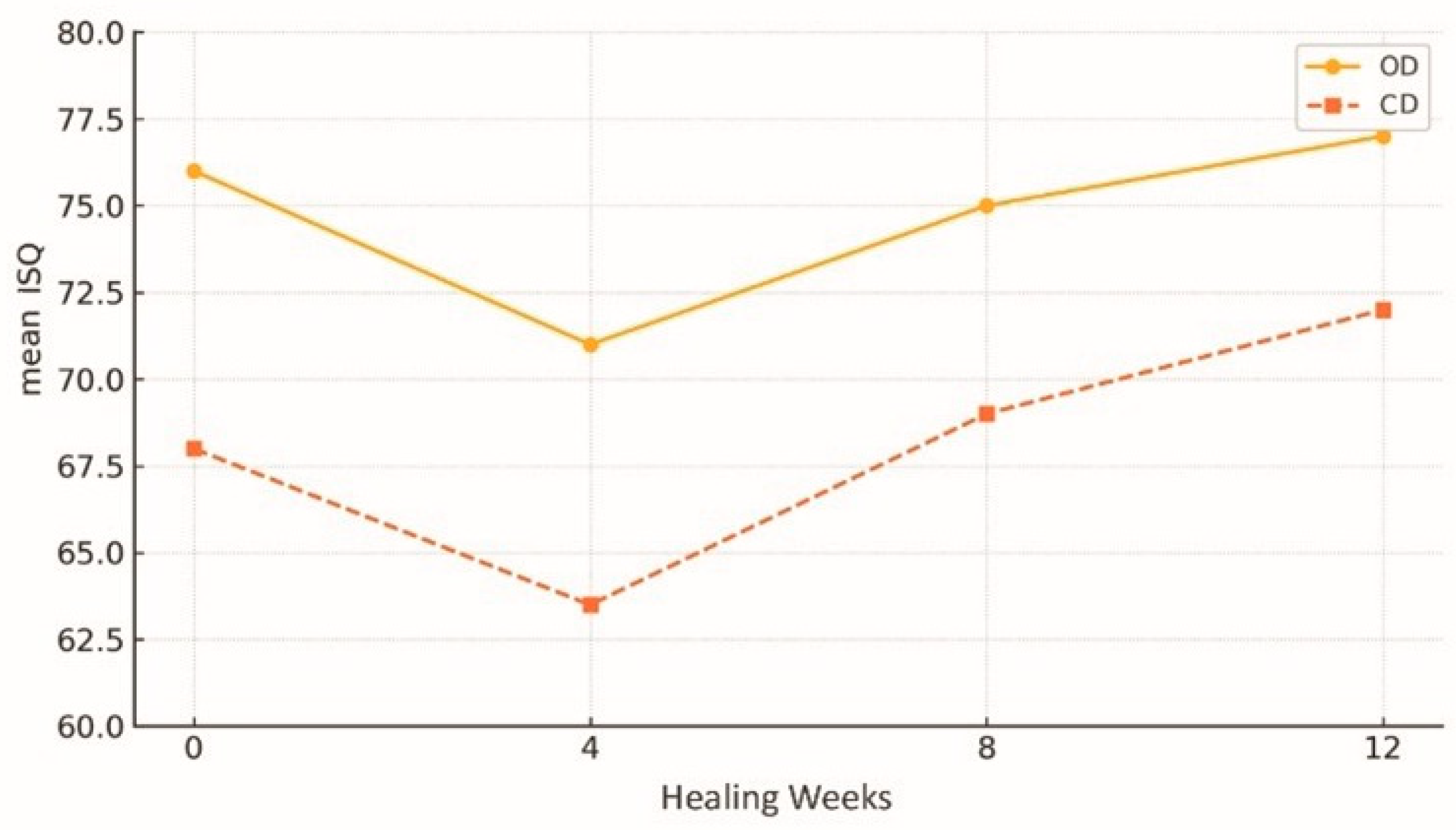
3.2. Increased Primary Stability and Insertion Torque
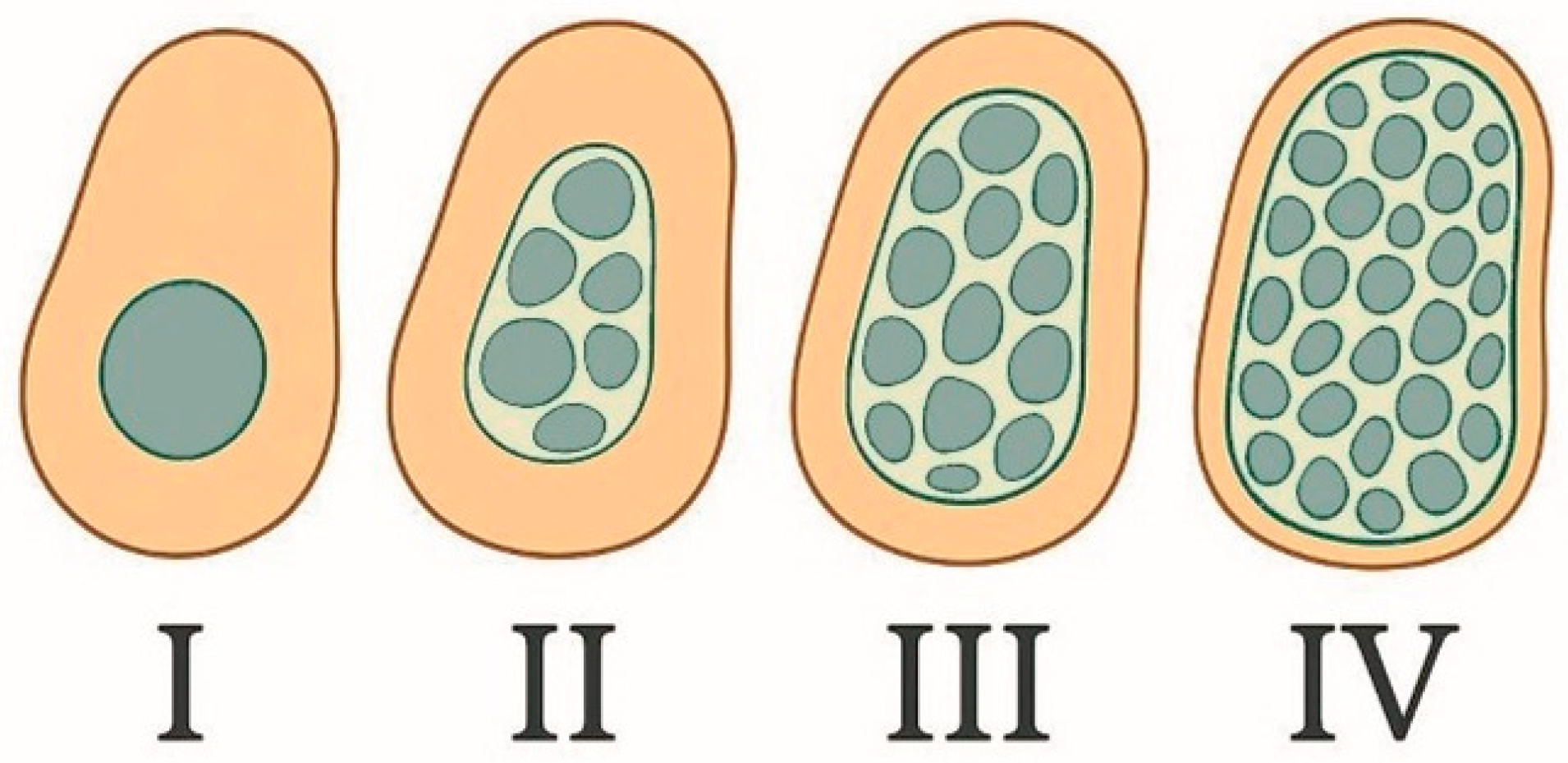
3.3. Histological and Morphometric Changes
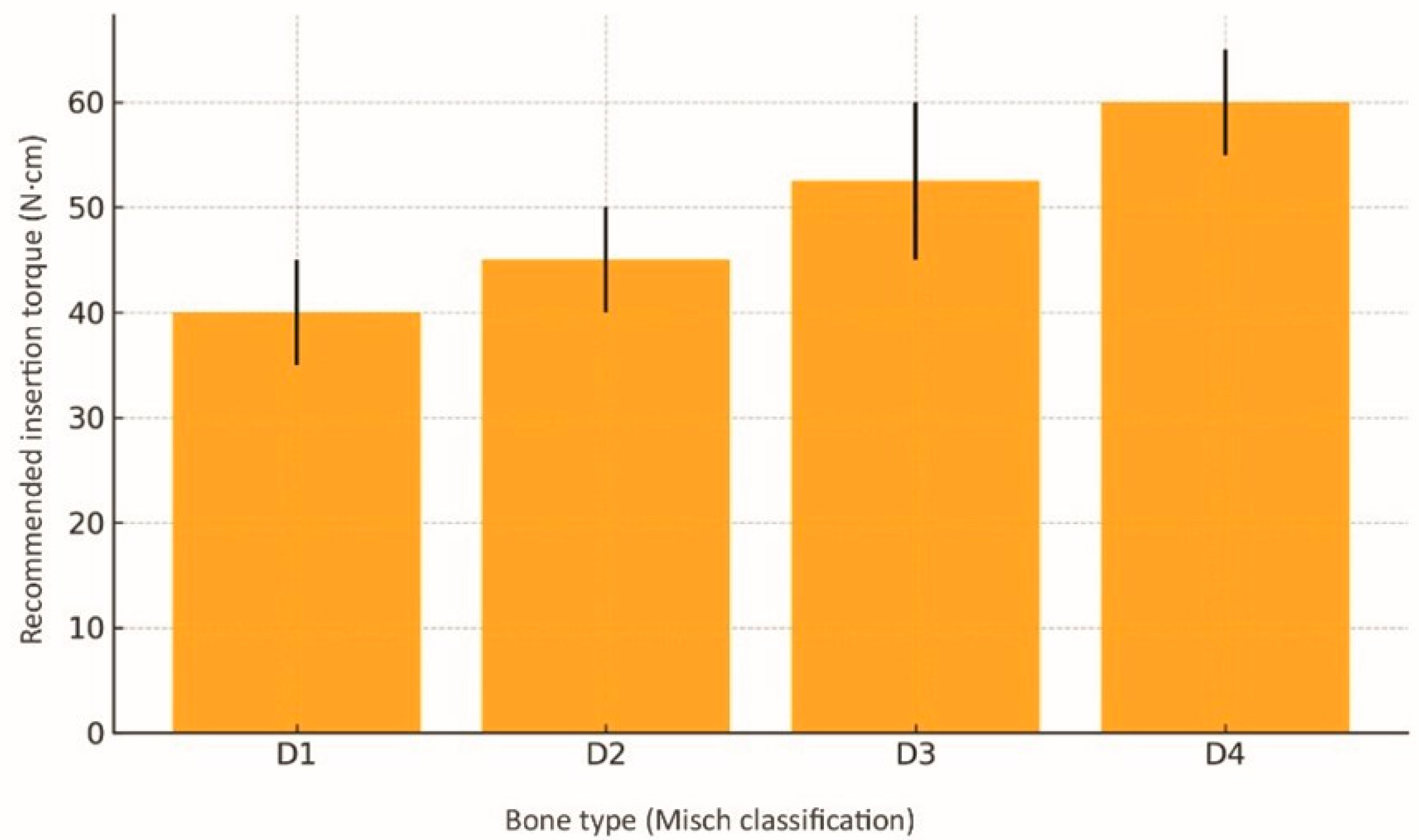
3.4. Clinical Applications in Different Bone Types
3.5. Benefits of Sinus Lift Procedures and Atrophic Jaws
3.6. Reported Complications and Operational Limitations
4. Discussion
4.1. Impact of Osseodensification on Primary Stability and Osseointegration
4.2. Clinical Applicability of OD in Different Bone Densities
4.3. OD as an Alternative to Invasive Reconstructive Procedures
4.4. Complications and Technical Challenges: Limitations of the Technique
4.5. Challenges and Strategies for Bone Densification in Patients with Systemic and Bone Limitations
4.6. Limitations
4.7. Clinical Implementation and Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| BAFO | Bone area fraction occupancy |
| BIC | Bone-to-implant contact |
| BMD | Bone mineral density |
| CBCT | Cone beam computed tomography |
| CD | Conventional drilling |
| GBR | Guided bone regeneration |
| ISQ | Implant stability quotient |
| NR | Not reported |
| OD | Osseodensification |
| RCT | Randomised controlled trial |
| SD | Standard deviation |
References
- Huwais, S.; Meyer, E.G. A novel osseous densification approach in implant osteotomy preparation to increase biomechanical primary stability, bone mineral density, and bone-to-implant contact. Int. J. Oral Maxillofac. Implant. 2016, 31, 601–610. [Google Scholar] [CrossRef]
- Oliveira, P.G.F.P.; Bergamo, E.T.P.; Neiva, R.; Bonfante, E.A.; Witek, L.; Tovar, N.; Coelho, P.G. Osseodensification outperforms conventional implant subtractive instrumentation: A study in sheep. Mater. Sci. Eng. C 2018, 90, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Lahens, B.; Neiva, R.; Tovar, N.; Alifarag, A.M.; Jimbo, R.; Bonfante, E.A.; Bowers, M.M.; Cuppini, M.; Freitas, H.; Witek, L.; et al. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone: An experimental study in sheep. J. Mech. Behav. Biomed. Mater. 2016, 63, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Witek, L.; Neiva, R.; Alifarag, A.; Shahraki, F.; Sayah, G.; Tovar, N.; Lopez, C.D.; Gil, L.; Coelho, P.G. Absence of healing impairment in osteotomies prepared via osseodensification drilling. Int. J. Periodontics Restor. Dent. 2019, 39, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Jimbo, R.; Witek, L.; Tovar, N.; Neiva, R.; Torroni, A.; Coelho, P.G. Biomaterial and biomechanical considerations to prevent risks in implant therapy. Periodontol. 2000 2019, 81, 139–151. [Google Scholar] [CrossRef]
- Macari, J.; Araújo, W.P.; Bella, A.P.G.S.N.; Ferreira, M.S.; Moerbeck-Filho, P.; Coelho, M.G.; Silva, L.C.; Moura, C.C.L.; Cruz, R.M.; Andrade, I.S.; et al. Avanços da osseodensificação na implantodontia: Benefícios clínicos, eficácia na osseointegração e perspectivas futuras. Braz. J. Implantol. Health Sci. 2024, 6, 1729–1738. [Google Scholar] [CrossRef]
- Machado, R.C.M.; Gama, C.S.; Batista, S.H.; Rizzo, D.; Valiense, H.; Moreira, R.F. Tomographic and clinical findings of osseodensification in immediate implant treatment. Int. J. Growth Factors Stem Cells Dent. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Tretto, P.H.W.; Fabris, V.; Cericato, G.O.; Sarkis-Onofre, R.; Bacchi, A. Does the instrument used for the implant site preparation influence the bone–implant interface? A systematic review of clinical and animal studies. Int. J. Oral Maxillofac. Surg. 2018, 47, 1441–1451. [Google Scholar] [CrossRef]
- Cardozo, C.G.T.; Cardoso, J.M.; Zacharías, A.D.; Fontão, F.N.G.K.; Oliveira, G.J.P.L.; Marcantonio Júnior, E. Comparação da expansão óssea promovida pela técnica de osseodensificação com dois tipos de conjunto de fresas. Rev. Odontol. UNESP 2022, 51, e20220044. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Joshi, A.A.; Nadgere, J.B. Biomechanical and histomorphometric analysis of endosteal implants placed by using the osseodensification technique in animal models: A systematic review and meta-analysis. J. Prosthet. Dent. 2022, 127, 61–70. [Google Scholar] [CrossRef]
- Yu, X.; Chang, C.; Guo, W.; Wu, Y.; Zhou, W.; Yu, D. Primary implant stability based on alternative site preparation techniques: A systematic review and meta-analysis. Clin. Implant Dent. Relat. Res. 2022, 24, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, E.T.P.; Zahoui, A.; Barrera, R.B.; Huwais, S.; Coelho, P.G.; Karateew, E.D.; Bonfante, E.A. Osseodensification effect on implants primary and secondary stability: Multicenter controlled clinical trial. Clin. Implant Dent. Relat. Res. 2021, 23, 317–328. [Google Scholar] [CrossRef]
- Slete, F.B.; Olin, P.; Prasad, H. Histomorphometric comparison of 3 osteotomy techniques. Implant Dent. 2018, 27, 424–428. [Google Scholar] [CrossRef]
- Bittar, B.F.; Sotto-Maior, B.S.; Devito, K.L.; Rabelo, G.D.; Machado, A.S.; Lopes, R.T.; Assis, N.M.S.P. Assessing peri-implant bone microarchitecture: Conventional vs. osseodensification drilling—Ex vivo analysis. Braz. Dent. J. 2024, 35, e245599. [Google Scholar] [CrossRef]
- Koutouzis, T.; Huwais, S.; Hasan, F.; Trahan, W.; Waldrop, T.; Neiva, R. Alveolar ridge expansion by osseodensification-mediated plastic deformation and compaction autografting: A multicenter retrospective study. Implant Dent. 2019, 28, 349–355. [Google Scholar] [CrossRef]
- Elsaid, M.G.; Elwaseef, S.; Abdelrahman, A.O.A.; Shuman, M.A. Transcrestal sinus lift with simultaneous implant placement using osseodensification in posterior maxilla with residual bone height of 4–6 mm. Braz. Dent. Sci. 2022, 25, e3403. [Google Scholar] [CrossRef]
- Arafat, S.W.; Elbaz, M.A. Clinical and radiographic evaluation of osseodensification versus osteotome for sinus floor elevation in partially atrophic maxilla: A prospective long-term study. Egypt. Dent. J. 2019, 65, 189–195. [Google Scholar] [CrossRef]
- Gaspar, J.; Botelho, J.; Proença, L.; Machado, V.; Chambrone, L.; Neiva, R.; Mendes, J.J. Osseodensification versus lateral window technique for sinus floor elevation with simultaneous implant placement: A randomized clinical trial on patient-reported outcome measures. Clin. Implant Dent. Relat. Res. 2024, 26, 113–126. [Google Scholar] [CrossRef]
- Salgar, N. Osseodensified crestal sinus window augmentation: An alternative procedure to the lateral window technique. J. Oral Implantol. 2021, 47, 45–52. [Google Scholar] [CrossRef]
- Stacchi, C.; Troiano, G.; Montaruli, G.; Mozzati, M.; Lamazza, L.; Antonelli, A.; Giudice, A.; Lombardi, T. Changes in implant stability using different site preparation techniques: Osseodensification drills versus piezoelectric surgery. Clin. Implant Dent. Relat. Res. 2023, 25, 133–140. [Google Scholar] [CrossRef]
- Mello-Machado, R.C.; Sartoretto, S.C.; Granjeiro, J.M.; Calasans-Maia, J.A.; Uzeda, M.J.P.G.; Mourão, C.F.A.B.; Ghiraldini, B.; Bezerra, F.J.B.; Senna, P.M.; Calasans-Maia, M.D. Osseodensification enables bone healing chambers with improved low-density bone site primary stability. Sci. Rep. 2021, 11, 15436. [Google Scholar] [CrossRef]
- Ibrahim, A.; Ayad, S.; ElAshwah, A. The effect of osseodensification technique on implant stability (clinical trial). Alexandria Dent. J. 2020, 45, 1–7. [Google Scholar] [CrossRef]
- Kanathila, H.; Pangi, A. An insight into the concept of osseodensification—Enhancing the implant stability and success. J. Clin. Diagn. Res. 2018, 12, ZE01–ZE03. [Google Scholar] [CrossRef]
- Lahens, B.; Lopez, C.D.; Neiva, R.F.; Bowers, M.M.; Jimbo, R.; Bonfante, E.A.; Morcos, J.; Witek, L.; Tovar, N.; Coelho, P.G. The effect of osseodensification drilling for endosteal implants with different surface treatments: A study in sheep. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 1928–1935. [Google Scholar] [CrossRef]
- Alifarag, A.M.; Lopez, C.D.; Neiva, R.F.; Tovar, N.; Witek, L.; Coelho, P.G. Temporal osseointegration: Early biomechanical stability through osseodensification. J. Orthop. Res. 2018, 36, 2636–2643. [Google Scholar] [CrossRef]
- Trisi, P.; Berardini, M.; Falco, A.; Podaliri Vulpiani, M. New osseodensification implant site preparation method to increase bone density in low-density bone: In vivo evaluation in sheep. Implant Dent. 2016, 25, 24–31. [Google Scholar] [CrossRef]
- Rodrigues, E.D.R.; De Morais, A.L.; Valença Freire, B.K.; Da Silva, M.E.L.; Vasconcelos, B.C.E. Instalação de implante em região posterior de maxila através da técnica de Summers e osseodensificação: Relato de caso. Rev. Cir. Traumatol. Buco-Maxilo-Fac. 2023, 23, 49–52. [Google Scholar]
- Ferreira Amancio, M.K.M.; Hipólito da Silva, S.D.B. Os benefícios da osseodensificação em implantes de carga imediata. Braz. J. Health Rev. 2024, 7, 256–266. [Google Scholar] [CrossRef]
- Tian, J.H.; Neiva, R.; Coelho, P.G.; Witek, L.; Tovar, N.; Lo, I.C.; Gil, L.F.; Torroni, A. Alveolar ridge expansion: Comparison of osseodensification and conventional osteotome techniques. J. Craniofac. Surg. 2018, 29, e559–e563. [Google Scholar] [CrossRef]
- Bleyan, S.; Gaspar, J.; Huwais, S.; Schwimer, C.; Mazor, Z.; Mendes, J.J.; Neiva, R. Molar septum expansion with osseodensification for immediate implant placement: Retrospective multicenter study with up-to-5-year follow-up, introducing a new molar socket classification. J. Funct. Biomater. 2021, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Samir, M.; Bissar, M.W.; Abuel-Ela, H.A. Osseodensification versus piezoelectric internal sinus elevation (PISE) technique in delayed implant placement: A randomized controlled clinical trial. BMC Oral Health 2024, 24, 1306. [Google Scholar] [CrossRef]
- Andrade, L.P.; Valiense, H.B. Osseodensificação como abordagem previsível para reabilitação em região posterior de maxila—Relato de caso. Rev. Ciênc. Saúde 2023, 28. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Barel, K.Z.; Maluf, P.S.Z. Maxillary sinus floor augmentation through bone densification. J. Surg. Surg. Res. 2020, 6, 149–151. [Google Scholar] [CrossRef]
- Baron, T.K.; Venkatraman, N. Minimally invasive crestal approach sinus floor elevation using Densah burs, and hydraulic lift utilising putty graft in cartridge delivery. Clin. Oral Implants Res. 2017, 28, 203. [Google Scholar] [CrossRef]
- Jarikian, S.; Jaafo, M.H.; Al-Nerabieah, Z. Clinical evaluation of two techniques for narrow alveolar ridge expansion: Clinical study. J. Dent. Oral Sci. 2021, 8, 1047–1052. [Google Scholar] [CrossRef]
- Guentsch, A.; An, H.; Dentino, A.R. Precision and trueness of computer-assisted implant placements using static surgical guides with open and closed sleeves: An in vitro analysis. Clin. Oral Implant. Res. 2022, 33, 441–450. [Google Scholar] [CrossRef]
- Mele, R.E.; Kurtzman, G.M. Feline dental implants: New paradigm shift in maxillary alveolar osteitis treatment planning with osseodensification. J. Osseointegr. 2019, 11, 485–492. [Google Scholar] [CrossRef]
- Cisternas Covarrubias, S.; Sánchez Varela, M.; Brenner Agosín, C. Evaluación de cambios dimensionales en ancho y altura del reborde óseo alveolar mediante la técnica de oseodensificación en zonas posteriores del maxilar: Reporte de serie de casos. Int. J. Odontostomatol. 2023, 17, 55–63. [Google Scholar] [CrossRef]
- Mello-Machado, R.C.; Mourão, C.F.A.B.; Javid, K.; Ferreira, H.T.; Montemezzi, P.; Calasans-Maia, M.D.; Senna, P.M. Clinical assessment of dental implants placed in low-quality bone sites prepared for the healing chamber with osseodensification concept: A double-blind, randomized clinical trial. Appl. Sci. 2021, 11, 640. [Google Scholar] [CrossRef]
- Torroni, A.; Lima, P.E.; Witek, L.; Hacquebord, J.H.; Coelho, P.G. Osseodensification drilling vs conventional manual instrumentation technique for posterior lumbar fixation: Ex vivo mechanical and histomorphological analysis in an ovine model. J. Orthop. Res. 2021, 39, 1463–1469. [Google Scholar] [CrossRef]
- Rosa, J.C.M.d.; Rosa, A.C.P.d.O.; Huwais, S. Use of the immediate dentoalveolar restoration technique combined with osseodensification in periodontally compromised extraction sites. Int. J. Periodontics Restor. Dent. 2019, 39, 527–534. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; López-Píriz, R.; Peñarrocha, M. Preoperative evaluation and treatment planning: Zygomatic implant critical zone (ZICZ) location. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2021, 29, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Olivo, A.; de Paz, V.; Kraus, D.; Martin Luque, M.; Crooke, E.; Simon, P.; Simon, M.; Ferreira, J.; Sakima Serrano, A.; et al. The zygoma anatomy-guided approach (ZAGA) for rehabilitation of the atrophic maxilla. Clin. Dent. Rev. 2022, 6, 2. [Google Scholar] [CrossRef]
- Tanello, B.; Huwais, S.; Tawil, I.; Rosen, P.; Neiva, R. Osseodensification protocols for enhancement of primary and secondary implant stability: A retrospective five-year follow-up multi-center study. Clin. Oral Implant. Res. 2019, 30, 414. [Google Scholar] [CrossRef]
- Lopez, C.D.; Alifarag, A.M.; Torroni, A.; Tovar, N.; Diaz-Siso, J.R.; Witek, L.; Rodriguez, E.D.; Coelho, P.G. Osseodensification for enhancement of spinal surgical hardware fixation. J. Mech. Behav. Biomed. Mater. 2017, 69, 275–281. [Google Scholar] [CrossRef]
- Pai, U.Y.; Rodrigues, S.J.; Talreja, K.S.; Mundathaje, M. Osseodensification—A novel approach in implant dentistry. J. Indian Prosthodont. Soc. 2018, 18, 196–200. [Google Scholar] [CrossRef]
- Althobaiti, A.K.; Ashour, A.W.; Halteet, F.A.; Alghamdi, S.I.; AboShetaih, M.M.; Al-Hayazi, A.M.; Saaduddin, A.M. A comparative assessment of primary implant stability using osseodensification vs. conventional drilling methods: A systematic review. Cureus 2023, 15, e46841. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, N. Tuning surface properties of bone biomaterials to manipulate osteoblastic cell adhesion and the signaling pathways for the enhancement of early osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontol. 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Goriainov, V.; Cook, R.; Latham, J.M.; Dunlop, D.G.; Oreffo, R.O. Bone and metal: An orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014, 10, 4043–4057. [Google Scholar] [CrossRef]
- Sartoretto, S.C.; Alves, A.T.; Resende, R.F.; Calasans-Maia, J.; Granjeiro, J.M.; Calasans-Maia, M.D. Early osseointegration driven by the surface chemistry and wettability of dental implants. J. Appl. Oral Sci. 2015, 23, 279–287. [Google Scholar] [CrossRef]
- Jones, S.E.; Nichols, L.; Elder, S.H.; Priddy, L.B. Laser microgrooving and resorbable blast texturing for enhanced surface function of titanium alloy for dental implant applications. Biomed. Eng. Adv. 2023, 5, 100090. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of dental implant surface modifications on osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]




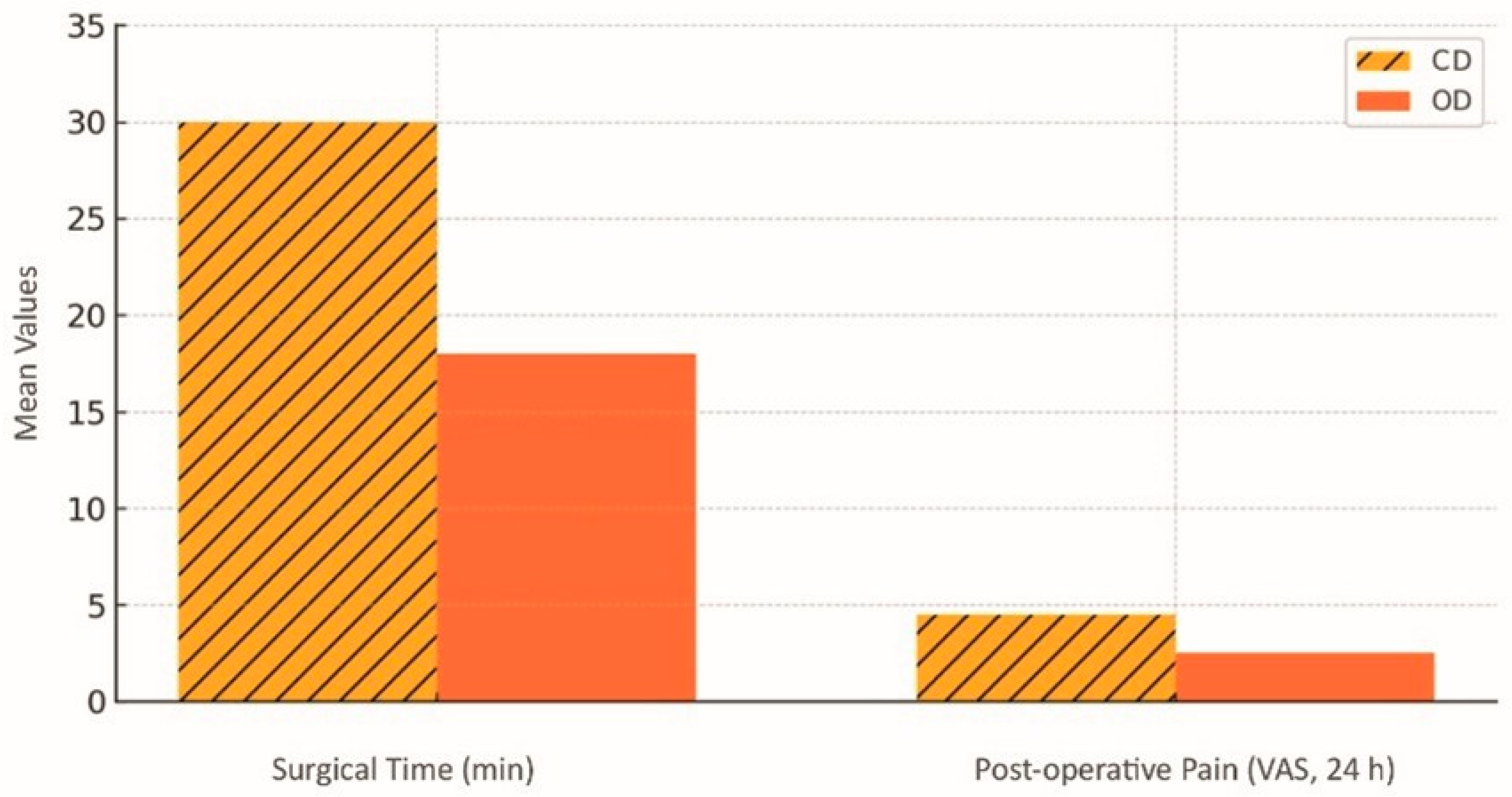

| Misch Bone Type | Typical Cortical/ Trabecular Aspect | Pilot Drill ø (mm) | Osseodensifying Bur Sequence | Densification Speed (rpm, Counterclockwise) | Target Insertion Torque (N·cm) | Axial Pressure and Irrigation/Clinical Remarks |
|---|---|---|---|---|---|---|
| D1 (dense cortical) anterior mandible | Cortical ≥ 2 mm, scant medullary bone | 2.0 | 2.5 → 3.0 (optional)—avoid oversizing | 600–800 | 35–45 | Light pressure: abundant irrigation with minimal under-preparation; risk of overcompression/thermal necrosis. |
| D2 (thick cortical, medium trabeculae) | Cortical 1–2 mm | 2.0 | 2.5 → 3.5 | 800–1000 | 40–50 | Light to moderate pressure; “pumping” 2 s/2 s constant flushing; pause every 3 mm to dissipate heat. |
| D3 (thin cortical, moderate trabeculae) | Cortical ≤ 1 mm | 1.8 | 2.0 → 3.0 → 4.0 | 1000–1300 | 45–60 | Moderate pressure, pumping 3 s/2 s allows ridge expansion of 1–2 mm without microfractures; ideal for the posterior maxilla. |
| D4 (thin cortical, sparse trabeculae) | Cortical < 0.5 mm | 1.8 | 2.0 → 2.8 → 3.8 (implant Ø 4.2) | 1300–1500 | 55–65 | Firm, controlled pressure; dual-pass recommended; use prolonged reverse rotation (4 s) to maximise compaction; facilitates immediate loading. |
| No. | Study (Year/Design) | n (Implants) | Anatomical Region/Bone Type | Mean Torque (N·cm) | Initial ISQ (OD/CD) | 1-Year Success (OD/CD) | Key Observations |
|---|---|---|---|---|---|---|---|
| 1 | Bergamo et al. (2021) [12]—multicentre controlled | 75 | Maxilla and mandible/D3–D4 | 50 ± 12 | 78/69 | 98.7%/96.0% | Significant gain in primary and secondary stability |
| 2 | Koutouzis & Huwais (2019) [15]—retrospective | 56 | Posterior maxilla/ridge ≤ 3 mm | 61 ± 14 | 77/NR | 92.8%/— | Mean ridge expansion of 2.8 mm without grafts |
| 3 | Elsaid et al. (2022) [16]—RCT transcrestal sinus | 20 | Posterior maxilla/height 4–6 mm | 45 ± 10 | 61 → 80 | 100%/— | Zero membrane perforations; bone gain +3.5 mm |
| 4 | Arafat & Elbaz (2019) [17]—prospective clinical study | 40 | Posterior maxilla/residual bone height 4–7 mm | 48 ± 10/42 ± 9 | 71/66 | 97.5%/95.% | OD achieved greater bone gain and higher implant survival compared with the osteotome technique |
| 5 | Gaspar et al. (2024) [18]—RCT sinus floor | 60 | Posterior maxilla/D4 | 48 ± 11 | 75/70 | 96.7%/94.5% | Lower self-reported pain in OD group |
| 6 | Salgar (2021) [19]—case series | 25 | Posterior maxilla/height ≤ 1.5 mm | 50 | NR | 96%/— | Vertical gain 10–14 mm (crestal OD) |
| 7 | Stacchi et al. (2023) [20]—RCT (OD vs. piezosurgery) | 60 | Various/D2–D4 | 40 ± 9/38 ± 8 | 70/72 | 98.3%/98.3% | Only 1 failure (OD) within 90 days |
| 8 | Mello-Machado et al. (2021) [21]—double-blind RCT | 30 | Posterior mandible/D3–D4 | 46 ± 8/33 ± 7 | 73/65 | 100%/— | Healing chambers enhance BAFO↑ |
| No. | Study/Model | Primary Metric | OD (Mean ± SD) | CD (Mean ± SD) | Δ OD vs. CD |
|---|---|---|---|---|---|
| 1 | Lahens (2016) [3]—ovine, 6 weeks | BIC (%) | 58 ± 6 | 43 ± 7 | +35% |
| 2 | Oliveira (2018) [2]—ovine, 12 weeks | BAFO (%) | 41 ± 5 | 30 ± 4 | +37% |
| 3 | Alifarag (2018) [25]—ovine, 3 and 6 weeks | Pull-out torque (N·cm) | 76 ± 9 | 54 ± 8 | +41% |
| 4 | Trisi (2016) [26]—ovine, 8 weeks | Peri-implant bone density (g/cm3) | 0.82 ± 0.07 | 0.62 ± 0.05 | +32% |
| 5 | Witek (2019) [4]—ovine, 4 weeks | Trabecular thickness (mm) | 0.23 ± 0.02 | 0.17 ± 0.02 | +35% |
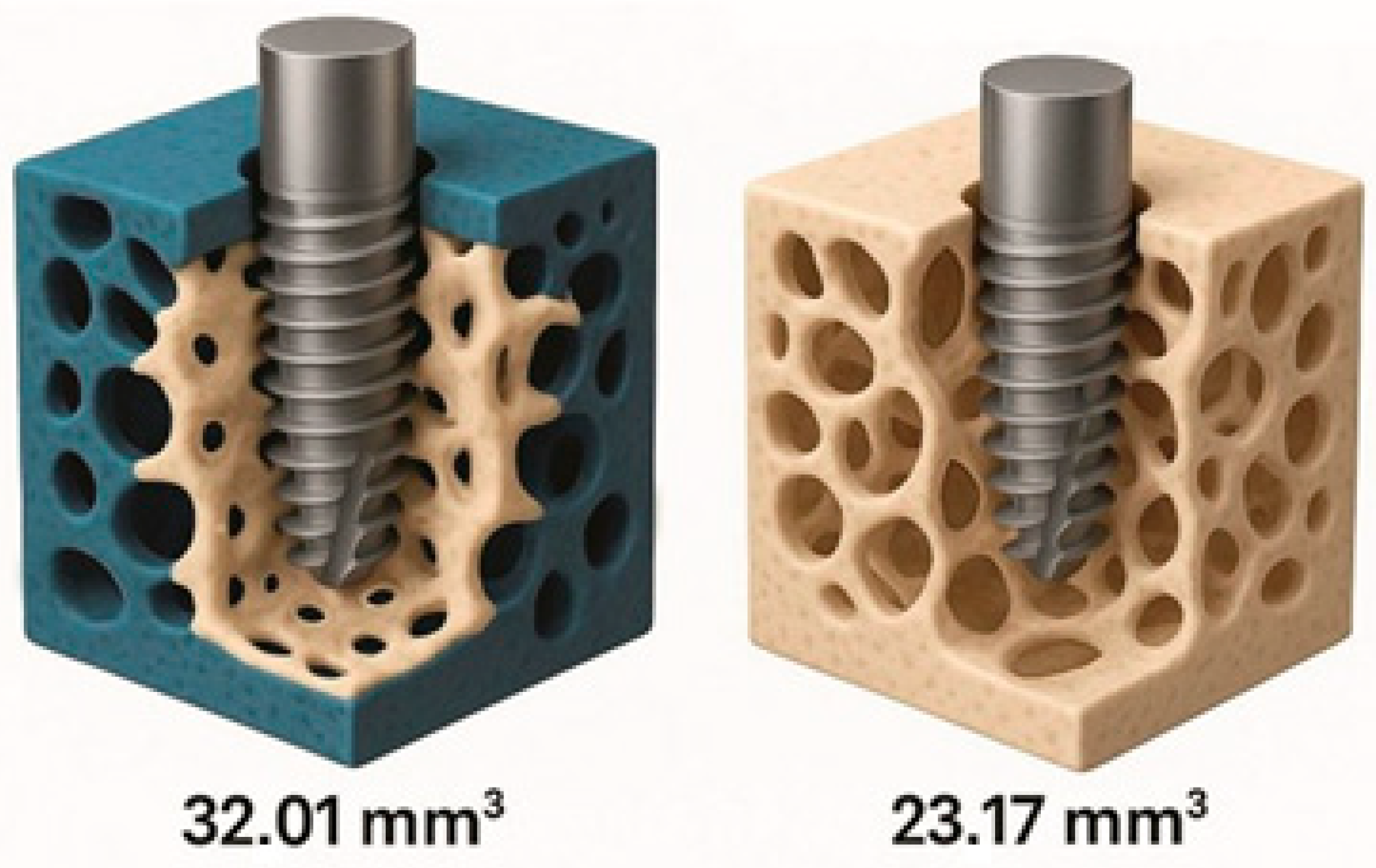
| 6 | Bittar (2024) [14]—porcine ex vivo | Peri-implant bone volume 3 mm (mm3) | 32.0 ± 5.8 | 23.2 ± 3.4 | +38% |
| No. | Surgical Indication | Associated OD Procedure | Documented Advantages | Limitations/Precautions | Key Evidence (Year)/Level |
|---|---|---|---|---|---|
| 1 | Transcrestal sinus lift in posterior maxilla (residual height ≤ 6 mm) | OD burs drill 1 mm below sinus floor → compaction + biomaterial pumping |
| Thick membrane or large septa may require a lateral window; ensure copious irrigation. | Elsaid 2022; Gaspar 2024; Salgar 2021/1B [16,18,19] |
| 2 | Horizontal ridge expansion (≤4 mm) | OD sequence 1–2 diameters below final implant, “spring-back” 1–3 mm |
| Very thin cortical (<0.5 mm) at risk of fractures; moderate pressure advised. | Koutouzis 2019; Cisternas 2023/2B [15,29] |
| 3 | Immediate loading in D3–D4 bone | Compaction until torque 55–65 N·cm; ISQ ≥ 70 immediately after insertion |
| Requires balanced occlusion; bruxist patients need splints. | Bergamo 2021; Ferreira Amancio 2024/2A [12,28] |
| 4 | Atrophic post-extraction socket (Types III–IV) | OD concurrent with septum drilling → healing chambers |
| Check integrity of thin buccal plate; gradual torque advised. | Bleyan 2021; Mello-Machado 2021/2B [21,30] |
| 5 | Minimally invasive lateral sinus lift (height ≤ 3 mm) | OD perforates thin lateral wall + compacts graft into sinus |
| Long learning curve; limited visibility. | Samir 2024 [31]/3B |
| No. | Potential Complication | Typical Risk Scenario | OD-Specific Preventive/Corrective Strategy | Key Evidence (Year) † | Reported Incidence |
|---|---|---|---|---|---|
| 1 | Thermal necrosis | Rotation > 1500 rpm without continuous irrigation, especially in D1 |
| Huwais 2016; Witek 2019 [1,4] | <1% |
| 2 | Cortical overcompression/microcrack fracture | Use of full OD sequence in thick D1 bone |
| Bergamo 2021 [12] | Isolated cases |
| 3 | Sinus membrane perforation | Transcrestal lift in high septa or thin membrane |
| Elsaid 2022; Gaspar 2024 [16,18] | 0–2% |
| 4 | Buccal cortical plate fracture | Ridge expansion ≥ +3 mm in cortical < 0.5 mm |
| Koutouzis 2019 [15] | 3–5% |
| 5 | Excessive torque → implant rotation | D4 bone under aggressive under-preparation |
| Lahens et al., 2016 [3] | <1% |
| 6 | Increased postoperative oedema/pain | Continuous axial pressure > 15 N in D3 bone |
| Salgar 2021 [19] | Similar to CD |
| 7 | Micromovement during immediate loading | Improper prosthetic adjustment/bruxism |
| Bergamo 2021 [12] | 2–4% early failures |
| 8 | Late peri-implantitis due to initial overheating | Inadequate irrigation + rough implant surface |
| Mello-Machado 2021 [21] | Not quantified |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, R.; Maurício, P.; Mascarenhas, P.S. Densifying the Future: A Critical Review of Osseodensification and Implant Dentistry. Dent. J. 2025, 13, 461. https://doi.org/10.3390/dj13100461
Ortiz R, Maurício P, Mascarenhas PS. Densifying the Future: A Critical Review of Osseodensification and Implant Dentistry. Dentistry Journal. 2025; 13(10):461. https://doi.org/10.3390/dj13100461
Chicago/Turabian StyleOrtiz, Rafael, Paulo Maurício, and Paulo Sobral Mascarenhas. 2025. "Densifying the Future: A Critical Review of Osseodensification and Implant Dentistry" Dentistry Journal 13, no. 10: 461. https://doi.org/10.3390/dj13100461
APA StyleOrtiz, R., Maurício, P., & Mascarenhas, P. S. (2025). Densifying the Future: A Critical Review of Osseodensification and Implant Dentistry. Dentistry Journal, 13(10), 461. https://doi.org/10.3390/dj13100461








