Abstract
Peri-implantitis can affect the longevity of successfully integrated implants. Implant success is dependent on reducing the peri-implantitis risk or successfully managing peri-implantitis. Further understanding of peri-implantitis can be derived from its prevalence, microbial and diagnostic findings, existing therapies, and the effects of systemic health issues and medication. Based on published information: (1) peri-implantitis is higher in patients who have periodontitis or smoke as well as in implants with 5 years of function; (2) peri-implantitis microflora is different from periodontitis; (3) peri-implantitis risk is increased in patients with cardiovascular diseases and uncontrolled diabetes; (4) most reported peri-implantitis therapies may result in resolution, but the best peri-implantitis treatment is still to be determined; (5) more frequent peri-implant maintenance may reduce risk for peri-implantitis.
1. Introduction
Dental-implant-supported fixed restorations of full and partial edentulism are predictable alternatives to removable dentures and fixed tooth-supported bridges [1,2]. Dental implants reported 92.8–97.1% survival rates but can be prone to peri-implantitis during a patient’s lifetime [3,4]. Peri-implantitis may damage the soft and hard tissue around dental implants leading to bone loss, periodontal pocketing, and loss of osseointegration around the implant [5]. An understanding of the available research on peri-implantitis can improve clinical care and reduce the risk of implant failure [6,7].
2. Definition of Peri-Implantitis
Peri-implantitis is defined inconsistently in research studies and clinical reports. These are some common peri-implantitis definitions: (1) the classification of implant diseases by American Academy of Periodontology and the European Federation of Periodontology [8]; (2) the consensus definition by the 1st European Workshop on Periodontology [9]; (3) supporting bone loss in the presence of peri-implant mucosa inflammation and bleeding on probing with or without pus [5]; (4) progressing peri-implant bone loss greater than 2 mm in presence of purulence, bleeding on probing, and greater than 6 mm probings; (5) peri-implant suppuration, bleeding, probings ≥ 5 mm, and radiographic bone loss ≥ 2.5 mm or beyond the first three threads [10]; (6) peri-implant bleeding on probing and probings > 5 mm; and (7) peri-implant mucosa inflammation with peri-implant crestal bone loss [11].
3. Potential Etiologies and Risk Factors
The American Academy of Periodontology and the European Federation of Periodontology jointly published a classification of peri-implantitis [12]. The new classification included implant health, peri-implant mucositis, peri-implantitis, and hard- and soft-tissue deformities. Hard- and soft-tissue deformities of dental implants may result in peri-implantitis; the potential factors include improper implant placement, inadequate bone quality, inadequate bone quantity, and traumatic occlusion [13].
Improper implant placement can result in compromised hard-tissue and soft-tissue defects around an implant; this can develop relatively quickly to bone loss around an implant and a diagnosis of peri-implantitis.
Improper surgical implant placement is an iatrogenic risk factor for peri-implantitis. Common examples of improper implant placement include being too close to a contiguous tooth or another dental implant and not having adequate 1.5 to 2.0 mm of bone buccal and lingual to the placed implant.
Inadequate alveolar bone quality may also be a contributing factor. Bone density is classified as D1, D2, D3, and D4 bone [14]. This classification pertains to the density of bone related to trabecular and cortical bone. If implants are placed in inadequate bone quality classified as D3 and D4 bone, the implant is at a higher risk of peri-implantitis. Other situations contributing to this lack of bone quality include osteoporosis, osteopenia, or other bone diseases [14].
Inadequate bone quantity can be correlated to improper implant placement. Inadequate bone quantity results from lack of proper diagnosis and poor surgical implant placement into a site where the bone is insufficient to support the stability of the dental implant.
Poor angulation and positioning of dental implants result from improper diagnosis or clinician error. Angulation and positioning of the implant that results in a thin buccal plate between the implant and the buccal bone is highly susceptible to peri-implantitis. When there is bone loss, dehiscence or fenestration of the implant can occur.
Poor treatment planning generally leads to poor design of the implant prosthesis. The implant prosthesis with a large occlusal table can negatively impact the support of the dental implant. The implant prosthesis with poorly designed shape and contour can negatively impact the patient’s oral hygiene. In addition, appropriate structures of the implant prosthesis must be designed so that the angulation of occlusal force is parallel to the long axis of the tooth. Extensions of the implant prosthesis due to cantilevers, missing teeth, or other factors can negatively impact the occlusal factors of a dental implant.
Occlusion is a contributing factor for peri-implantitis. Occlusal trauma has a positive correlation to increased peri-implant bone loss around dental implants [13]. Heavy implant occlusal factors like parafunction and bruxism could cause early failure of the dental implant. Occlusal overload is often regarded as one of the main causes of peri-implant bone loss and implant prosthesis failures. Radiographically, this results in crestal bone loss, mobility of the implant, and damage to the prosthesis.
Early loading of dental implants may disrupt the physiologic osteointegration process, interfering with optimal bone remodeling. Interference with the osteointegration can result in inadequate bone formation and loss of crestal bone around a dental implant, leading to peri-implantitis.
Smoking is a well-known risk factor for multiple diseases including cancer, heart disease, and dental implant diseases. Dental implants are negatively impacted by smoking. Smoking changes the microbiome and the immune response around dental implants. Electronic cigarettes also have an adverse effect on implant success [15]. Therefore, smoking and tobacco negatively affect the outcome of virtually all therapeutic procedures, including dental implants. The failure rate of implant osteointegration is considerably higher among smokers. Oral hygiene around implants and the peri-implantitis risk are adversely affected by smoking.
Cement negatively impacts on the overall health of a dental implant. Peri-implantitis is frequently the result of cement left around an implant prosthesis [16]. The European Federation of Periodontology consensus report and other systematic reviews concluded that cement is the most common reason for peri-implantitis [17,18].
The host response is an integral part of implant maintenance and osteointegration success [19]. Any compromised immune response may lead to improper osteointegration and inadequate host defense mechanisms against bacterial colonies around the dental implants. Different inflammatory components can also lead to excessive destructive cytokines and host response cells that can initiate peri-implantitis and ultimately impact the success of the bone-to-implant interface.
Systemic diseases can also have a significant role in peri-implantitis and implant failures. Diabetes mellitus is the most exhaustively explored factor. The impact of diabetes and glycemic control on the osteointegration of dental implants is well recognized [20]. Successful dental implant osteointegration can be accomplished in subjects with diabetes with good metabolic control, which is a hemoglobin A1C of 7% or less. Diabetic patients with a controlled health status have a similar osteointegration pattern as subjects without diabetes mellitus.
Osteoporosis is another systemic disease that has a major impact on implant success. Based on the DEXA dual X-ray absorptiometry and resulting T-score of the patient, we can predictably look for stability of our dental implants upon surgical placement [21]. A healthy patient with ideal bone density of a T-score within 1.5 standard deviations of the norm may improve osteointegration. In osteopenic patients with a diagnosis of 1.5 to 2.5 standard deviations from the mean, osteointegration may be successful but may take a longer time. In patients with osteoporosis with a diagnosis of 2.5 or greater standard deviations from the mean, they may have a higher risk for peri-implantitis.
Other factors affecting peri-implantitis have been associated with selected medications predominantly involving implant prognosis including antiresorptive drugs. Dental practitioners should become increasingly aware of implant failures associated with oral bisphosphonate use. Implant failure and implant complications related to bisphosphonates are increasingly being reported [22]. With regard to the pharmacology of bisphosphates, medications that involve interruption of the homeostasis of bone can ultimately impact on implant success [23].
Periodontal disease is related to peri-implantitis. Patients with a history of periodonitis may have an increased risk of peri-implantitis [7,24]. A dysbiotic microbial community due to improper oral hygiene or other oral factors such as xerostomia may lead to quantities of red complex and orange complex bacteria around dental implants. However, patients with treated periodontitis who receive implants appear to have satisfactory implant longevity. Patients with a history of periodontitis are more likely to develop peri-implantitis [7,24].
Improper oral hygiene could result in a dysbiotic microbial flora. This dysbiotic microbial flora can include pathogenic forms of bacteria colonies [25]. Increased red complex and orange complex bacterial clusters can lead to increased risk of peri-implantitis.
Maintenance of dental implants, especially lack of follow-up care and poor oral hygiene, are risk factors for peri-implantitis. Quarterly maintenance visits are recommended for every patient having a dental implant. Implant maintenance visits should include periodontal probings and radiographic analysis that are planned sequentially and at appropriate time intervals [26,27,28,29,30,31,32,33].
Metal corrosion from titanium implants (Figure 1) may be an initiating factor for inflammation soft-tissue modifications and bone resorption. The mechanisms are not completely understood but may include titanium metal fatigue and stress, reaction to acidic by-products of the bacterial microbiome, chemical reactions to antimicrobial mouth rinses, mechanical damage to debridement by dental practitioners, and chemical reaction to certain diets and alcohol. Further research is required to elucidate these factors [34].
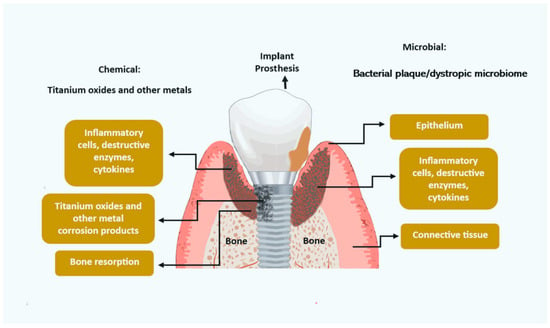
Figure 1.
Potential etiologies for peri-implantitis.
4. Prevalence
Peri-implantitis in the population has a reported prevalence of 1–47% and a mean prevalence of 22% [35]. The peri-implantitis prevalence affecting implants ranges from 0 to 3.4% and is most probable after 5 years of function [36]. Beyond 10 years of function, the peri-implantitis prevalence affecting implants increased to 5.8–16.9%, and the prevalence in the population increased to 10.7–47.2% [36]. The peri-implantitis prevalence is also higher in patients with a history of periodontitis and smokers [36].
The incidence of peri-implantitis affecting patients with implants was 18.8%, and the peri-implantitis incidence affecting dental implants was 9.6% [27]. Periodontal maintenance reduced peri-implantitis incidence to 14.3% [27]. The peri-implantitis incidence in smokers increased to 36.6% [27]. Compared to non-smokers, smokers have a higher peri-implantitis risk [37].
The peri-implantitis incidence varies with periodontal health status (Figure 2). The incidence of peri-implantitis in periodontally healthy patients was reported as 10% [38]. Periodontally healthy patients have lower peri-implantitis incidence and less marginal bone loss around dental implants compared to patients with a history of periodontitis (Figure 2) [38,39,40]. In patients with aggressive periodontitis, the peri-implantitis incidence is higher at 26% [38]. Patients who have residual probings after periodontal treatment have more sites affected by peri-implantitis than treated periodontal patients without residual probings [40].
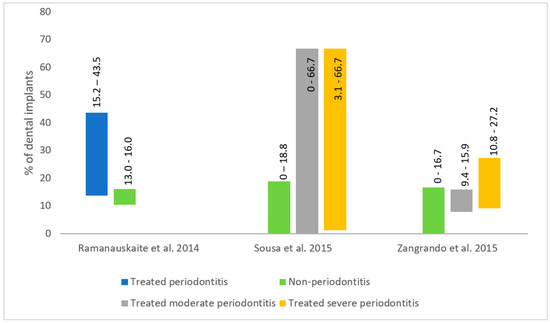
Figure 2.
Peri-implantitis incidence based on periodontal treatment status [38,39,40].
Patient risk factors for osteoporosis which relate to peri-implantitis may include increasing age, Asian or Caucasian ethnicity, genetic predisposition, thin or frail body type [41], poor oral hygiene and habits, smoking [42], and lifestyle lacking in exercise [43].
5. Pathogenesis
Bacterial challenges from dental plaque resulting in loss of attachment on dental implants proceed differently in periodontitis and peri-implantitis. This pathogenic mechanism is not clearly defined but may be related to the anatomic difference between the soft- and hard-tissue attachment around teeth and implants [44].
Several anatomical considerations may explain the difference in pathogenesis. There are two predominant reasons why implants are more susceptible to bacterial challenge than teeth, these include the attachment and vascularity around dental implants. In periodontitis around natural teeth, there is the presence of the periodontal ligament, an epithelial attachment, connective tissue attachment, and alveolar bone. In peri-implantitis around dental implants, there is the presence of epithelial attachment and alveolar bone. Epithelial attachment and bone without the periodontal ligament and the connective tissue attachment increases the dental implant susceptibility to assault by bacterial plaque [44].
In addition, the vascularity around dental implants is different from natural teeth. There are three primary sources of vascularity around teeth: alveolar bone, periodontal ligament, and periodontal soft tissues. In dental implants, there are alveolar bone and periodontal soft tissue excluding the periodontal ligament as sources of vascularity reflecting the immune response and wound healing capabilities. Since there is no periodontal ligament around dental implants, the network of vascularity including nervous bundles of sensory components is lacking. Therefore, a major source of wound healing and immune response capabilities is lacking for dental implants [44].
6. Immunologic Findings
Proinflammatory cytokines including TNF-α and IL-1β were statistically greater in peri-implantitis compared to peri-implant tissue in health. Increased probing depth, gingival index, and bone loss were linked to increased levels of TNF-α and IL-1β in peri-implantitis crevicular fluid. However, IL-1β levels between peri-implantitis and peri-implant mucositis were not statistically different. Other cytokines found in peri-implantitis include IL-4, IL-6,IL-8, IL-10, IL-12, and IL-17 [45].
7. Microbial Findings
The microbial flora in peri-implantitis is different from periodontitis [46,47]. The microflora associated with periodontitis consists of the red complex group (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola), Fusobacterium species, Bacteroides fragilis, and Prevotella intermedia [48,49]. The microflora around implants with peri-implantitis is opportunistic and consists of Gram-negative anaerobes, asaccharolytic Gram-positive anaerobes, as well as herpesviruses like Epstein–Barr virus (EBV) [46].
The microflora in peri-implantitis also differs from peri-implant health. Compared to peri-implant health, the following microbial pathogens were more prevalent around implants with peri-implantitis: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, Prevotella intermedia, human cytomegalovirus, human herpesvirus 4 and 5, and Epstein–Barr virus 1 [50].
The healthy peri-implant sites have lower mean colony-forming units than peri-implantitis sites. The colony-forming units in peri-implantitis consist of opportunistic microorganisms like Staphylococcus intermedius, Staphylococcus aureus, Streptococcus mitis, and Haemophilus influenzae [51]. Furthermore, 30% of the microflora in peri-implantitis consists of Porphyromonas gingivalis, Tannerella forsythia, Treponema socranskii, Staphylococcus anaerobius, Staphylococcus intermedius, Staphylococcus aureus, and Streptococcus mitis [51].
8. Effect of Systemic Disease
The peri-implantitis risk is higher in diabetic patients [52]. Type 1 diabetes makes up 5–10% of diabetic patients, and the other 90–95% consist of Type 2 diabetes [53]. Patients with well-controlled diabetes have lower probing depths, gingival index, and bone loss compared to uncontrolled diabetes [54]. Poorly controlled diabetes increases the risk of peri-implantitis compared to well controlled diabetes or healthy patients [55].
The peri-implantitis risk is greater in cardiovascular disease patients. These patients have a higher risk of harboring Epstein–Barr virus, a herpesvirus associated with aggressive periodontal disease [56].
Based on statistical evaluation, peri-implantitis is not associated with rheumatoid arthritis [56].
Osteoporosis and osteopenia are recognized contributing factors for peri-implantitis and dental implant failures [57]. Poor bone density is a risk factor for peri-implant disease [58]. The quality of the cortical bone is related to the stability of the marginal bone around the implant for the long term [59]. Implant surgical placement in osteoporotic bone results in increased bone loss around the implant collar 1–3 years post-surgical-placement. In a large-scale clinical report on osteoporosis, periodontitis and peri-implant disease risk were reported to be elevated [57,59]. Therefore, the risk factors for osteoporosis and the medication increasing the risk of osteoporosis should be factored in for dental implant treatment planning and maintenance [43,57].
Other systemic conditions like high systolic blood pressure and obesity were also associated with peri-implantitis [60].
9. Impact of Medications on Peri-Implantitis
Selected medications may impact soft and hard tissue around implants [61]. Medication-related factors impacting peri-implantitis include steroids, organ transplant medications, anti-neoplastic medications, antacids, androgen-deprivation therapy (Lupron), and selective serotonin reuptake inhibitors (SSRIs).
SSRIs used to alleviate depression were reported to increase implant disease and failures [62]. The pharmacological mechanism for this observation is not clearly understood.
Bisphosphonates were commonly used to manage osteoporosis, Paget’s disease, and breast cancer therapy, and have also been reported to be associated with implant failure. In addition, bisphosphonates and other antiresorptive medications may increase the risk of osteonecrosis in the jaw from dental implant procedures [22].
Heartburn medications like proton pump inhibitors, antacids, and H2 blockers can increase the risk of osteoporosis and the associated peri-implant disease [63].
10. Treatment of Peri-Implantitis
Peri-implantitis treatments may include adjunctive therapy and be surgical or non-surgical. Non-surgical treatment detoxifies and cleanses the implant surface with or without the utilization of adjunctive antibacterial medicaments. Non-surgical treatments used to treat peri-implantitis may include ultra-sonic or manual debridement, air-abrasion, systemic or local antimicrobial therapy, chlorhexidine therapy, local antiseptics therapy, laser therapy, and host modulation.
Manual debridement can reduce inflammation around dental implants. Manual debridement using titanium instruments or ultrasonics showed reduced plaque and bleeding scores but had no effect on probing depths. There were also no significant treatment differences between the use of ultrasonics or titanium instruments [64]. However, repetitive treatment with oscillating brushes and curettes produced a significantly reduced bleeding index and probing depths at 6 and 12 months compared to baseline [65,66].
Air-abrasives with glycine or erythritol powder decontamination of peri-implant surfaces were shown to be comparable to ultrasonics in treating peri-implantitis [67,68,69]. Similarly, air-abrasives compared to erbium-doped yttrium aluminum garnet (Er:YAG, Biolase, Inc. Foothill, CA, USA) laser debridement reported comparable clinical results [70,71]. However, air-abrasives were more effective in reducing peri-implant inflammation when compared to chemical disinfection with chlorhexidine (CHX).
Peri-implant antimicrobial therapy with adjunctive systemic antibiotics like amoxicillin, azithromycin, or metronidazole may also have beneficial effects. Metronidazole used in conjunction with manual debridement significantly improved probing depths, clinical attachment, and bone fill compared to control 12 months after treatment [72].
Adjunctive therapy with antimicrobial therapy and antiseptic mouthrinses may improve peri-implantitis outcomes [73]. Antimicrobial therapy with debridement resulted in greater reduced probing depth compared to debridement alone [74]. Mechanical debridement with minocycline resulted in better peri-implantitis resolution compared to chlorohexidine and debridement at 12-month follow-up [75,76].
Peri-implant therapy with adjunctive antiseptic mouth rinse like chlorohexidine (CHX), sodium chloride (NaCl), hypochlorite, and herbal oral rinses may be effective against peri-implant mucositis and peri-implantitis [77,78]. However, in some peri-implantitis cases, the results may be limited [79].
Adjunctive therapy with laser disinfection may also improve peri-implantitis outcomes. Carbon dioxide (CO2) lasers and erbium-doped yttrium aluminum garnet (Er:YAG) lasers were found to clinically improve peri-implant parameters up to 6 months [75,80]. Er:YAG laser treatment compared to debridement with chlorhexidine resulted in greater inflammation reduction. Er:YAG lasers compared to glycine powder may produce similar clinical peri-implantitis resolution [76].
Laser decontamination is often used as an adjunct to manual debridement and is a useful option to decontaminate and remove bacterial biofilm on rough implant surfaces [81,82,83]. Diode lasers were reported to have some beneficial adjunctive effects [83]. Photodynamic therapy when used adjunctively for reducing peri-implant inflammation was as effective as adjunctive minocycline microspheres for up to 12 months [84].
Innovative approaches for non-surgical peri-implantitis treatment include enamel matrix (EMD) and probiotics [85,86,87]. Adjunctive use of EMD compared to manual debridement was more effective in reducing peri-implant inflammation [85]. Use of Lactobacillus reuteri with manual debridement produced improved clinical parameters like bleeding on probing and probing depths around implants with peri-implantitis [86]. However, oral probiotics seem to have limited effects on the microbiota around the dental implant [86].
Surface decontamination with implantoplasty or dental lasers may result in similar treatment outcomes compared to other surface decontamination techniques [75,76,88,89]. In general, non-surgical debridement with adjunctive therapy of peri-implantitis may be superior to debridement alone [74].
Non-surgical therapy was effective for debriding irritants around implants in peri-implantitis sites. This may have a positive impact on implant inflammation [65,66]. However, non-surgical therapy may not have an effect on osseous defects [90,91].
Surgical treatment involved elevation of the flap to expose the contaminated implant surface, cleaning and detoxifying with antimicrobial therapy or antiseptic solution, and grafting the osseous defects with or without bone graft and membranes. Surgical protocols may include open-flap debridement, resective osseous peri-implant procedures, or regenerative techniques. Open-flap peri-implantitis procedures may include implant debriding with ultrasonic scalers, curettes, air abrasion, curettes, burs, or laser treatment.
Resective peri-implant procedures may include peri-implant pocket elimination and implantoplasty. After 3 yrs, the implant survival rate of implantoplasty and resective peri-implantitis treatment was reported to be 100% [92]. At 24 months, implantoplasty of contaminated implants during resective peri-implantitis treatment improved probing depth, attachment levels, and the bleeding index. However, the treatment resulted in a higher recession index in the treated implants [92].
Regenerative peri-implant techniques may include biomaterials like synthetic membranes, porcine/bovine membranes, bone graft, bone substitutes, platelet concentrates, calcium carbonate, or hydroxyapatite. Surgical peri-implantitis therapy can reduce peri-implant probing depth by 30–50% [89,93]. There are conflicting reports for the use of biomaterials in surgical peri-implantitis treatment. The use of enamel matrix derivative in the treatment of peri-implantitis showed no improvement in probing depths or bone fill [94]. Similarly, open-flap surgery with cancellous bone and 10% purified porcine collagen did not improve bleeding on probing or probing depths but significantly reduced buccal gingival recession [95].
However, other studies show that regenerative materials when used in combination with open-flap surgery were more effective in peri-implantitis resolution than without [95,96,97,98].
Regenerative procedures may produce radiographic bone fill of 2–2.17 mm [89,93,99,100]. Radiographic bone fill is often enhanced with the use of regenerative materials, but it is important to discern if the radiographic improvement equates to the clinical improvement of the peri-implant defect [101].
Laser peri-implantitis treatment may stimulate bone fill in peri-implantitis bony defects and may reduce inflammation and probing depths. The diode, Er:YAG, neodymium-doped yttrium aluminum garnet (Nd:YAG), and O2 lasers have studies reporting marginal bone fill in peri-implantitis defects [102].
The Nd:YAG lasers utilizing laser-assisted peri-implantitis surgical protocols may potentially rescue failing dental implants by clinical attachment gain and radiographic bone fill [103]. Nd:YAG-induced histologic evidence of regeneration in periodontitis-affected teeth may parallel the clinical response of Nd:YAG-induced peri-implantitis resolution [104,105]. Peri-implantitis treatment may be successful if treated implants report resolution of inflammation and no progressing bone loss.
With Er:YAG laser-assisted regenerative surgical therapy, although there were no significant radiographic bone changes, the probing depth was significantly improved [106].
However, there are conflicting outcomes for different peri-implantitis treatments. Heitz-Mayfield et al. reported peri-implantitis resolution in 75–93% of implants and 76–100% of patients after 12 months [107]. Their peri-implantitis treatment included non-surgical and surgical intervention with different combinations of adjunctive treatments. Other studies report less than 50% of infected implant respond to conventional surgical peri-implantitis debridement [108,109]. And guided bone regeneration with bone graft and membranes reported unpredictable outcomes [89,99,110,111].
In general, clinical attachment levels and probing depth were improved with surgical interventions compared to non-surgical approaches [7,112]. In addition, poor oral hygiene practices may have a direct negative effect on implant success [113].
11. Conclusions
Patients with periodontal disease or treated periodontal disease may have a higher peri-implantitis incidence. Implants in function after 5 years also have a higher peri-implantitis incidence. Smokers, patients with cardiovascular disease, and those with uncontrolled diabetes may have a higher peri-implantitis risk. Proinflammatory cytokines including TNF-α and IL-1β were significantly elevated in peri-implantitis. The microflora involved in peri-implantitis differs from periodontitis and may consist of opportunistic pathogens including Porphyromonas gingivalis, Prevotella intermedia, Aggregatibacter actinomycetemcomitans, Treponema denticola, Treponema socranskii, Tannerella forsythia, Streptococcus mitis, Staphylococcus anaerobius, Staphylococcus aureus, Staphylococcus intermedius, Epstein–Barr virus, human cytomegalovirus, and human herpesvirus 4 and 5.
All combinations of adjunctive treatments for non-surgical and surgical approaches may result in successful peri-implantitis resolution and are better than debridement alone. Surgical intervention may reduce peri-implant probing depths. However, guided bone regeneration (GBR) may be technique-sensitive and unpredictable. Meticulous post-implant maintenance may reduce peri-implantitis in high-risk patients. To determine better treatments for peri-implantitis, additional randomized controlled trials are required.
Author Contributions
Conceptualization, M.T. and J.B.S.; methodology, M.T.; software, M.T.; validation, M.T. and J.B.S.; formal analysis, M.T.; investigation, M.T. and J.B.S.; resources, M.T. and J.B.S.; data curation, J.B.S.; writing—original draft preparation, M.T. and J.B.S.; writing—review and editing, M.T. and J.B.S.; visualization, M.T.; supervision, J.B.S.; project administration, M.T. and J.B.S.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest in the publication of this paper.
References
- Lang, N.P.; Pjetursson, B.E.; Tan, K.; Bragger, U.; Egger, M.; Zwahlen, M. A systematic review of the survival and complication rates of fixed partial dentures (fpds) after an observation period of at least 5 years. Ii. Combined tooth-implant-supported fpds. Clin. Oral Implants Res. 2004, 15, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Tan, W.C.; Tan, K.; Brägger, U.; Zwahlen, M.; Lang, N.P. A systematic review of the survival and complication rates of resin-bonded bridges after an observation period of at least 5 years. Clin. Oral Implant. Res. 2007, 19, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Vazquez, L.; Rieder, P.; Moraguez, O.; Bernard, J.; Belser, U.C. Survival rates of short (6 mm) micro-rough surface implants: A review of literature and meta-analysis. Clin. Oral Implant. Res. 2014, 25, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Klinge, B.; Meyle, J. Peri-implant tissue destruction. The third eao consensus conference 2012. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 108–110. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.U.; Berglundh, T. Definition and prevalence of peri-implant diseases. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 286–291. [Google Scholar] [CrossRef]
- Ting, M.; Suzuki, J. Antiresorptive Medication Effects on Dental Implant Survival and Implant-Related Jaw Osteonecrosis. Oral Health J. 2023. Available online: https://www.oralhealthgroup.com/features/antiresorptive-medication-effects-on-dental-implant-survival-and-implant-related-jaw-osteonecrosis/ (accessed on 12 January 2024).
- Ting, M.; Craig, J.; Balkin, B.E.; Suzuki, J.B. Peri-implantitis: A comprehensive overview of systematic reviews. J. Oral Implant. 2018, 44, 225–247. [Google Scholar] [CrossRef] [PubMed]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Periodontol. 2018, 89 (Suppl. 1), S173–S182. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Isidor, F.; Lang, N.P.; Karring, T. Consensus report of session iv. In 1st European Workshop on Periodontology; Quintessence Publishing Co Ltd.: London, UK, 1994; pp. 365–369. [Google Scholar]
- Ong, C.T.T.; Ivanovski, S.; Needleman, I.G.; Retzepi, M.; Moles, D.R.; Tonetti, M.S.; Donos, N. Systematic review of implant outcomes in treated periodontitis subjects. J. Clin. Periodontol. 2008, 35, 438–462. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Berglundh, T. Working Group 4 of Seventh European Workshop on P. Periimplant diseases: Where are we now?—Consensus of the seventh european workshop on periodontology. J. Clin. Periodontol. 2011, 38 (Suppl. 11), 178–181. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef]
- Misch, C.E.; Suzuki, J.B.; Misch-Dietsh, F.M.; Bidez, M.W. A positive correlation between occlusal trauma and peri-implant bone loss: Literature support. Implant. Dent. 2005, 14, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E. Misch’s Contemporary Implant Dentistry: Misch’s Contemporary Implant Dentistry E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007; Chapter 42; pp. 1073–1085. [Google Scholar]
- Guney, Z.; Altingoz, S.; Has, H.; Serdar, M.A.; Kurgan, S. The impact of electronic cigarettes on peri-implant health: A systematic review and meta-analysis. J. Dent. 2024, 143, 104883. [Google Scholar] [CrossRef] [PubMed]
- Misch, C.E.; Perel, M.L.; Wang, H.-L.; Sammartino, G.; Galindo-Moreno, P.; Trisi, P.; Steigmann, M.; Rebaudi, A.; Palti, A.; Pikos, M.A.; et al. Implant success, survival, and failure: The international congress of oral implantologists (icoi) pisa consensus conference. Implant. Dent. 2008, 17, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Staubli, N.; Walter, C.; Schmidt, J.C.; Weiger, R.; Zitzmann, N.U. Excess cement and the risk of peri-implant disease—A systematic review. Clin. Oral Implants Res. 2017, 28, 1278–1290. [Google Scholar] [CrossRef] [PubMed]
- Giovannoli, J.-L.; Roccuzzo, M.; Albouy, J.-P.; Duffau, F.; Lin, G.-H.; Serino, G. Local risk indicators—Consensus report of working group 2. Int. Dent. J. 2019, 69 (Suppl. 2), 7–11. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pimentel, S.P.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z. Host response and peri-implantitis. Braz. Oral Res. 2019, 33 (Suppl. 1), e066. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Romanos, G.E. Impact of diabetes mellitus and glycemic control on the osseointegration of dental implants: A systematic literature review. J. Periodontol. 2009, 80, 1719–1730. [Google Scholar] [CrossRef]
- Alsadi, W.; AbouSulaiman, A.; AlSabbagh, M.M. Association of dental implants success in bone density classification of postmenopausal women with osteoporosis—A clinical and radiographic prospective study. J. Indian. Acad. Oral Med. Radiol. 2021, 33, 428–434. [Google Scholar] [CrossRef]
- Yip, J.K.; Borrell, L.N.; Cho, S.; Francisco, H.; Tarnow, D.P. Association between oral bisphosphonate use and dental implant failure among middle-aged women. J. Clin. Periodontol. 2012, 39, 408–414. [Google Scholar] [CrossRef]
- Lee, C.Y.; Suzuki, J.B. Medication-related osteonecrosis of the jaws from once per year intravenous zoledronic acid (reclast): Report of 4 cases. Implant. Dent. 2015, 24, 227–231. [Google Scholar] [CrossRef]
- Lombardo, G.; Signoriello, A.; Pardo, A.; Romero, X.Z.S.; Sierra, L.A.V.; Tovar, L.A.; Marincola, M.; Nocini, P.F. Short and ultra-short (<6-mm) locking-taper implants supporting single crowns in posterior areas (part II): A 5-year retrospective study on periodontally healthy patients and patients with a history of periodontitis. Clin. Implant. Dent. Relat. Res. 2022, 24, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Salcetti, J.M.; Moriarty, J.D.; Cooper, L.F.; Smith, F.W.; Collins, J.G.; Socransky, S.S.; Offenbacher, S. The clinical, microbial, and host response characteristics of the failing implant. Int. J. Oral Maxillofac. Implants 1997, 12, 32–42. [Google Scholar] [PubMed]
- Algraffee, H.; Borumandi, F.; Cascarini, L. Peri-implantitis. Br. J. Oral Maxillofac. Surg. 2012, 50, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Atieh, M.A.; Alsabeeha, N.H.M.; Faggion, C.M., Jr.; Duncan, W.J. The frequency of peri-implant diseases: A systematic review and meta-analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A. Peri-implant diseases: Diagnosis and risk indicators. J. Clin. Periodontol. 2008, 35 (Suppl. 8), 292–304. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.M.; Knight, E.T.; Russell, A.A.; Tawse-Smith, A.; Leichter, J.W. Peri-implant disease: Current understanding and future direction. N. Z. Dent. J. 2013, 109, 55–62. [Google Scholar] [PubMed]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (fdps) after a mean observation period of at least 5 years. Clin. Oral Implants Res. 2012, 23 (Suppl. 6), 22–38. [Google Scholar] [CrossRef]
- Naert, I.; Koutsikakis, G.; Quirynen, M.; Duyck, J.; van Steenberghe, D.; Jacobs, R. Biologic outcome of implant-supported restorations in the treatment of partial edentulism. Part 2: A longitudinal radiographic study. Clin. Oral Implants Res. 2002, 13, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Roccuzzo, M.; Bonino, F.; Gaudioso, L.; Zwahlen, M.; Meijer, H.J. What is the optimal number of implants for removable reconstructions? A systematic review on implant-supported overdentures. Clin. Oral Implant. Res. 2012, 23, 229–237. [Google Scholar] [CrossRef]
- Serino, G.; Turri, A.; Lang, N.P. Probing at implants with peri-implantitis and its relation to clinical peri-implant bone loss. Clin. Oral Implant. Res. 2013, 24, 91–95. [Google Scholar] [CrossRef]
- Suárez-López del Amo, F.; Garaicoa-Pazmiño, C.; Fretwurst, T.; Castilho, R.M.; Squarize, C.H. Dental implants-associated release of titanium particles: A systematic review. Clin. Oral Implant. Res. 2018, 29, 1085–1100. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158––S171. [Google Scholar] [CrossRef]
- de Waal, Y.C.M.; van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M.; Winkel, E.G. Differences in peri-implant conditions between fully and partially edentulous subjects: A systematic review. J. Clin. Periodontol. 2013, 40, 266–286. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Smoking and the risk of peri-implantitis. A systematic review and meta-analysis. Clin. Oral Implants Res. 2015, 26, e62–e67. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Mardas, N.; Farias, B.; Petrie, A.; Needleman, I.; Spratt, D.; Donos, N. A systematic review of implant outcomes in treated periodontitis patients. Clin. Oral Implant. Res. 2016, 27, 787–844. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Baseviciene, N.; Wang, H.L.; Tozum, T.F. Effect of history of periodontitis on implant success: Meta-analysis and systematic review. Implant. Dent. 2014, 23, 687–696. [Google Scholar] [CrossRef]
- Zangrando, M.S.; Damante, C.A.; Sant’Ana, A.C.; Rubo de Rezende, M.L.; Greghi, S.L.; Chambrone, L. Long-term evaluation of periodontal parameters and implant outcomes in periodontally compromised patients: A Systematic review. J. Periodontol. 2015, 86, 201–221. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2020 update. Endocr. Pract. 2020, 26 (Suppl. 1), 1–46. [Google Scholar] [CrossRef]
- Strietzel, F.P.; Reichart, P.A.; Kale, A.; Kulkarni, M.; Wegner, B.; Küchler, I. Smoking interferes with the prognosis of dental implant treatment: A systematic review and meta-analysis. J. Clin. Periodontol. 2007, 34, 523–544. [Google Scholar] [CrossRef]
- International Osteoporosis Foundation. Available online: https://www.Osteoporosis.Foundation (accessed on 6 August 2023).
- Misch, C. Misch’s Contemporary Implant Dentistry: Misch’s Contemporary Implant Dentistry E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2007; Chapter 37; pp. 870–904. [Google Scholar]
- Duarte, P.M.; Serrão, C.R.; Miranda, T.S.; Zanatta, L.C.S.; Bastos, M.F.; Faveri, M.; Figueiredo, L.C.; Feres, M. Could cytokine levels in the peri-implant crevicular fluid be used to distinguish between healthy implants and implants with peri-implantitis? A systematic review. J. Periodontal Res. 2016, 51, 689–698. [Google Scholar] [CrossRef]
- Rakic, M.; Grusovin, M.; Canullo, L. The microbiologic profile associated with peri-implantitis in humans: A systematic review. Int. J. Oral Maxillofac. Implant. 2016, 31, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.; Suzuki, J. Peri-implantitis: How to reduce risk and manage implant failure? Oral Health J. 2021. Oralhealthgroup.com. Available online: https://www.oralhealthgroup.com/features/peri-implantitis-how-to-reduce-risk-and-manage-implant-failure/ (accessed on 8 August 2023).
- Scapoli, L.; Girardi, A.; Palmieri, A.; Testori, T.; Zuffetti, F.; Monguzzi, R.; Lauritano, D.; Carinci, F. Microflora and periodontal disease. Dent. Res. J. 2012, 9 (Suppl. 2), S202–S206. [Google Scholar] [CrossRef]
- Benachinmardi, K.K.; Nagamoti, J.; Kothiwale, S.; Metgud, S.C. Microbial flora in chronic periodontitis: Study at a tertiary health care center from north karnataka. J. Lab. Physicians 2015, 7, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chaparro, P.J.; Duarte, P.M.; Shibli, J.A.; Montenegro, S.; Heluy, S.L.; Figueiredo, L.C.; Faveri, M.; Feres, M. The current weight of evidence of the microbiologic profile associated with peri-implantitis: A systematic review. J. Periodontol. 2016, 87, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Padial-Molina, M.; López-Martínez, J.; O’Valle, F.; Galindo-Moreno, P. Microbial profiles and detection techniques in peri-Implant diseases: A systematic review. J. Oral Maxillofac. Res. 2016, 7, e10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turri, A.; Rossetti, P.; Canullo, L.; Grusovin, M.; Dahlin, C. Prevalence of peri-implantitis in medically compromised patients and smokers: A systematic review. Int. J. Oral Maxillofac. Implant. 2016, 31, 111–118. [Google Scholar] [CrossRef]
- Nibali, L.; Gkranias, N.; Mainas, G.; Di Pino, A. Periodontitis and implant complications in diabetes. Periodontology 2000 2022, 90, 88–105. [Google Scholar] [CrossRef]
- Tan, S.J.; Baharin, B.; Nabil, S.; Mohd, N.; Zhu, Y. Does glycemic control have a dose-response relationship with implant outcomes? a comprehensive systematic review and meta-analysis. J. Evid. Based Dent. Pract. 2021, 21, 101543. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Spille, J.H.; Wiltfang, J.; Naujokat, H. Systematic review on diabetes mellitus and dental implants: An update. Int. J. Implant. Dent. 2022, 8, 1. [Google Scholar] [CrossRef]
- Tseng, K.C.; Zheng, X.Y.; Qu, X.H.; Lu, E.Y. Risk of peri-implantitis in patients with diabetes mellitus: A meta-analysis. Int. J. Clin. Exp. Med. 2016, 9, 15986–15995. [Google Scholar]
- LaMonte, M.J.; Hovey, K.M.; Genco, R.J.; Millen, A.E.; Trevisan, M.; Wactawski-Wende, J. Five-year changes in periodontal disease measures among postmenopausal females: The buffalo osteoperio study. J. Periodontol. 2013, 84, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Parel, S.M.; Phillips, W.R. A risk assessment treatment planning protocol for the four implant immediately loaded maxilla: Preliminary findings. J. Prosthet. Dent. 2011, 106, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Simons, W.; De Smit, M.; Duyck, J.; Coucke, W.; Quirynen, M. The proportion of cancellous bone as predictive factor for early marginal bone loss around implants in the posterior part of the mandible. Clin. Oral Implant. Res. 2014, 26, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.B.; Coelho, T.d.R.C.; de Azevedo, R.A.; Dos Santos, J.N.; Neves, F.S.; Cury, P.R. Systemic risk indicators for peri-implant diseases in individuals with implant-supported fixed prostheses: A cross-sectional study. Int. J. Oral Implantol. 2020, 13, 255–266. [Google Scholar]
- Romanos, G.E.; Vaglica, M.; Sculean, A. Drug-associated bone resorption with potential dental and implant implications. Periodontology 2000 2022, 90, 236–246. [Google Scholar] [CrossRef]
- Wu, X.; Al-Abedalla, K.; Rastikerdar, E.; Abi Nader, S.; Daniel, N.G.; Nicolau, B.; Tamimi, F. Selective serotonin reuptake inhibitors and the risk of osseointegrated implant failure: A cohort study. J. Dent. Res. 2014, 93, 1054–1061. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Lewis, J.D.; Epstein, S.; Metz, D.C. Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 2006, 296, 2947–2953. [Google Scholar] [CrossRef]
- Renvert, S.; Samuelsson, E.; Lindahl, C.; Persson, G.R. Mechanical non-surgical treatment of peri-implantitis: A double-blind randomized longitudinal clinical study. I: Clinical results. J. Clin. Periodontol. 2009, 36, 604–609. [Google Scholar] [CrossRef]
- Khan, S.N.; Koldsland, O.C.; Roos-Jansåker, A.; Wohlfahrt, J.C.; Verket, A.; Mdala, I.; Magnusson, A.; Salvesen, E.; Hjortsjö, C. Non-surgical treatment of mild to moderate peri-implantitis using an oscillating chitosan brush or a titanium curette-A randomized multicentre controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 1254–1264. [Google Scholar] [CrossRef]
- Khan, S.N.; Koldsland, O.C.; Roos-Jansåker, A.; Wohlfahrt, J.C.; Verket, A.; Mdala, I.; Magnusson, A.; Salvesen, E.; Hjortsjö, C. Non-surgical treatment of mild to moderate peri-implantitis with an oscillating chitosan brush or a titanium curette-12-month follow-up of a multicenter randomized clinical trial. Clin. Oral Implant. Res. 2023, 34, 684–697. [Google Scholar] [CrossRef]
- Ji, Y.; Tang, Z.; Wang, R.; Cao, J.; Cao, C.; Jin, L. Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: A pilot clinical trial. Clin. Oral Implant. Res. 2014, 25, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Hentenaar, D.F.M.; De Waal, Y.C.M.; Stewart, R.E.; Van Winkelhoff, A.J.; Meijer, H.J.A.; Raghoebar, G.M. Erythritol airpolishing in the non-surgical treatment of peri-implantitis: A randomized controlled trial. Clin. Oral Implant. Res. 2021, 32, 840–852. [Google Scholar] [CrossRef] [PubMed]
- Riben-Grundstrom, C.; Norderyd, O.; André, U.; Renvert, S. Treatment of peri-implant mucositis using a glycine powder air-polishing or ultrasonic device: A randomized clinical trial. J. Clin. Periodontol. 2015, 42, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Roos-Jansaker, A.M.; Lindahl, C.; Renvert, S. Microbiologic results after non-surgical erbium-doped:Yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: A randomized clinical trial. J. Periodontol. 2011, 82, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Lindahl, C.; Jansåker, A.-M.R.; Persson, G.R. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: A randomized clinical trial. J. Clin. Periodontol. 2011, 38, 65–73. [Google Scholar] [CrossRef]
- Blanco, C.; Pico, A.; Dopico, J.; Gandara, P.; Blanco, J.; Linares, A. Adjunctive benefits of systemic metronidazole on non-surgical treatment of peri-implantitis. A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2022, 49, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Becker, K.; Sager, M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J. Clin. Periodontol. 2015, 42 (Suppl. 16), S202–S213. [Google Scholar] [CrossRef]
- Faggion, C.M., Jr.; Listl, S.; Frühauf, N.; Chang, H.; Tu, Y. A systematic review and Bayesian network meta-analysis of randomized clinical trials on non-surgical treatments for peri-implantitis. J. Clin. Periodontol. 2014, 41, 1015–1025. [Google Scholar] [CrossRef]
- Kotsovilis, S.; Karoussis, I.K.; Trianti, M.; Fourmousis, I. Therapy of peri-implantitis: A systematic review. J. Clin. Periodontol. 2008, 35, 621–629. [Google Scholar] [CrossRef]
- Muthukuru, M.; Zainvi, A.; Esplugues, E.O.; Flemmig, T.F. Non-surgical therapy for the management of peri-implantitis: A systematic review. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 77–83. [Google Scholar] [CrossRef]
- Alqutub, M.N.; Alhumaidan, A.A.; Alali, Y.; Al-Aali, K.A.; Javed, F.; Vohra, F.; Abduljabbar, T. Comparison of the postoperative anti-inflammatory efficacy of chlorhexidine, saline rinses and herbal mouthwashes after mechanical debridement in patients with peri-implant mucositis: A randomized controlled trial. Int. J. Dent. Hyg. 2023, 21, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Alzoman, H.; Alojaym, T.G.; Chalikkandy, S.N.; Mehmood, A.; Rashed, F.; Divakar, D.D. Comparison of an herbal-and a 0.12% chlorhexidine-based oral rinse as adjuncts to nonsurgical mechanical debridement in the management of peri-implant mucositis: A randomised controlled trial. Oral Health Prev. Dent. 2020, 18, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Menezes, K.M.; Fernandes-Costa, A.N.; Silva-Neto, R.D.; Calderon, P.S.; Gurgel, B.C. Efficacy of 0.12% chlorhexidine gluconate for non-surgical treatment of peri-implant mucositis. J. Periodontol. 2016, 87, 1305–1313. [Google Scholar] [CrossRef]
- Natto, Z.S.; Aladmawy, M.; Levi, P.A.; Wang, H.-L. Comparison of the efficacy of different types of lasers for the treatment of peri-implantitis: A systematic review. Int. J. Oral Maxillofac. Implant. 2015, 30, 338–345. [Google Scholar] [CrossRef]
- Alpaslan Yayli, N.Z.; Talmac, A.C.; Keskin Tunc, S.; Akbal, D.; Altindal, D.; Ertugrul, A.S. Erbium, chromium-doped: Yttrium, scandium, gallium, garnet and diode lasers in the treatment of peri-implantitis: Clinical and biochemical outcomes in a randomized-controlled clinical trial. Lasers Med. Sci. 2022, 37, 665–674. [Google Scholar] [CrossRef]
- Roccuzzo, A.; Klossner, S.; Stähli, A.; Imber, J.C.; Eick, S.; Sculean, A.; Salvi, G.E. Non-surgical mechanical therapy of peri-implantitis with or without repeated adjunctive diode laser application. A 6-month double-blinded randomized clinical trial. Clin. Oral Implants Res. 2022, 33, 900–912. [Google Scholar] [CrossRef]
- Tenore, G.; Montori, A.; Mohsen, A.; Mattarelli, G.; Palaia, G.; Romeo, U. Evaluation of adjunctive efficacy of diode laser in the treatment of peri-implant mucositis: A randomized clinical trial. Lasers Med. Sci. 2020, 35, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Schär, D.; Wicki, B.; Eick, S.; Ramseier, C.A.; Arweiler, N.B.; Sculean, A.; Salvi, G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin. Oral Implant. Res. 2014, 25, 279–287. [Google Scholar] [CrossRef]
- Kashefimehr, A.; Pourabbas, R.; Faramarzi, M.; Zarandi, A.; Moradi, A.; Tenenbaum, H.C.; Azarpazhooh, A. Effects of enamel matrix derivative on non-surgical management of peri-implant mucositis: A double-blind randomized clinical trial. Clin. Oral Investig. 2017, 21, 2379–2388. [Google Scholar] [CrossRef]
- Galofré, M.; Palao, D.; Vicario, M.; Nart, J.; Violant, D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J. Periodontal Res. 2018, 53, 378–390. [Google Scholar] [CrossRef]
- Laleman, I.; Pauwels, M.; Quirynen, M.; Teughels, W. The usage of a lactobacilli probiotic in the non-surgical therapy of peri-implantitis: A randomized pilot study. Clin. Oral Implant. Res. 2019, 31, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Monje, A.; Schwarz, F. Principles of combined surgical therapy for the management of peri-implantitis. Clin. Adv. Periodontics 2022, 12, 57–63. [Google Scholar] [CrossRef]
- Chan, H.; Lin, G.; Suarez, F.; MacEachern, M.; Wang, H. Surgical management of peri-implantitis: A systematic review and meta-analysis of treatment outcomes. J. Periodontol. 2014, 85, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Mahato, N.; Wu, X.; Wang, L. Management of peri-implantitis: A systematic review, 2010–2015. SpringerPlus 2016, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez Del Amo, F.; Yu, S.H.; Wang, H.L. Non-surgical therapy for peri-implant diseases: A systematic review. J. Oral Maxillofac. Res. 2016, 7, e13. [Google Scholar] [CrossRef] [PubMed]
- Romeo, E.; Lops, D.; Chiapasco, M.; Ghisolfi, M.; Vogel, G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part ii: Radiographic outcome. Clin. Oral Implants Res. 2007, 18, 179–187. [Google Scholar] [CrossRef]
- Khoshkam, V.; Chan, H.L.; Lin, G.H.; MacEachern, M.P.; Monje, A.; Suarez, F.; Giannobile, W.V.; Wang, H.L. Reconstructive procedures for treating peri-implantitis: A systematic review. J. Dent. Res. 2013, 92 (Suppl. 12), 131S–138S. [Google Scholar] [CrossRef] [PubMed]
- Isehed, C.; Holmlund, A.; Renvert, S.; Svenson, B.; Johansson, I.; Lundberg, P. Effectiveness of enamel matrix derivative on the clinical and microbiological outcomes following surgical regenerative treatment of peri-implantitis. A randomized controlled trial. J. Clin. Periodontol. 2016, 43, 863–873. [Google Scholar] [CrossRef]
- Derks, J.; Ortiz-Vigón, A.; Guerrero, A.; Donati, M.; Bressan, E.; Ghensi, P.; Schaller, D.; Tomasi, C.; Karlsson, K.; Abrahamsson, I.; et al. Reconstructive surgical therapy of peri-implantitis: A multicenter randomized controlled clinical trial. Clin. Oral Implant. Res. 2022, 33, 921–944. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S.; Laine, M.L.; Anssari Moin, D.; Pilloni, A.; Zeza, B.; Sanz, M.; Ortiz-Vigon, A.; Roos-Jansåker, A.M.; Renvert, S. Reconstruction of peri-implant osseous defects: A multicenter randomized trial. J. Dent. Res. 2016, 95, 58–66. [Google Scholar] [CrossRef]
- Wohlfahrt, J.C.; Lyngstadaas, S.P.; Rønold, H.J.; Saxegaard, E.; Ellingsen, J.E.; Karlsson, S.; Aass, A.M. Porous titanium granules in the surgical treatment of peri-implant osseous defects: A randomized clinical trial. Int. J. Oral Maxillofac. Implant. 2012, 27, 401–410. [Google Scholar]
- Emanuel, N.; Machtei, E.E.; Reichart, M.; Shapira, L. D-plex500: A local biodegradable prolonged release doxycycline-formulated bone graft for the treatment for peri-implantitis. A randomized controlled clinical study. Quintessence Int. 2020, 51, 546–553. [Google Scholar] [PubMed]
- Khoshkam, V.; Del Amo, F.S.-L.; Monje, A.; Lin, G.-H.; Chan, H.-L.; Wang, H.-L. Long-term radiographic and clinical outcomes of regenerative approach for treating peri-implantitis: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Implant. 2016, 31, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Daugela, P.; Juodzbalys, G. Treatment of peri-implantitis: Meta-analysis of findings in a systematic literature review and novel protocol proposal. Quintessence Int. 2016, 47, 379–393. [Google Scholar]
- Schwarz, F.; Sahm, N.; Bieling, K.; Becker, J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: A four-year clinical follow-up report. J. Clin. Periodontol. 2009, 36, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.; Alluri, L.S.C.; Sulewski, J.G.; Suzuki, J.B.; da Silva, A.P.B. Laser treatment of peri-implantitis: A systematic review of radiographic outcomes. Dent. J. 2022, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.B. Salvaging implants with an nd:Yag laser: A novel approach to a growing problem. Compend Contin Educ. Dent. 2015, 36, 756–761. [Google Scholar] [PubMed]
- Yukna, R.A.; Carr, R.L.; Evans, G.H. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int. J. Periodontics Restor. Dent. 2007, 27, 577–587. [Google Scholar]
- Nevins, M.L.; Camelo, M.; Schupbach, P.; Kim, S.-W.; Kim, D.M.; Nevins, M. Human clinical and histologic evaluation of laser-assisted new attachment procedure. Int. J. Periodontics Restor. Dent. 2012, 32, 497–507. [Google Scholar]
- Wang, C.; Ashnagar, S.; Di Gianfilippo, R.; Arnett, M.; Kinney, J.; Wang, H. Laser-assisted regenerative surgical therapy for peri-implantitis: A randomized controlled clinical trial. J. Periodontol. 2021, 92, 378–388. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E.; Mombelli, A.; Loup, P.J.; Heitz, F.; Kruger, E.; Lang, N.P. Supportive peri-implant therapy following anti-infective surgical peri-implantitis treatment: 5-year survival and success. Clin. Oral Implants Res. 2018, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wagner, T.P.; Pires, P.R.; Rios, F.S.; de Oliveira, J.A.P.; Costa, R.d.S.A.; Cunha, K.F.; Silveira, H.L.D.; Pimentel, S.; Casati, M.Z.; Rosing, C.K.; et al. Surgical and non-surgical debridement for the treatment of peri-implantitis: A two-center 12-month randomized trial. Clin. Oral Investig. 2021, 25, 5723–5733. [Google Scholar] [CrossRef]
- de Waal, Y.C.M.; Raghoebar, G.M.; Meijer, H.J.A.; Winkel, E.G.; van Winkelhoff, A.J. Prognostic indicators for surgical peri-implantitis treatment. Clin. Oral Implant. Res. 2016, 27, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Daugela, P.; Cicciù, M.; Saulacic, N. Surgical regenerative treatments for peri-implantitis: Meta-analysis of recent findings in a systematic literature review. J. Oral Maxillofac. Res. 2016, 7, e15. [Google Scholar] [CrossRef] [PubMed]
- Sahrmann, P.; Attin, T.; Schmidlin, P.R. Regenerative treatment of peri-implantitis using bone substitutes and membrane: A systematic review. Clin. Implant. Dent. Relat. Res. 2011, 13, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Faggion, C.M.; Chambrone, L.; Listl, S.; Tu, Y. Network meta-analysis for evaluating interventions in implant dentistry: The case of peri-implantitis treatment. Clin. Implant. Dent. Relat. Res. 2013, 15, 576–588. [Google Scholar] [CrossRef]
- Lombardo, G.; D’agostino, A.; Nocini, P.F.; Signoriello, A.; Zangani, A.; Pardo, A.; Lonardi, F.; Trevisiol, L. Clinical outcomes and periodontal conditions of dental implants placed in free fibula flaps (fff): A retrospective study with a mean follow-up of 6 years. Clin. Oral Investig. 2023, 27, 7737–7751. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).