The Oral Microbiome of Peri-Implant Health and Disease: A Narrative Review
Abstract
1. Introduction
2. Methodology
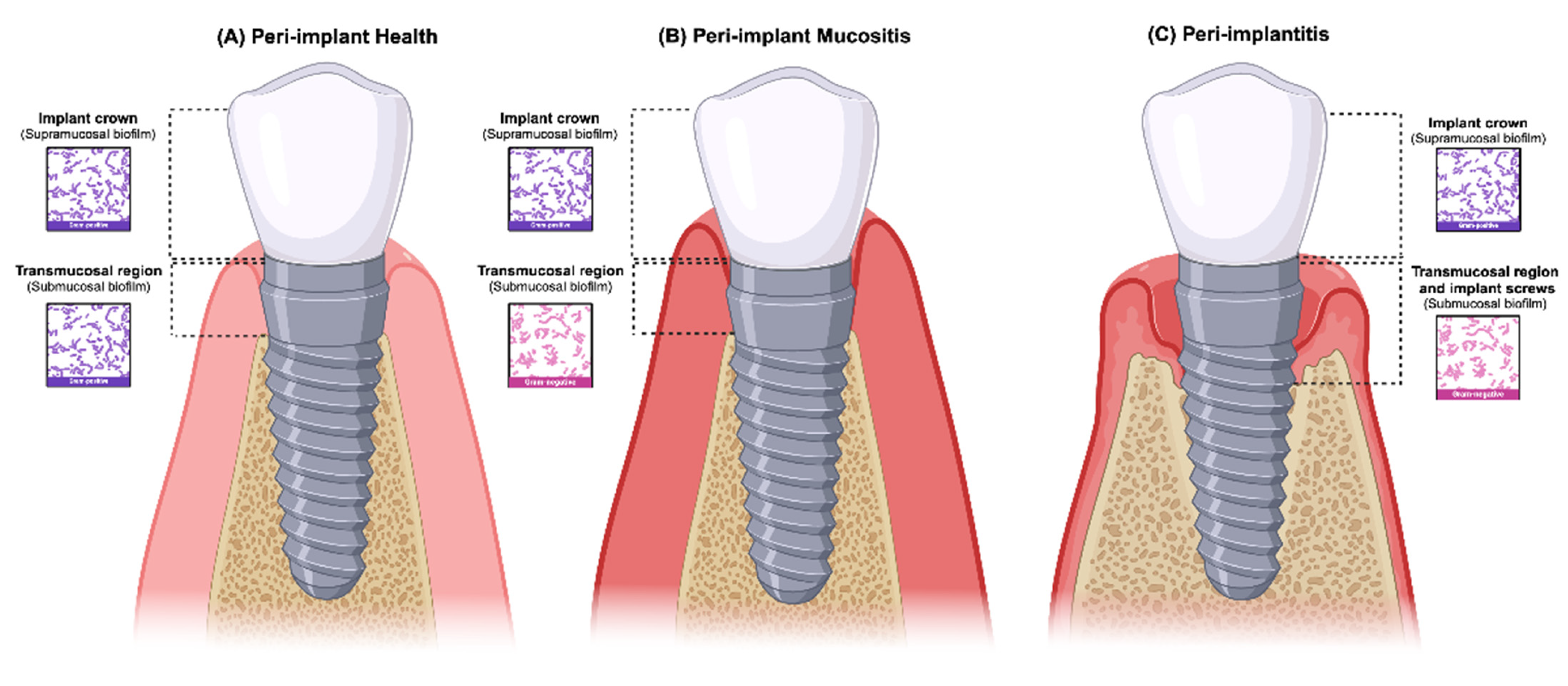
3. Peri-Implant Supramucosal and Submucosal Definition and Standardization
4. What Do We Know about the Peri-Implant Biofilm?
4.1. Peri-Implant Biofilm Formation and Colonization
4.2. The Peri-Implant Biofilm Structure
5. Peri-Implant Microbial Profile
- (a)
- Healthy implant microbiome profile
- (b)
- Peri-implant disease
| Supramucosal | Submucosal | |
|---|---|---|
| Peri-implant Health [22,33,44,58,88,89,90,91,92,93,94,104] | A. massiliensis K. oralis L. mirabilis P. multiformis | A. massiliensis A. meyeri A. naeslundii A. oris Actinomyces sp. Actinomycetia class Bacilli class (genus Granulicatella) C. sputigena C. matruchotii E. corrodens Epsilonproteobacteria class (genus Campylobacter) Fusobacterium sp. Gammaproteobacteria class (genus Vibrio) genus Acinetobacter genus Bradyrhizobium genus Dialister genus Filifactor genus Mogibacterium genus Paludibacter genus Propionibacterium genus Staphylococcus H. parainfluenzae L. hofstadii L. wadei M. salivarium Neisseria sp. P. melaninogenica R. aeria R. dentocariosa Rothia sp. S. oralis S. salivaris S. sanguinis Streptococcus sp. V. dispar Veillonella sp. |
| Peri-implant mucositis [89,105,106,107,108] | - | A. gerencseriae C. rectus C. ochracea D. pneumosintes Fusobacterium sp. Genera Fusobacterium Genera Prevotella P. micros P. gingivalis P. denticola P. intermedia T. forsythia T. denticola |
| Peri-implantitis [18,22,23,31,58,72,81,83,90,95,104,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121] | - | Actinobacteria class (genus Micrococcus) A. actinomycetemcomitans Bacteroidia class Candida sp. C. leadbetteri Chloroflexi spp. Clostridia class (species Catonella morbi and Clostridiales spp. HOT-093) Deltaproteobacteria class D. invisus E. aerogenes E. cloacae Epstein–Barr virus E. coli E. saphenum F. alocis F. fastidiosum Fretibacterium HMT 360 F. nucleatum Gammaproteobacteria (genus Moraxella and Acinetobacter) class H. pylori Human cytomegalovirus L. lactis Mitsuokella spp. HOT 131 Neisseria sp. Peptostreptococcus sp. P. endodontali P. gingivalis P. nigrescens P. oris Porphyromonas spp. HOT-395 P. intermedia P. aeruginosa Pseudomonas sp. Spirochaetes S. aureus S. epidermidis Synergistia class (species Syergistetes spp. HOT-360) T. forsythia Tenericutes spp. T. denticola T. maltophilum |
6. The Interaction between the Host Immune System and the Peri-Implant Microbiota
7. Role of Implant Surface on the Microbiota of Peri-Implantitis
8. Evidence of the Titanium Particle Effect on the Peri-Implant Microbiome
9. Similarities and Differences between Peri-Implantitis and Periodontitis
10. Conclusions
11. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, J.; Meyle, J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 282–285. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological complications with dental implants: Their prevention, diagnosis and treatment. Clin. Oral. Implants Res. 2000, 11 (Suppl. S1), 146–155. [Google Scholar] [CrossRef] [PubMed]
- Pokrowiecki, R.; Mielczarek, A.; Zaręba, T.; Tyski, S. Oral microbiome and peri-implant diseases: Where are we now? Ther. Clin. Risk Manag. 2017, 13, 1529–1542. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S313–S318. [Google Scholar] [CrossRef]
- Alves, C.H.; Russi, K.L.; Rocha, N.C.; Bastos, F.; Darrieux, M.; Parisotto, T.M.; Girardello, R. Host-microbiome interactions regarding peri-implantitis and dental implant loss. J. Transl. Med. 2022, 20, 425. [Google Scholar] [CrossRef]
- Cecchinato, D.; Parpaiola, A.; Lindhe, J. A cross-sectional study on the prevalence of marginal bone loss among implant patients. Clin. Oral. Implant. Res. 2013, 24, 87–90. [Google Scholar] [CrossRef]
- Marrone, A.; Lasserre, J.; Bercy, P.; Brecx, M.C. Prevalence and risk factors for peri-implant disease in Belgian adults. Clin. Oral. Implant. Res. 2013, 24, 934–940. [Google Scholar] [CrossRef]
- Mir-Mari, J.; Mir-Orfila, P.; Figueiredo, R.; Valmaseda-Castellón, E.; Gay-Escoda, C. Prevalence of peri-implant diseases. A cross-sectional study based on a private practice environment. J. Clin. Periodontol. 2012, 39, 490–494. [Google Scholar] [CrossRef]
- Diaz, P.; Gonzalo, E.; Villagra, L.J.G.; Miegimolle, B.; Suarez, M.J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health 2022, 22, 449. [Google Scholar] [CrossRef] [PubMed]
- Vernon, J.; Raif, E.L.M.; Aw, J.; Attenborough, E.; Jha, A.; Do, T. Dental implant surfaces and their interaction with the oral microbiome. Dent. Rev. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Daubert, D.M.; Weinstein, B.F. Biofilm as a risk factor in implant treatment. Periodontol. 2000 2019, 81, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Săndulescu, M.; Sîrbu, V.D.; Popovici, I.A. Bacterial species associated with peri-implant disease—A literature review. Germs 2023, 13, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Giro, G.; Tebar, A.; Franco, L.; Racy, D.; Bastos, M.F.; Shibli, J.A. Treg and TH17 link to immune response in individuals with peri-implantitis: A preliminary report. Clin. Oral. Investig. 2021, 25, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Saremi, L.; Shafizadeh, M.; Esmaeilzadeh, E.; Ghaffari, M.E.; Mahdavi, M.H.; Amid, R.; Kadkhodazadeh, M. Assessment of IL-10, IL-1ß and TNF-α gene polymorphisms in patients with peri-implantitis and healthy controls. Mol. Biol. Rep. 2021, 48, 2285–2290. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, J.; Ma, L.; Bai, N.; Xu, H. Wnt5a is involved in LOX-1 and TLR4 induced host inflammatory response in peri-implantitis. J. Periodontal. Res. 2020, 55, 199–208. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Periodontol. 2018, 89 (Suppl. S1), S267–S290. [Google Scholar] [CrossRef]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef]
- Ganesan, S.M.; Joshi, V.; Fellows, M.; Dabdoub, S.M.; Nagaraja, H.N.; O’Donnell, B.; Deshpande, N.R.; Kumar, P.S. A tale of two risks: Smoking, diabetes and the subgingival microbiome. ISME J. 2017, 11, 2075–2089. [Google Scholar] [CrossRef]
- Kensara, A.; Hefni, E.; Williams, M.A.; Saito, H.; Mongodin, E.; Masri, R. Microbiological Profile and Human Immune Response Associated with Peri-Implantitis: A Systematic Review. J. Prosthodont. 2021, 30, 210–234. [Google Scholar] [CrossRef] [PubMed]
- Kensara, A.; Saito, H.; Mongodin, E.F.; Masri, R. Microbiological profile of peri-implantitis: Analyses of peri-implant microbiome. J. Prosthodont. 2024, 33, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, É.B.S.; Romandini, M.; Sadilina, S.; Sant’Ana, A.C.P.; Sanz, M. Microbiota associated with peri-implantitis-A systematic review with meta-analyses. Clin. Oral. Implant. Res. 2023, 34, 1176–1187. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Wennström, J.L.; Lindhe, J. Long-term outcome of surgical treatment of peri-implantitis. A 2-11-year retrospective study. Clin. Oral. Implant. Res. 2018, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Romandini, M.; Lima, C.; Pedrinaci, I.; Araoz, A.; Soldini, M.C.; Sanz, M. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study. Clin. Oral. Implant. Res. 2021, 32, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Chaparro, P.J.; Duarte, P.M.; Shibli, J.A.; Montenegro, S.; Lacerda Heluy, S.; Figueiredo, L.C.; Faveri, M.; Feres, M. The Current Weight of Evidence of the Microbiologic Profile Associated with Peri-Implantitis: A Systematic Review. J. Periodontol. 2016, 87, 1295–1304. [Google Scholar] [CrossRef]
- Pontoriero, R.; Tonelli, M.P.; Carnevale, G.; Mombelli, A.; Nyman, S.R.; Lang, N.P. Experimentally induced peri-implant mucositis. A clinical study in humans. Clin. Oral. Implant. Res. 1994, 5, 254–259. [Google Scholar] [CrossRef]
- Salvi, G.E.; Aglietta, M.; Eick, S.; Sculean, A.; Lang, N.P.; Ramseier, C.A. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin. Oral. Implant. Res. 2012, 23, 182–190. [Google Scholar] [CrossRef]
- Schwarz, F.; Mihatovic, I.; Golubovic, V.; Eick, S.; Iglhaut, T.; Becker, J. Experimental peri-implant mucositis at different implant surfaces. J. Clin. Periodontol. 2014, 41, 513–520. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Mombelli, A.; Décaillet, F. The characteristics of biofilms in peri-implant disease. J. Clin. Periodontol. 2011, 38 (Suppl. S11), 203–213. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.; Nagay, B.E.; Bertolini, M.; Costa-Oliveira, B.E.; Sampaio, A.A.; Retamal-Valdes, B.; Shibli, J.A.; Feres, M.; Barão, V.A.R.; Souza, J.G.S. Fitting pieces into the puzzle: The impact of titanium-based dental implant surface modifications on bacterial accumulation and polymicrobial infections. Adv. Colloid. Interface Sci. 2021, 298, 102551. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Yang, Y.; Wang, Y.; Cao, X.; Jin, Y.; Xu, Y.; Li, S.C.; Zhou, Q. Periodontal and Peri-Implant Microbiome Dysbiosis Is Associated with Alterations in the Microbial Community Structure and Local Stability. Front. Microbiol. 2021, 12, 785191. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Kozak, M.; Pawlik, A. The Role of the Oral Microbiome in the Development of Diseases. Int. J. Mol. Sci. 2023, 24, 5231. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef]
- Kuramitsu, H.K.; He, X.; Lux, R.; Anderson, M.H.; Shi, W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 2007, 71, 653–670. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S12–S22. [Google Scholar] [CrossRef]
- Chawhuaveang, D.D.; Yu, O.Y.; Yin, I.X.; Lam, W.Y.; Mei, M.L.; Chu, C.H. Acquired salivary pellicle and oral diseases: A literature review. J. Dent. Sci. 2021, 16, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral. Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Edgerton, M.; Lo, S.E.; Scannapieco, F.A. Experimental salivary pellicles formed on titanium surfaces mediate adhesion of streptococci. Int. J. Oral. Maxillofac. Implant. 1996, 11, 443–449. [Google Scholar]
- Quirynen, M.; Vogels, R.; Pauwels, M.; Haffajee, A.D.; Socransky, S.S.; Uzel, N.G.; van Steenberghe, D. Initial subgingival colonization of ‘pristine’ pockets. J. Dent. Res. 2005, 84, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yamada, M.; Ueda, T.; Sakurai, K. Reduction of biofilm formation on titanium surface with ultraviolet-C pre-irradiation. J. Biomater. Appl. 2014, 29, 161–171. [Google Scholar] [CrossRef]
- Boks, N.P.; Norde, W.; van der Mei, H.C.; Busscher, H.J. Forces involved in bacterial adhesion to hydrophilic and hydrophobic surfaces. Microbiology 2008, 154 Pt 10, 3122–3133. [Google Scholar] [CrossRef]
- Charalampakis, G.; Belibasakis, G.N. Microbiome of peri-implant infections: Lessons from conventional, molecular and metagenomic analyses. Virulence 2015, 6, 183–187. [Google Scholar] [CrossRef]
- Gristina, A.G.; Naylor, P.; Myrvik, Q. Infections from biomaterials and implants: A race for the surface. Med. Prog. Technol. 1988, 14, 205–224. [Google Scholar]
- Costerton, J.W.; Montanaro, L.; Arciola, C.R. Biofilm in implant infections: Its production and regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bächle, M.; Hannig, C.; Han, J.S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral. Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef]
- Pye, A.D.; Lockhart, D.E.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N. Microbiological and immuno-pathological aspects of peri-implant diseases. Arch. Oral. Biol. 2014, 59, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S.; Kim, H.Y.; Lim, K.S.; Han, J.S. Implant surface factors and bacterial adhesion: A review of the literature. Int. J. Artif. Organs 2012, 35, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, P.; Sánchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz Alonso, M. Biofilm formation on dental implants with different surface micro-topography: An in vitro study. Clin. Oral. Implant. Res. 2019, 30, 725–734. [Google Scholar] [CrossRef]
- Costa, F.O.; Ferreira, S.D.; Cortelli, J.R.; Lima, R.P.E.; Cortelli, S.C.; Cota, L.O.M. Microbiological profile associated with peri-implant diseases in individuals with and without preventive maintenance therapy: A 5-year follow-up. Clin. Oral. Investig. 2019, 23, 3161–3171. [Google Scholar] [CrossRef]
- Yu, X.L.; Chan, Y.; Zhuang, L.; Lai, H.C.; Lang, N.P.; Keung Leung, W.; Watt, R.M. Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease. Clin. Oral. Implant. Res. 2019, 30, 760–776. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Olmedo, D.G. Peri-implantitis is not periodontitis: Scientific discoveries shed light on microbiome-biomaterial interactions that may determine disease phenotype. Periodontol. 2000 2021, 86, 231–240. [Google Scholar] [CrossRef]
- Lima, E.M.; Koo, H.; Vacca Smith, A.M.; Rosalen, P.L.; Del Bel Cury, A.A. Adsorption of salivary and serum proteins, and bacterial adherence on titanium and zirconia ceramic surfaces. Clin. Oral. Implant. Res. 2008, 19, 780–785. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Llama-Palacios, A.; Fernández, E.; Figuero, E.; Marín, M.J.; León, R.; Blanc, V.; Herrera, D.; Sanz, M. An in vitro biofilm model associated to dental implants: Structural and quantitative analysis of in vitro biofilm formation on different dental implant surfaces. Dent. Mater. 2014, 30, 1161–1171. [Google Scholar] [CrossRef]
- Tsigarida, A.A.; Dabdoub, S.M.; Nagaraja, H.N.; Kumar, P.S. The Influence of Smoking on the Peri-Implant Microbiome. J. Dent. Res. 2015, 94, 1202–1217. [Google Scholar] [CrossRef] [PubMed]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Chapple, I.L. Clinical research on peri-implant diseases: Consensus report of Working Group 4. J. Clin. Periodontol. 2012, 39 (Suppl. S12), 202–206. [Google Scholar] [CrossRef]
- Shibli, J.A.; Melo, L.; Ferrari, D.S.; Figueiredo, L.C.; Faveri, M.; Feres, M. Composition of supra- and subgingival biofilm of subjects with healthy and diseased implants. Clin. Oral. Implant. Res. 2008, 19, 975–982. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, D.; Zhao, Y.; Chen, S.; Li, Y. Inflammatory cytokine profiles in the crevicular fluid around clinically healthy dental implants compared to the healthy contralateral side during the early stages of implant function. Arch. Oral. Biol. 2019, 108, 104509. [Google Scholar] [CrossRef] [PubMed]
- Villar, C.; Huynh-Ba, G.; Mills, M.; Cochran, D. Wound Healing Around Dental Implants. Endod. Top 2013, 25, 44–62. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin. Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef]
- Gravel, D.; Canard, E.; Guichard, F.; Mouquet, N. Persistence increases with diversity and connectance in trophic metacommunities. PLoS ONE 2011, 6, e19374. [Google Scholar] [CrossRef]
- Kulkarni, D.; De Laender, F. The combined effects of biotic and abiotic stress on species richness and connectance. PLoS ONE 2017, 12, e0172828. [Google Scholar] [CrossRef]
- Gardner, M.R.; Ashby, W.R. Connectance of large dynamic (cybernetic) systems: Critical values for stability. Nature 1970, 228, 784. [Google Scholar] [CrossRef]
- Stacy, A.; Everett, J.; Jorth, P.; Trivedi, U.; Rumbaugh, K.P.; Whiteley, M. Bacterial fight-and-flight responses enhance virulence in a polymicrobial infection. Proc. Natl. Acad. Sci. USA 2014, 111, 7819–7824. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.N.; Manoil, D. Microbial Community-Driven Etiopathogenesis of Peri-Implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; Marsh, P.D.; Mira, A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018, 97, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Serino, G.; Ström, C. Peri-implantitis in partially edentulous patients: Association with inadequate plaque control. Clin. Oral. Implant. Res. 2009, 20, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Frisch, E.; Ziebolz, D.; Vach, K.; Ratka-Krüger, P. Supportive post-implant therapy: Patient compliance rates and impacting factors: 3-year follow-up. J. Clin. Periodontol. 2014, 41, 1007–1014. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Cury, J.A.; Ricomini Filho, A.P.; Feres, M.; Faveri, M.; Barão, V.A.R. Effect of sucrose on biofilm formed in situ on titanium material. J. Periodontol. 2019, 90, 141–148. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Costa Oliveira, B.E.; Bertolini, M.; Lima, C.V.; Retamal-Valdes, B.; de Faveri, M.; Feres, M.; Barão, V.A.R. Titanium particles and ions favor dysbiosis in oral biofilms. J. Periodontal Res. 2020, 55, 258–266. [Google Scholar] [CrossRef]
- Costa, R.C.; Souza, J.G.S.; Bertolini, M.; Retamal-Valdes, B.; Feres, M.; Barão, V.A.R. Extracellular biofilm matrix leads to microbial dysbiosis and reduces biofilm susceptibility to antimicrobials on titanium biomaterial: An in vitro and in situ study. Clin. Oral. Implant. Res. 2020, 31, 1173–1186. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pimentel, S.P.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z. Host response and peri-implantitis. Braz. Oral. Res. 2019, 33 (Suppl. S1), e066. [Google Scholar] [CrossRef]
- Rams, T.E.; Link, C.C., Jr. Microbiology of failing dental implants in humans: Electron microscopic observations. J. Oral. Implantol. 1983, 11, 93–100. [Google Scholar]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral. Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.S.; Torosian, J.P.; Slots, J. Microbial differences in 2 clinically distinct types of failures of osseointegrated implants. Clin. Oral. Implant. Res. 1991, 2, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Buser, D.; Lang, N.P. Colonization of osseointegrated titanium implants in edentulous patients. Early results. Oral. Microbiol. Immunol. 1988, 3, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Mericske-Stern, R. Microbiological features of stable osseointegrated implants used as abutments for overdentures. Clin. Oral. Implant. Res. 1990, 1, 1–7. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal microbial ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Ogata, Y. Risk Factors for Peri-Implant Diseases; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Bower, R.C.; Radny, N.R.; Wall, C.D.; Henry, P.J. Clinical and microscopic findings in edentulous patients 3 years after incorporation of osseointegrated implant-supported bridgework. J. Clin. Periodontol. 1989, 16, 580–587. [Google Scholar] [CrossRef]
- Korsch, M.; Marten, S.M.; Stoll, D.; Prechtl, C.; Dötsch, A. Microbiological findings in early and late implant loss: An observational clinical case-controlled study. BMC Oral. Health 2021, 21, 112. [Google Scholar] [CrossRef]
- Zhou, N.; Huang, H.; Liu, H.; Li, Q.; Yang, G.; Zhang, Y.; Ding, M.; Dong, H.; Mou, Y. Microbiota analysis of peri-implant mucositis in patients with periodontitis history. Clin. Oral. Investig. 2022, 26, 6223–6233. [Google Scholar] [CrossRef]
- Sanz-Martin, I.; Doolittle-Hall, J.; Teles, R.P.; Patel, M.; Belibasakis, G.N.; Hämmerle, C.H.F.; Jung, R.E.; Teles, F.R.F. Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J. Clin. Periodontol. 2017, 44, 1274–1284. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Charalampakis, G.; Bostanci, N.; Stadlinger, B. Peri-implant infections of oral biofilm etiology. Adv. Exp. Med. Biol. 2015, 830, 69–84. [Google Scholar] [CrossRef]
- Elter, C.; Heuer, W.; Demling, A.; Hannig, M.; Heidenblut, T.; Bach, F.W.; Stiesch-Scholz, M. Supra- and subgingival biofilm formation on implant abutments with different surface characteristics. Int. J. Oral. Maxillofac. Implant. 2008, 23, 327–334. [Google Scholar]
- Quirynen, M.; Gijbels, F.; Jacobs, R. An infected jawbone site compromising successful osseointegration. Periodontol. 2000 2003, 33, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Rutger Persson, G. Infection at titanium implants with or without a clinical diagnosis of inflammation. Clin. Oral. Implant. Res. 2007, 18, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Renvert, S. Cluster of bacteria associated with peri-implantitis. Clin. Implant. Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Casado, P.L.; Otazu, I.B.; Balduino, A.; de Mello, W.; Barboza, E.P.; Duarte, M.E. Identification of periodontal pathogens in healthy periimplant sites. Implant. Dent. 2011, 20, 226–235. [Google Scholar] [CrossRef]
- Lee, K.H.; Tanner, A.C.; Maiden, M.F.; Weber, H.P. Pre- and post-implantation microbiota of the tongue, teeth, and newly placed implants. J. Clin. Periodontol. 1999, 26, 822–832. [Google Scholar] [CrossRef]
- Hultin, M.; Gustafsson, A.; Hallström, H.; Johansson, L.A.; Ekfeldt, A.; Klinge, B. Microbiological findings and host response in patients with peri-implantitis. Clin. Oral. Implant. Res. 2002, 13, 349–358. [Google Scholar] [CrossRef]
- Devides, S.L.; Franco, A.T. Evaluation of peri-implant microbiota using the polymerase chain reaction in completely edentulous patients before and after placement of implant-supported prostheses submitted to immediate load. Int. J. Oral. Maxillofac. Implant. 2006, 21, 262–269. [Google Scholar]
- Van Assche, N.; Van Essche, M.; Pauwels, M.; Teughels, W.; Quirynen, M. Do periodontopathogens disappear after full-mouth tooth extraction? J. Clin. Periodontol. 2009, 36, 1043–1047. [Google Scholar] [CrossRef]
- Fernandes, C.B.; Aquino, D.R.; Franco, G.C.; Cortelli, S.C.; Costa, F.O.; Cortelli, J.R. Do elderly edentulous patients with a history of periodontitis harbor periodontal pathogens? Clin. Oral. Implants Res. 2010, 21, 618–623. [Google Scholar] [CrossRef]
- Quirynen, M.; Van Assche, N. Microbial changes after full-mouth tooth extraction, followed by 2-stage implant placement. J. Clin. Periodontol. 2011, 38, 581–589. [Google Scholar] [CrossRef]
- Van Assche, N.; Pittayapat, P.; Jacobs, R.; Pauwels, M.; Teughels, W.; Quirynen, M. Microbiological outcome of two screw-shaped titanium implant systems placed following a split-mouth randomised protocol, at the 12th year of follow-up after loading. Eur. J. Oral. Implantol. 2011, 4, 103–116. [Google Scholar] [PubMed]
- Gao, X.; Zhou, J.; Sun, X.; Li, X.; Zhou, Y. Diversity analysis of subgingival microbial bacteria in peri-implantitis in Uygur population. Medicine 2018, 97, e9774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, L.; Wang, Z.; Li, L.; Zhang, J.; Zhang, Q.; Chen, T.; Lin, J.; Chen, F. Subgingival microbiome in patients with healthy and ailing dental implants. Sci. Rep. 2015, 5, 10948. [Google Scholar] [CrossRef] [PubMed]
- Emrani, J.; Chee, W.; Slots, J. Bacterial colonization of oral implants from nondental sources. Clin. Implant. Dent. Relat. Res. 2009, 11, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Máximo, M.B.; de Mendonça, A.C.; Renata Santos, V.; Figueiredo, L.C.; Feres, M.; Duarte, P.M. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin. Oral. Implant. Res. 2009, 20, 99–108. [Google Scholar] [CrossRef]
- Apatzidou, D.; Lappin, D.F.; Hamilton, G.; Papadopoulos, C.A.; Konstantinidis, A.; Riggio, M.P. Microbiome of peri-implantitis affected and healthy dental sites in patients with a history of chronic periodontitis. Arch. Oral. Biol. 2017, 83, 145–152. [Google Scholar] [CrossRef]
- Mulla, M.; Mulla, M.; Hegde, S.; Koshy, A.V. In vitro assessment of the effect of probiotic lactobacillus reuteri on peri-implantitis microflora. BMC Oral. Health 2021, 21, 408. [Google Scholar] [CrossRef]
- Komatsu, K.; Shiba, T.; Takeuchi, Y.; Watanabe, T.; Koyanagi, T.; Nemoto, T.; Shimogishi, M.; Shibasaki, M.; Katagiri, S.; Kasugai, S.; et al. Discriminating Microbial Community Structure Between Peri-Implantitis and Periodontitis With Integrated Metagenomic, Metatranscriptomic, and Network Analysis. Front. Cell Infect. Microbiol. 2020, 10, 596490. [Google Scholar] [CrossRef]
- Kumar, P.S.; Mason, M.R.; Brooker, M.R.; O’Brien, K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J. Clin. Periodontol. 2012, 39, 425–433. [Google Scholar] [CrossRef]
- Maruyama, N.; Maruyama, F.; Takeuchi, Y.; Aikawa, C.; Izumi, Y.; Nakagawa, I. Intraindividual variation in core microbiota in peri-implantitis and periodontitis. Sci. Rep. 2014, 4, 6602. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Zhang, Q.; Zheng, H.; Xu, L.X.; Wang, X.Y.; Chen, F. Pyrosequencing of the subgingival microbiome in peri-implantitis after non-surgical mechanical debridement therapy. J. Periodontal Res. 2020, 55, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Leonhardt, A.; Dahlén, G.; Renvert, S. Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J. Periodontol. 2003, 74, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral. Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Persson, G.R.; Samuelsson, E.; Lindahl, C.; Renvert, S. Mechanical non-surgical treatment of peri-implantitis: A single-blinded randomized longitudinal clinical study. II. Microbiological results. J. Clin. Periodontol. 2010, 37, 563–573. [Google Scholar] [CrossRef]
- Thurnheer, T.; Belibasakis, G.N. Incorporation of staphylococci into titanium-grown biofilms: An in vitro “submucosal” biofilm model for peri-implantitis. Clin. Oral. Implants Res. 2016, 27, 890–895. [Google Scholar] [CrossRef]
- Kensara, A.; Saito, H.; Mongodin, E.F.; Masri, R. Microbiological profile of peri-implantitis: Analyses of microbiome within dental implants. J. Prosthodont. 2023, 32, 783–792. [Google Scholar] [CrossRef]
- Mussano, F.; Ferrocino, I.; Gavrilova, N.; Genova, T.; Dell’Acqua, A.; Cocolin, L.; Carossa, S. Apical periodontitis: Preliminary assessment of microbiota by 16S rRNA high throughput amplicon target sequencing. BMC Oral. Health 2018, 18, 55. [Google Scholar] [CrossRef]
- Kaboré, W.A.D.; Dembélé, R.; Bagré, T.S.; Konaté, A.; Boisramé, S.; Chevalier, V.; Konsem, T.; Traoré, A.S.; Barro, N. Characterization and Antimicrobial Susceptibility of Lactococcus lactis Isolated from Endodontic Infections in Ouagadougou, Burkina Faso. Dent. J. 2018, 6, 69. [Google Scholar] [CrossRef]
- Jankovic, S.; Aleksic, Z.; Dimitrijevic, B.; Lekovic, V.; Milinkovic, I.; Kenney, B. Correlation between different genotypes of human cytomegalovirus and Epstein-Barr virus and peri-implant tissue status. Aust. Dent. J. 2011, 56, 382–388. [Google Scholar] [CrossRef]
- de Melo, F.; Milanesi, F.C.; Angst, P.D.M.; Oppermann, R.V. A systematic review of the microbiota composition in various peri-implant conditions: Data from 16S rRNA gene sequencing. Arch. Oral. Biol. 2020, 117, 104776. [Google Scholar] [CrossRef] [PubMed]
- Sousa, V.; Nibali, L.; Spratt, D.; Dopico, J.; Mardas, N.; Petrie, A.; Donos, N. Peri-implant and periodontal microbiome diversity in aggressive periodontitis patients: A pilot study. Clin. Oral. Implant. Res. 2017, 28, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Jiang, J.; Li, J.; Zhou, C.; Chen, Y.; Lu, H.; He, F. The Characteristics of Microbiome and Cytokines in Healthy Implants and Peri-Implantitis of the Same Individuals. J. Clin. Med. 2022, 11, 5817. [Google Scholar] [CrossRef] [PubMed]
- Pallos, D.; Sousa, V.; Feres, M.; Retamal-Valdes, B.; Chen, T.; Curtis, M.; Boaventura, R.M.; Tanaka, M.H.; Salomão, G.; Zanella, L.; et al. Salivary Microbial Dysbiosis Is Associated with Peri-Implantitis: A Case-Control Study in a Brazilian Population. Front. Cell Infect. Microbiol. 2021, 11, 696432. [Google Scholar] [CrossRef]
- Teng, F.; Darveekaran Nair, S.S.; Zhu, P.; Li, S.; Huang, S.; Li, X.; Xu, J.; Yang, F. Impact of DNA extraction method and targeted 16S-rRNA hypervariable region on oral microbiota profiling. Sci. Rep. 2018, 8, 16321. [Google Scholar] [CrossRef]
- Rahnama-Hezavah, M.; Mertowska, P.; Mertowski, S.; Skiba, J.; Krawiec, K.; Łobacz, M.; Grywalska, E. How Can Imbalance in Oral Microbiota and Immune Response Lead to Dental Implant Problems? Int. J. Mol. Sci. 2023, 24, 17620. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Liu, Y.; Qv, W.; Ma, Y.; Zhang, Y.; Ding, C.; Chu, M.; Chen, F. The interplay between oral microbes and immune responses. Front. Microbiol. 2022, 13, 1009018. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Baseri, M.; Radmand, F.; Hamedi, R.; Yousefi, M.; Kafil, H.S. Immunological Aspects of Dental Implant Rejection. Biomed. Res. Int. 2020, 2020, 7279509. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Garaicoa-Pazmino, C.; Collins, A.; Ong, H.S.; Chudri, R.; Giannobile, W.V. Protein biomarkers and microbial profiles in peri-implantitis. Clin. Oral. Implants Res. 2016, 27, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Widén, C.; Persson, G.R. Cytokine expression in peri-implant crevicular fluid in relation to bacterial presence. J. Clin. Periodontol. 2015, 42, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Venza, I.; Visalli, M.; Cucinotta, M.; De Grazia, G.; Teti, D.; Venza, M. Proinflammatory gene expression at chronic periodontitis and peri-implantitis sites in patients with or without type 2 diabetes. J. Periodontol. 2010, 81, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Hashim, A.; Alsam, A.; Payne, M.A.; Aduse-Opoku, J.; Curtis, M.A.; Joseph, S. Loss of Neutrophil Homing to the Periodontal Tissues Modulates the Composition and Disease Potential of the Oral Microbiota. Infect. Immun. 2021, 89, e0030921. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, R.; Kambara, H.; Ma, F.; Luo, H.R. The role of CXCR2 in acute inflammatory responses and its antagonists as anti-inflammatory therapeutics. Curr. Opin. Hematol. 2019, 26, 28–33. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Fretwurst, T.; Garaicoa-Pazmino, C.; Nelson, K.; Giannobile, W.V.; Squarize, C.H.; Larsson, L.; Castilho, R.M. Characterization of macrophages infiltrating peri-implantitis lesions. Clin. Oral. Implant. Res. 2020, 31, 274–281. [Google Scholar] [CrossRef]
- Galarraga-Vinueza, M.E.; Obreja, K.; Ramanauskaite, A.; Magini, R.; Begic, A.; Sader, R.; Schwarz, F. Macrophage polarization in peri-implantitis lesions. Clin. Oral. Investig. 2021, 25, 2335–2344. [Google Scholar] [CrossRef]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 2009, 10, 241–247. [Google Scholar] [CrossRef]
- Padial-Molina, M.; Montalvo-Acosta, S.; Martín-Morales, N.; Pérez-Carrasco, V.; Magan-Fernandez, A.; Mesa, F.; O’Valle, F.; Garcia-Salcedo, J.A.; Galindo-Moreno, P. Correlation between Inflammasomes and Microbiota in Peri-Implantitis. Int. J. Mol. Sci. 2024, 25, 961. [Google Scholar] [CrossRef] [PubMed]
- Jiao, P.; Li, Z.; Li, B.; Jiao, X. The Role of Caspase-11 and Pyroptosis in the Regulation of Inflammation in Peri-Implantitis. J. Inflamm. Res. 2023, 16, 4471–4479. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Carinci, F.; Palmieri, A.; Cura, F.; Caruso, S.; Candotto, V. Reuterinos® as adjuvant for peri-implant treatment: A pilot study. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419827745. [Google Scholar] [CrossRef]
- Robitaille, N.; Reed, D.N.; Walters, J.D.; Kumar, P.S. Periodontal and peri-implant diseases: Identical or fraternal infections? Mol. Oral. Microbiol. 2016, 31, 285–301. [Google Scholar] [CrossRef]
- Kniha, K.; Heussen, N.; Modabber, A.; Hölzle, F.; Möhlhenrich, S.C. The effect of zirconia and titanium surfaces on biofilm formation and on host-derived immunological parameters. Int. J. Oral. Maxillofac. Surg. 2021, 50, 1361–1374. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Matos, G.R.M. Surface Roughness of Dental Implant and Osseointegration. J. Maxillofac. Oral. Surg. 2021, 20, 1–4. [Google Scholar] [CrossRef]
- Quirynen, M. The clinical meaning of the surface roughness and the surface free energy of intra-oral hard substrata on the microbiology of the supra- and subgingival plaque: Results of in vitro and in vivo experiments. J. Dent. 1994, 22 (Suppl. S1), S13–S16. [Google Scholar] [CrossRef]
- Quirynen, M.; van der Mei, H.C.; Bollen, C.M.; Schotte, A.; Marechal, M.; Doornbusch, G.I.; Naert, I.; Busscher, H.J.; van Steenberghe, D. An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J. Dent. Res. 1993, 72, 1304–1309. [Google Scholar] [CrossRef]
- Amoroso, P.F.; Adams, R.J.; Waters, M.G.; Williams, D.W. Titanium surface modification and its effect on the adherence of Porphyromonas gingivalis: An in vitro study. Clin. Oral. Implant. Res. 2006, 17, 633–637. [Google Scholar] [CrossRef]
- Kim, M.L.; Jeong, C.M.; Jeon, Y.C.; Byon, E.; Jeong, Y.; Cho, L.R. The effects of Mg-ion implantation and sandblasting on Porphyromonas gingivalis attachment. Clin. Oral. Implant. Res. 2012, 23, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Fröjd, V.; Linderbäck, P.; Wennerberg, A.; Chávez de Paz, L.; Svensäter, G.; Davies, J.R. Effect of nanoporous TiO2 coating and anodized Ca2+ modification of titanium surfaces on early microbial biofilm formation. BMC Oral. Health 2011, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Badihi Hauslich, L.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The adhesion of oral bacteria to modified titanium surfaces: Role of plasma proteins and electrostatic forces. Clin. Oral. Implants Res. 2013, 24 (Suppl. SA100), 49–56. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Flores, A.; Olivares-Navarrete, R.; Wieland, M.; Ximénez-Fyvie, L.A.; Schwartz, Z.; Boyan, B.D. Influence of topography and hydrophilicity on initial oral biofilm formation on microstructured titanium surfaces in vitro. Clin. Oral. Implant. Res. 2012, 23, 301–307. [Google Scholar] [CrossRef]
- Grössner-Schreiber, B.; Griepentrog, M.; Haustein, I.; Müller, W.D.; Lange, K.P.; Briedigkeit, H.; Göbel, U.B. Plaque formation on surface modified dental implants. An in vitro study. Clin. Oral. Implant. Res. 2001, 12, 543–551. [Google Scholar] [CrossRef]
- Tillmanns, H.W.; Hermann, J.S.; Cagna, D.R.; Burgess, A.V.; Meffert, R.M. Evaluation of three different dental implants in ligature-induced peri-implantitis in the beagle dog. Part I. Clinical evaluation. Int. J. Oral. Maxillofac. Implant. 1997, 12, 611–620. [Google Scholar]
- Shibli, J.A.; Martins, M.C.; Lotufo, R.F.; Marcantonio, E., Jr. Microbiologic and radiographic analysis of ligature-induced peri-implantitis with different dental implant surfaces. Int. J. Oral. Maxillofac. Implant. 2003, 18, 383–390. [Google Scholar]
- Sinjab, K.; Sawant, S.; Ou, A.; Fenno, J.C.; Wang, H.L.; Kumar, P. Impact of surface characteristics on the peri-implant microbiome in health and disease. J. Periodontol. 2024, 95, 244–255. [Google Scholar] [CrossRef]
- Matter, M.T.; Maliqi, L.; Keevend, K.; Guimond, S.; Ng, J.; Armagan, E.; Rottmar, M.; Herrmann, I.K. One-Step Synthesis of Versatile Antimicrobial Nano-Architected Implant Coatings for Hard and Soft Tissue Healing. ACS Appl. Mater. Interfaces 2021, 13, 33300–33310. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.B.; Albrektsson, T.; Francischone, C.E.; Schwartz Filho, H.O.; Wennerberg, A. The influence of surface treatment on the implant roughness pattern. J. Appl. Oral. Sci. 2012, 20, 550–555. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Xia, X.; Fares, C.; Ren, F.; Hsu, S.M.; Budei, D.; Aravindraja, C.; Kesavalu, L.; Esquivel-Upshaw, J.F. Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials 2021, 14, 4357. [Google Scholar] [CrossRef] [PubMed]
- D’Ercole, S.; Cellini, L.; Pilato, S.; Di Lodovico, S.; Iezzi, G.; Piattelli, A.; Petrini, M. Material characterization and Streptococcus oralis adhesion on Polyetheretherketone (PEEK) and titanium surfaces used in implantology. J. Mater. Sci. Mater. Med. 2020, 31, 84. [Google Scholar] [CrossRef] [PubMed]
- Bright, R.; Hayles, A.; Fernandes, D.; Visalakshan, R.M.; Ninan, N.; Palms, D.; Burzava, A.; Barker, D.; Brown, T.; Vasilev, K. In Vitro Bactericidal Efficacy of Nanostructured Ti6Al4V Surfaces is Bacterial Load Dependent. ACS Appl. Mater. Interfaces 2021, 13, 38007–38017. [Google Scholar] [CrossRef] [PubMed]
- Pantaroto, H.N.; Amorim, K.P.; Matozinho Cordeiro, J.; Souza, J.G.S.; Ricomini-Filho, A.P.; Rangel, E.C.; Ribeiro, A.L.R.; Vaz, L.G.; Barão, V.A.R. Proteome analysis of the salivary pellicle formed on titanium alloys containing niobium and zirconium. Biofouling 2019, 35, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Nagay, B.E.; Cordeiro, J.M.; Barao, V.A.R. Insight into Corrosion of Dental Implants: From Biochemical Mechanisms to Designing Corrosion-Resistant Materials. Curr. Oral. Health Rep. 2022, 9, 7–21. [Google Scholar] [CrossRef]
- Olmedo, D.G.; Paparella, M.L.; Spielberg, M.; Brandizzi, D.; Guglielmotti, M.B.; Cabrini, R.L. Oral mucosa tissue response to titanium cover screws. J. Periodontol. 2012, 83, 973–980. [Google Scholar] [CrossRef]
- Messous, R.; Henriques, B.; Bousbaa, H.; Silva, F.S.; Teughels, W.; Souza, J.C.M. Cytotoxic effects of submicron- and nano-scale titanium debris released from dental implants: An integrative review. Clin. Oral. Investig. 2021, 25, 1627–1640. [Google Scholar] [CrossRef]
- Berglundh, T.; Zitzmann, N.U.; Donati, M. Are peri-implantitis lesions different from periodontitis lesions? J. Clin. Periodontol. 2011, 38 (Suppl. S11), 188–202. [Google Scholar] [CrossRef]
- Carcuac, O.; Berglundh, T. Composition of human peri-implantitis and periodontitis lesions. J. Dent. Res. 2014, 93, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Li, Z.; Acharya, A.; Chen, D.; Chen, Z.; Mattheos, N.; Chen, Z.; Huang, B. Long non-coding RNA and mRNA expression profiles in peri-implantitis vs. periodontitis. J. Periodontal Res. 2020, 55, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Catania, D.; Neiva, K.; Wallet, S.M. Innate immune receptor expression in peri-implant tissues of patients with different susceptibility to periodontal diseases. J. Periodontol. 2013, 84, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Tay, J.R.H.; Balan, P.; Ong, M.M.A.; Bostanci, N.; Belibasakis, G.N.; Seneviratne, C.J. Metagenomic sequencing provides new insights into the subgingival bacteriome and aetiopathology of periodontitis. J. Periodontal Res. 2021, 56, 205–218. [Google Scholar] [CrossRef]
- Konstantinidis, I.K.; Kotsakis, G.A.; Gerdes, S.; Walter, M.H. Cross-sectional study on the prevalence and risk indicators of peri-implant diseases. Eur. J. Oral. Implantol. 2015, 8, 75–88. [Google Scholar]
- Dabdoub, S.M.; Tsigarida, A.A.; Kumar, P.S. Patient-specific analysis of periodontal and peri-implant microbiomes. J. Dent. Res. 2013, 92 (Suppl. S12), 168s–175s. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Renvert, S.; Polyzois, I. Risk indicators for peri-implant mucositis: A systematic literature review. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S172–S186. [Google Scholar] [CrossRef]
- Peyyala, R.; Ebersole, J.L. Multispecies biofilms and host responses: “discriminating the trees from the forest”. Cytokine 2013, 61, 15–25. [Google Scholar] [CrossRef]
- Shiba, T.; Watanabe, T.; Kachi, H.; Koyanagi, T.; Maruyama, N.; Murase, K.; Takeuchi, Y.; Maruyama, F.; Izumi, Y.; Nakagawa, I. Distinct interacting core taxa in co-occurrence networks enable discrimination of polymicrobial oral diseases with similar symptoms. Sci. Rep. 2016, 6, 30997. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Campos Kajimoto, N.; de Paiva Buischi, Y.; Mohamadzadeh, M.; Loomer, P. The Oral Microbiome of Peri-Implant Health and Disease: A Narrative Review. Dent. J. 2024, 12, 299. https://doi.org/10.3390/dj12100299
de Campos Kajimoto N, de Paiva Buischi Y, Mohamadzadeh M, Loomer P. The Oral Microbiome of Peri-Implant Health and Disease: A Narrative Review. Dentistry Journal. 2024; 12(10):299. https://doi.org/10.3390/dj12100299
Chicago/Turabian Stylede Campos Kajimoto, Natalia, Yvonne de Paiva Buischi, Mansour Mohamadzadeh, and Peter Loomer. 2024. "The Oral Microbiome of Peri-Implant Health and Disease: A Narrative Review" Dentistry Journal 12, no. 10: 299. https://doi.org/10.3390/dj12100299
APA Stylede Campos Kajimoto, N., de Paiva Buischi, Y., Mohamadzadeh, M., & Loomer, P. (2024). The Oral Microbiome of Peri-Implant Health and Disease: A Narrative Review. Dentistry Journal, 12(10), 299. https://doi.org/10.3390/dj12100299








