Hyaluronic Acid in Bone Regeneration: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Population, Intervention, Comparison, Outcomes, and Study Design
2.3. Inclusion and Exclusion Criteria
2.4. Types of Intervention
2.5. Outcome Measures
2.6. Search Strategy
2.7. Selection Criteria and Data Analysis
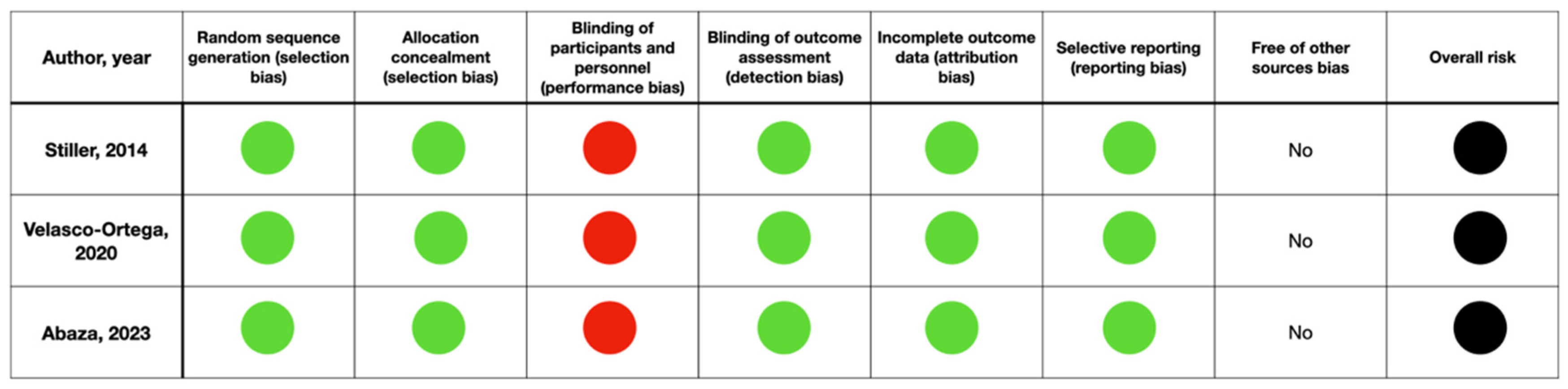
2.8. Risk of Bias
2.9. Statistical Analysis
3. Results
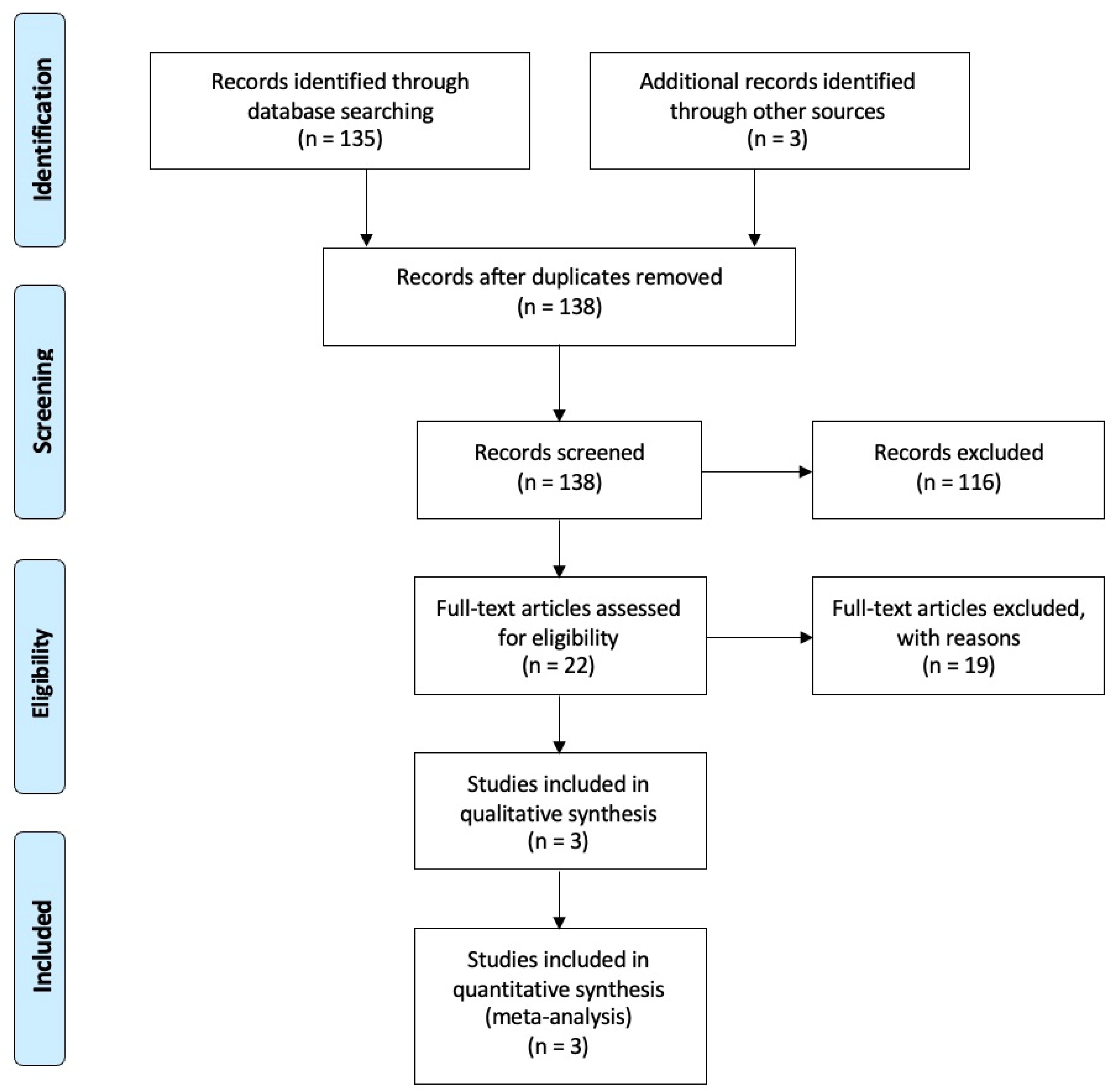
3.1. Included Studies
3.2. Excluded Studies
3.3. Study Characteristics
3.4. Included Studies’ Heterogeneity
3.5. New Bone Formation
3.6. Remaining Graft Particles
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, H.L.; Boyapati, L. “PASS” principles for predictable bone regeneration. Implant. Dent. 2006, 15, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Brighton, C.T.; Hunt, R.M. Early histological and ultrastructural changes in medullary fracture callus. J. Bone Jt. Surg. Am. 1991, 73, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Carosi, P.; Lorenzi, C.; Di Gianfilippo, R.; Papi, P.; Laureti, A.; Wang, H.L.; Arcuri, C. Immediate vs. Delayed Placement of Immediately Provisionalized Self-Tapping Implants: A Non-Randomized Controlled Clinical Trial with 1 Year of Follow-Up. J. Clin. Med. 2023, 12, 489. [Google Scholar] [CrossRef]
- Moussa, N.T.; Dym, H. Maxillofacial Bone Grafting Materials. Dent. Clin. N. Am. 2020, 64, 473–490. [Google Scholar] [CrossRef] [PubMed]
- De Risi, V.; Clementini, M.; Vittorini, G.; Mannocci, A.; De Sanctis, M. Alveolar ridge preservation techniques: A systematic review and meta-analysis of histological and histomorphometrical data. Clin. Oral Implants Res. 2015, 26, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The roles of signaling pathways in bone repair and regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, R.; Jelena, P. Hyaluronic acid: The reason for its variety of physiological and biochemical functional properties. Appl. Clin. Res. Clin. Trials Regul. Aff. 2019, 6, 112–159. [Google Scholar] [CrossRef]
- West, D.C.; Hampson, I.N.; Arnold, F.; Kumar, S. Angiogenesis induced by degradation products of hyaluronic acid. Science 1985, 228, 1324–1326. [Google Scholar] [CrossRef] [PubMed]
- Hemshekhar, M.; Thushara, R.M.; Chandranayaka, S.; Sherman, L.S.; Kemparaju, K.; Girish, K.S. Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine. Int. J. Biol. Macromol. 2016, 86, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta 2014, 1840, 2452–2459. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Y.Y.; Koo, P.L.; Lee, K.M.; Qin, L.; Cheng, J.C.Y.; Kumta, S.M. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell culture. J. Biomed. Mater. Res. A 2003, 66, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Yeh, Y.Y.; Lung, J.; Yang, Y.C.; Yuan, K. Mineralization Effect of Hyaluronan on Dental Pulp Cells via CD44. J. Endod. 2016, 42, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, E.R.; Boeckel, D.G.; Fulginiti, R.L.; Shinkai, R.S.A.; Machado, D. Mesenchymal stem cells and hyaluronic acid for bone grafting. Clin. Oral Implant. Res. 2018, 29, 12724. [Google Scholar] [CrossRef]
- Sudheesh Kumar, P.T.; Hashimi, S.; Saifzadeh, S.; Ivanovski, S.; Vaquette, C. Additively manufactured biphasic construct loaded with BMP-2 for vertical bone regeneration: A pilot study in rabbit. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Al-Khateeb, R.; Olszewska-Czyz, I. Biological molecules in dental applications: Hyaluronic acid as a companion biomaterial for diverse dental applications. Heliyon 2020, 6, e03722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, J.J.; Song, H.Y.; Ben Amara, H.; Kyung-Rim, K.; Koo, K.T. Hyaluronic Acid Improves Bone Formation in Extraction Sockets With Chronic Pathology: A Pilot Study in Dogs. J. Periodontol. 2016, 87, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Ben Amara, H.; Park, J.C.; Kim, S.; Kim, T.I.; Seol, Y.J.; Lee, Y.M.; Ku, Y.; Rhyu, I.C.; Koo, K.T. Biomodification of compromised extraction sockets using hyaluronic acid and rhBMP-2: An experimental study in dogs. J. Periodontol. 2019, 90, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.E.; Salter, D.M.; Simpson, R. CD44 expression in human bone: A novel marker of osteocytic differentiation. J. Bone Miner. Res. 1994, 9, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Bozic, D.; Grgurevic, L.; Erjavec, I.; Brkljacic, J.; Orlic, I.; Razdorov, G.; Grgurevic, I.; Vukicevic, S.; Plancak, D. The proteome and gene expression profile of cementoblastic cells treated by bone morphogenetic protein-7 in vitro. J. Clin. Periodontol. 2012, 39, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.; Yang, Y.; Yuan, K. Importance of CD44 in the proliferation and mineralization of periodontal ligament cells. J. Periodontal Res. 2014, 49, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Hakki, S.S.; Bozkurt, S.B.; Sculean, A.; Božić, D. Hyaluronic acid enhances cell migration, viability, and mineralized tissue-specific genes in cementoblasts. J. Periodontal Res. 2024, 59, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Mitsuhashi, N.; Klein, A.; Barsky, L.W.; Weinberg, K.; Barr, M.L.; Demetriou, A.; Wu, G.D. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem. Cells 2006, 24, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Gong, Y.; Lao, L.; Mao, Z.; Gao, C. Gelatin/chitosan/hyaluronan ternary complex scaffold containing basic fibroblast growth factor for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2007, 18, 1961–1968. [Google Scholar] [CrossRef] [PubMed]
- Romanò, C.L.; De Vecchi, E.; Bortolin, M.; Morelli, I.; Drago, L. Hyaluronic Acid and Its Composites as a Local Antimicrobial/Antiadhesive Barrier. J. Bone Jt. Infect. 2017, 2, 63–72. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stiller, M.; Kluk, E.; Bohner, M.; Lopez-Heredia, M.A.; Müller-Mai, C.; Knabe, C. Performance of β-tricalcium phosphate granules and putty, bone grafting materials after bilateral sinus floor augmentation in humans. Biomaterials 2014, 35, 3154–3163. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Ortega, E.; Valente, N.A.; Iezzi, G.; Petrini, M.; Derchi, G.; Barone, A. Maxillary sinus augmentation with three different biomaterials: Histological, histomorphometric, clinical, and patient-reported outcomes from a randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Abaza, G.; Abdel Gaber, H.K.; Afifi, N.S.; Adel-Khattab, D. Injectable platelet rich fibrin versus hyaluronic acid with bovine derived xenograft for alveolar ridge preservation. A randomized controlled clinical trial with histomorphometric analysis. Clin. Implant. Dent. Relat. Res. 2024, 26, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Cantore, S.; Capodiferro, S.; Grassi, F.R. Esterified hyaluronic acid and autologous bone in the surgical correction of the infra-bone defects. Int. J. Med. Sci. 2009, 6, 65–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Božić, D.; Ćatović, I.; Badovinac, A.; Musić, L.; Par, M.; Sculean, A. Treatment of Intrabony Defects with a Combination of Hyaluronic Acid and Deproteinized Porcine Bone Mineral. Materials 2021, 14, 6795. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Santana, R.B.; de Santana, C.M. Human intrabony defect regeneration with rhFGF-2 and hyaluronic acid—A randomized controlled clinical trial. J. Clin. Periodontol. 2015, 42, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Mamajiwala, A.S.; Sethi, K.S.; Raut, C.P.; Karde, P.A.; Mamajiwala, B.S. Clinical and radiographic evaluation of 0.8% hyaluronic acid as an adjunct to open flap debridement in the treatment of periodontal intrabony defects: Randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5257–5271. [Google Scholar] [CrossRef] [PubMed]
- Sehdev, B.; Bhongade, M.L.; Ganji, K.K. Evaluation of effectiveness of hyaluronic acid in combination with bioresorbable membrane (poly lactic acid-poly glycolic acid) for the treatment of infrabony defects in humans: A clinical and radiographic study. J. Indian Soc. Periodontol. 2016, 20, 50–56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Engström, P.E.; Shi, X.Q.; Tronje, G.; Larsson, A.; Welander, U.; Frithiof, L.; Engstrom, G.N. The effect of hyaluronan on bone and soft tissue and immune response in wound healing. J. Periodontol. 2001, 72, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Briguglio, E.; Briguglio, R.; Cafiero, C.; Isola, G. Treatment of infrabony periodontal defects using a resorbable biopolymer of hyaluronic acid: A randomized clinical trial. Quintessence Int. 2013, 44, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Rojas, M.A.; Marini, L.; Russo, P.; Shirakata, Y.; Sculean, A.; Iacono, R. Healing of intrabony defects following regenerative surgery by means of single-flap approach in conjunction with either hyaluronic acid or an enamel matrix derivative: A 24-month randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 5095–5107. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vanden Bogaerde, L. Treatment of infrabony periodontal defects with esterified hyaluronic acid: Clinical report of 19 consecutive lesions. Int. J. Periodontics Restorative Dent. 2009, 29, 315–323. [Google Scholar] [PubMed]
- Kaya, O.A.; Muglali, M.; Torul, D.; Kaya, I. Peri-implant bone defects: A 1-year follow-up comparative study of use of hyaluronic acid and xenografts. Niger. J. Clin. Pract. 2019, 22, 1388–1395. [Google Scholar] [CrossRef] [PubMed]
- D’Albis, G.; D’Albis, V.; Palma, M.; Plantamura, M.; Nizar, A.K. Use of hyaluronic acid for regeneration of maxillofacial bones. Genesis 2022, 60, e23497. [Google Scholar] [CrossRef] [PubMed]
- Ostos-Aguilar, B.I.; Pinheiro Furquim, C.; Muniz, F.W.M.G.; Faveri, M.; Meza-Mauricio, J. Clinical efficacy of hyaluronic acid in the treatment of periodontal intrabony defect: A systematic review and meta-analysis. Clin. Oral Investig. 2023, 27, 1923–1935. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Barbeck, M.; Kirkpatrick, C.J.; Sader, R.; Lerner, H.; Ghanaati, S. Injectable Bone Substitute Material on the Basis of β-TCP and Hyaluronan Achieves Complete Bone Regeneration While Undergoing Nearly Complete Degradation. Int. J. Oral Maxillofac. Implants 2018, 33, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Göçmen, G.; Atalı, O.; Aktop, S.; Sipahi, A.; Gönül, O. Hyaluronic Acid Versus Ultrasonic Resorbable Pin Fixation for Space Maintenance in Non-Grafted Sinus Lifting. J. Oral Maxillofac. Surg. 2016, 74, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Eeckhout, C.; Ackerman, J.; Glibert, M.; Cosyn, J. A randomized controlled trial evaluating hyaluronic acid gel as wound healing agent in alveolar ridge preservation. J. Clin. Periodontol. 2022, 49, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Husseini, B.; Friedmann, A.; Wak, R.; Ghosn, N.; Khoury, G.; El Ghoul, T.; Abboud, C.K.; Younes, R. Clinical and radiographic assessment of cross-linked hyaluronic acid addition in demineralized bovine bone based alveolar ridge preservation: A human randomized split-mouth pilot study. J. Stomatol. Oral Maxillofac. Surg. 2023, 124, 101426. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, C.E.P.; Castro, M.A.A.; Noronha, M.S.; Martins-Junior, P.A.; Mendes, R.M.; Caliari, M.V.; Mesquita, R.A.; Ferreira, A.J. Hyaluronic acid accelerates bone repair in human dental sockets: A randomized triple-blind clinical trial. Braz. Oral Res. 2018, 32, e84. [Google Scholar] [CrossRef] [PubMed]
- Baldini, A.; Zaffe, D.; Nicolini, G. Bone-defects healing by high-molecular hyaluronic acid: Preliminary results. Ann. Stomatol. 2010, 1, 2–7. [Google Scholar] [PubMed] [PubMed Central]
- Kauffmann, F.; Fickl, S.; Sculean, A.; Fischer, K.R.; Friedmann, A. Alveolar ridge alterations after lateral guided bone regeneration with and without hyaluronic acid: A prospective randomized trial with morphometric and histomorphometric evaluation. Quintessence Int. 2023, 54, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, C.; Lio, F.; Papi, P.; Mazzetti, V.; Laureti, A.; Arcuri, C. Clinical Reliability of Complete-Arch Fixed Prostheses Supported by Narrow-Diameter Implants to Support Complete-Arch Restorations. Appl. Sci. 2023, 13, 538. [Google Scholar] [CrossRef]
- Tan, W.L.; Wong, T.L.; Wong, M.C.; Lang, N.P. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin. Oral Implants Res. 2012, 23 (Suppl. 5), 1–21. [Google Scholar] [CrossRef] [PubMed]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restorative Dent. 2003, 23, 313–323. [Google Scholar] [PubMed]
- Galarraga-Vinueza, M.E.; Barootchi, S.; Nevins, M.L.; Nevins, M.; Miron, R.J.; Tavelli, L. Twenty-five years of recombinant human growth factors rhPDGF-BB and rhBMP-2 in oral hard and soft tissue regeneration. Periodontology 2000 2024, 94, 483–509. [Google Scholar] [CrossRef] [PubMed]
- Carosi, P.; Ottria, L.; Lio, F.; Laureti, A.; Papi, P. The health of soft tissues around four dental implants loaded immediately supporting a 4-year-old fixed screw-retained prosthesis. J. Biol. Regul. Homeost. Agents 2021, 35 (Suppl. 1), 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, A.; Carosi, P.; Gallucci, G.O.; Nagy, K.; Nardi, A.; Arcuri, L. Accuracy of complete-arch digital implant impression with intraoral optical scanning and stereophotogrammetry: An in vivo prospective comparative study. Clin. Oral Implants Res. 2023, 34, 1106–1117. [Google Scholar] [CrossRef]
- Leggeri, A.; Carosi, P.; Mazzetti, V.; Arcuri, C.; Lorenzi, C. Techniques to Improve the Accuracy of Intraoral Digital Impression in Complete Edentulous Arches: A Narrative Review. Appl. Sci. 2023, 13, 7068. [Google Scholar] [CrossRef]
- Domic, D.; Bertl, K.; Lang, T.; Pandis, N.; Ulm, C.; Stavropoulos, A. Hyaluronic acid in tooth extraction: A systematic review and meta-analysis of preclinical and clinical trials. Clin. Oral Investig. 2023, 27, 7209–7229. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carosi, P.; Ferrigno, N.; Arcuri, C.; Laureti, M. Computer-Aided Surgery and Immediate Loading to Rehabilitate Complete Arch with Four Dental Implants and Fixed Screw-Retained Prosthesis Up to 4 Years in Function: A Retrospective Study. Int. J. Oral Maxillofac. Implants 2021, 36, 1180–1187. [Google Scholar] [CrossRef]
- Zijderveld, S.A.; Zerbo, I.R.; van den Bergh, J.P.; Schulten, E.A.; ten Bruggenkate, C.M. Maxillary sinus floor augmentation using a beta-tricalcium phosphate (Cerasorb) alone compared to autogenous bone grafts. Int. J. Oral Maxillofac. Implants 2005, 20, 432–440. [Google Scholar] [PubMed]
- Kalk, W.W.; Raghoebar, G.M.; Jansma, J.; Boering, G. Morbidity from iliac crest bone harvesting. J. Oral Maxillofac. Surg. 1996, 54, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
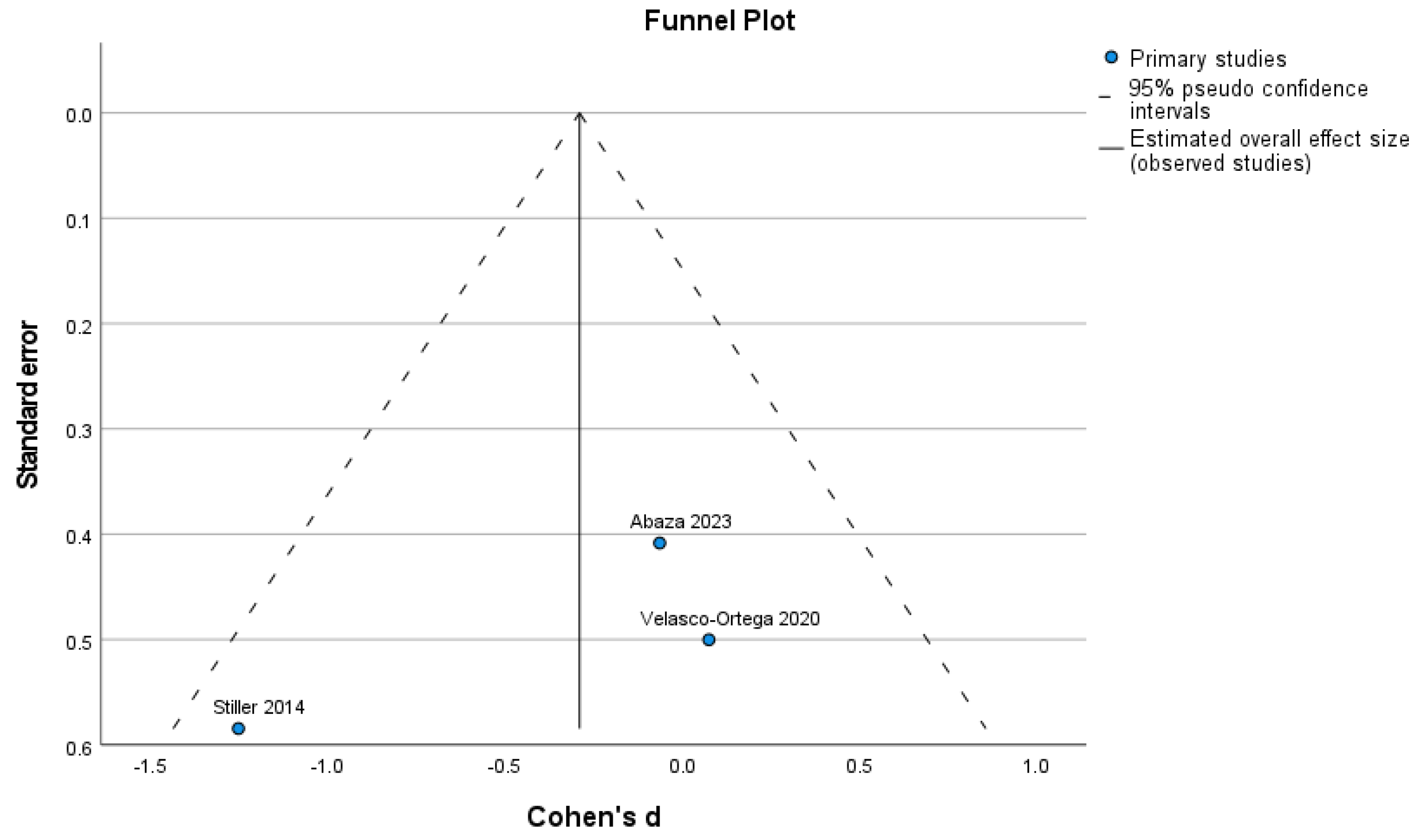
| Study | Study Design | Number of HA Cases | % New Bone HA | % Particles HA | Number of Control Cases | % New Bone Control | % Particles Control |
|---|---|---|---|---|---|---|---|
| Stiller et al., 2014 [27] | Randomized split-mouth | 7 | 30.1 | 29.5 | 7 | 17.4 | 32.9 |
| Velasco-Ortega et al., 2020 [28] | Randomized controlled trial | 8 | 23.29 | 7.17 | 8 | 23.85 | 7.17 |
| Abaza et al., 2023 [29] | Randomized controlled trial | 12 | 56.66 | 2.63 | 12 | 24.05 | 2.71 |
| Studies | Exclusion Reason |
|---|---|
| Ballini et al., 2009 [30] Božić et al., 2021 [31] de Santana et al., 2015 [32] Mamajiwala et al., 2021 [33] Sehdev et al., 2016 [34] Engström et al., 2001 [35] Briguglio et al., 2013 [36] Pilloni et al., 2021 [37] Vanden Bogaerde et al., [38] | The focus of these studies is periodontal surgery |
| Kaya et al., 2019 [39] | The main focus was not bone regeneration |
| D’Albis et al., 2022 [40] Ostos-Aguilar et al., 2023 [41] Lorenz et al., 2018 [42] | Study design different from RCTs |
| Göçmen et al., 2016 [43] Eeckhout et al., 2022 [44] | HA was not mixed with biomaterials |
| Husseini et al., 2023 [45] Alcântara et al., 2018 [46] Baldini et al., 2010 [47] | No histomorphometric data were reported |
| Kauffmann et al., 2023 [48] | Missing statistical data to be included in meta-analysis |
| Study | Bone Graft Materials | Purpose of the Study | Study Protocol | Histomorphometric Results |
|---|---|---|---|---|
| Stiller et al., 2014 [27] | TCP-G: CEROS TCP Granules, Mathys Ltd., Switzerland. Pure, synthetic b-TCP granules with a grain size of 700–1400 mm. TCP-P: CEROS TCP Putty, Mathys Ltd., Switzerland. Putty material composed of pure, synthetic b-TCP granules with two types of grain size ranges, i.e., 125–250 mm and 500–700 mm, embedded in a sodium HA hydrogel matrix with a b-TCP:HA ratio of 10:1. | Evaluate the effect of these two bone graft materials on bone formation, bone matrix maturation and osteoblast differentiation six months after MSA. | CBCT was performed preoperatively, post-operatively, and six months after MSA for a 3D assessment of the sinus floor anatomy and bone volume. Before the implant surgery, bone biopsies were performed for histomorphometric analyses. | Six months after SFA: TCP-G: Bone: 17.4 ± 3.3%, Particle: 32.9 ± 2.4% Marrow spaces: 49.7 ± 2.6%. TCP-P: Bone: 30.1 ± 3.1% Particle: 29.5 ± 3.0% Marrow spaces: 40.5 ± 3.2% |
| Velasco-Ortega et al., 2020 [28] | Control Group: Bio-Oss Cancellous, Geistlich, Wolhusen, Switzerland. Demineralized Bovine Bone Mineral Test group: Hyadent BG, Regedent. TCP in the test group plus crosslinked HA with a ratio of 2:1. | Evaluate and compare, histomorphometrically and clinically, different bone substitutes in the MSA. | A CBCT was performed before surgery and 9 months after the MSA before the implant surgery, where bone biopsies were performed for histomorphometric analyses. | Control Group: New bone: 25.97 ± 2.79% Particle: 32.19 ± 1.52% Marrow spaces: 41.99 ± 3.44% Test: New bone: 23.29 ± 2.01% Particle: 7.47 ± 3.59% Marrow spaces: 69.80 ± 2.51% |
| Abaza et al., 2023 [29] | Group 1: crosslinked HA solution (Perfecta) + cerabone®, Straumann, Germany. Group 2: cerabone®, Straumann, Germany. | Compare the effectiveness of HA in combination with xenografts for ARP versus xenografts alone. | Cone beam CT scans were performed preoperatively and 4 months post-operatively to measure radiographic bone gain. Histological assessment of core bone biopsies was performed 4 months post-operatively. | Group 1: New bone: 56.66 ± 7.35% (Mature bone: 18.26 ± 4.44%) Particle: 2.63 ± 1.27% Group 2: New bone: 24.05 ± 3.64% (Mature bone: 2.41 ± 1.36%) Particle: 2.71 ± 1.24% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzi, C.; Leggeri, A.; Cammarota, I.; Carosi, P.; Mazzetti, V.; Arcuri, C. Hyaluronic Acid in Bone Regeneration: Systematic Review and Meta-Analysis. Dent. J. 2024, 12, 263. https://doi.org/10.3390/dj12080263
Lorenzi C, Leggeri A, Cammarota I, Carosi P, Mazzetti V, Arcuri C. Hyaluronic Acid in Bone Regeneration: Systematic Review and Meta-Analysis. Dentistry Journal. 2024; 12(8):263. https://doi.org/10.3390/dj12080263
Chicago/Turabian StyleLorenzi, Claudia, Andrea Leggeri, Ilaria Cammarota, Paolo Carosi, Vincenzo Mazzetti, and Claudio Arcuri. 2024. "Hyaluronic Acid in Bone Regeneration: Systematic Review and Meta-Analysis" Dentistry Journal 12, no. 8: 263. https://doi.org/10.3390/dj12080263
APA StyleLorenzi, C., Leggeri, A., Cammarota, I., Carosi, P., Mazzetti, V., & Arcuri, C. (2024). Hyaluronic Acid in Bone Regeneration: Systematic Review and Meta-Analysis. Dentistry Journal, 12(8), 263. https://doi.org/10.3390/dj12080263