Effect of Bleaching Agents on Healthy Enamel, White Spots, and Carious Lesions: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion Criteria
- The review included randomized controlled trials, clinical trials, in vitro studies, and observational studies;
- Studies comparing at least two groups;
- In vitro studies investigating the effect of tooth bleaching agents on the demineralization of enamel analyzed with a confocal laser scanning microscope and/or measured via enamel surface roughness, and studies measuring the concentration of cariogenic and periodontal microflora in the surrounding tissues, as well as by the presence of initial or advanced forms of caries;
- Studies assess the effect of bleaching on bacterial adherence to enamel;
- Studies examining the aesthetic effect of bleaching on white or brown spots of enamel and the mechanical effects of bleaching on early caries lesions;
- Studies investigating any effect of bleaching agents on the prevalence of caries (primary or secondary).
2.3. Exclusion Criteria
- Animal studies, case reports, and case series;
- Studies without at least one control and one test group, and studies without a comprehensive protocol;
- Studies with ineligible results for this review (i.e., studies that, when reading the full text, the results did not relate to this study);
- Lack of access to the full text of a study after attempting to contact the author.
2.4. Data Extraction
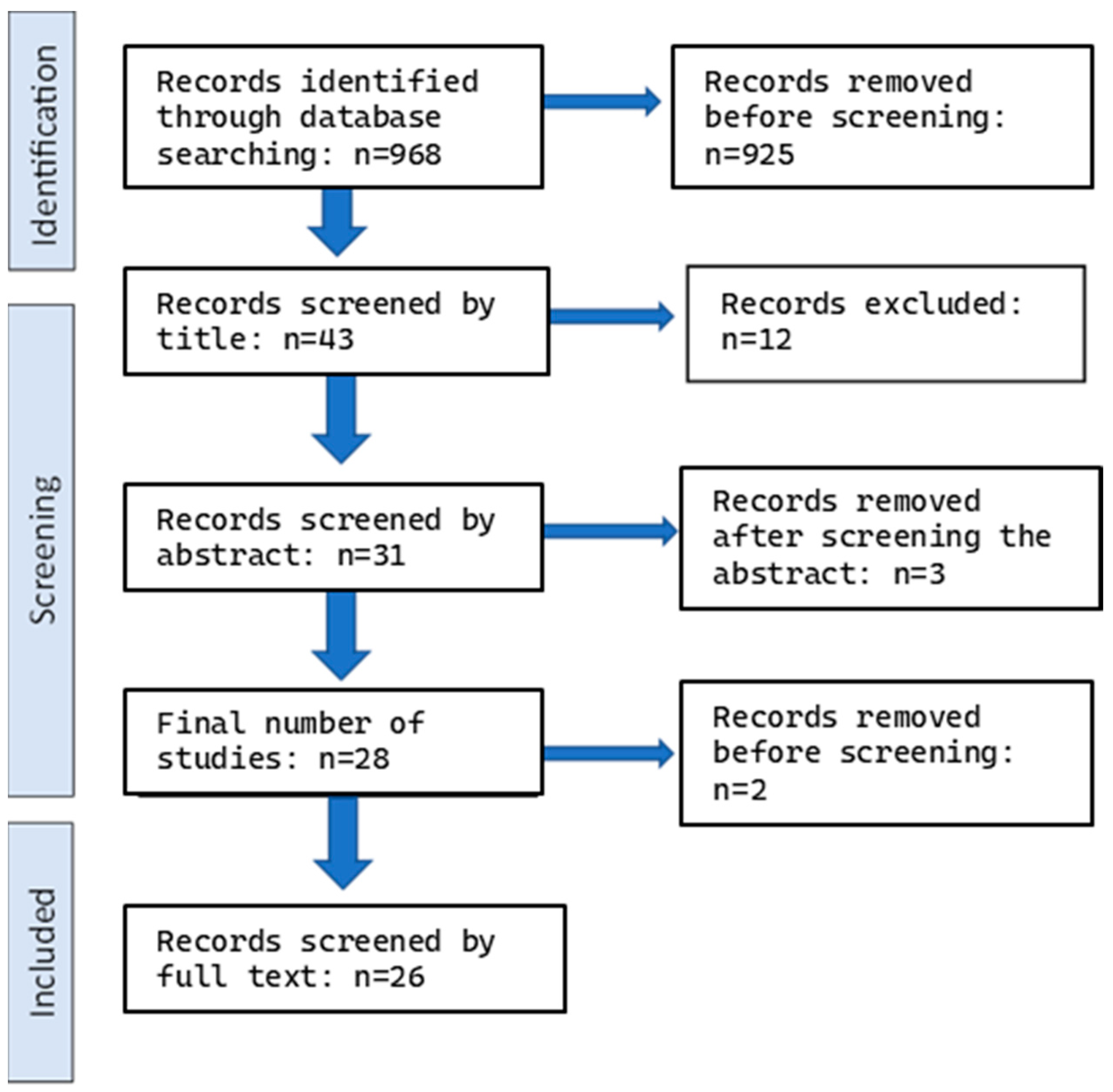
2.5. Screening and Eligibility Check
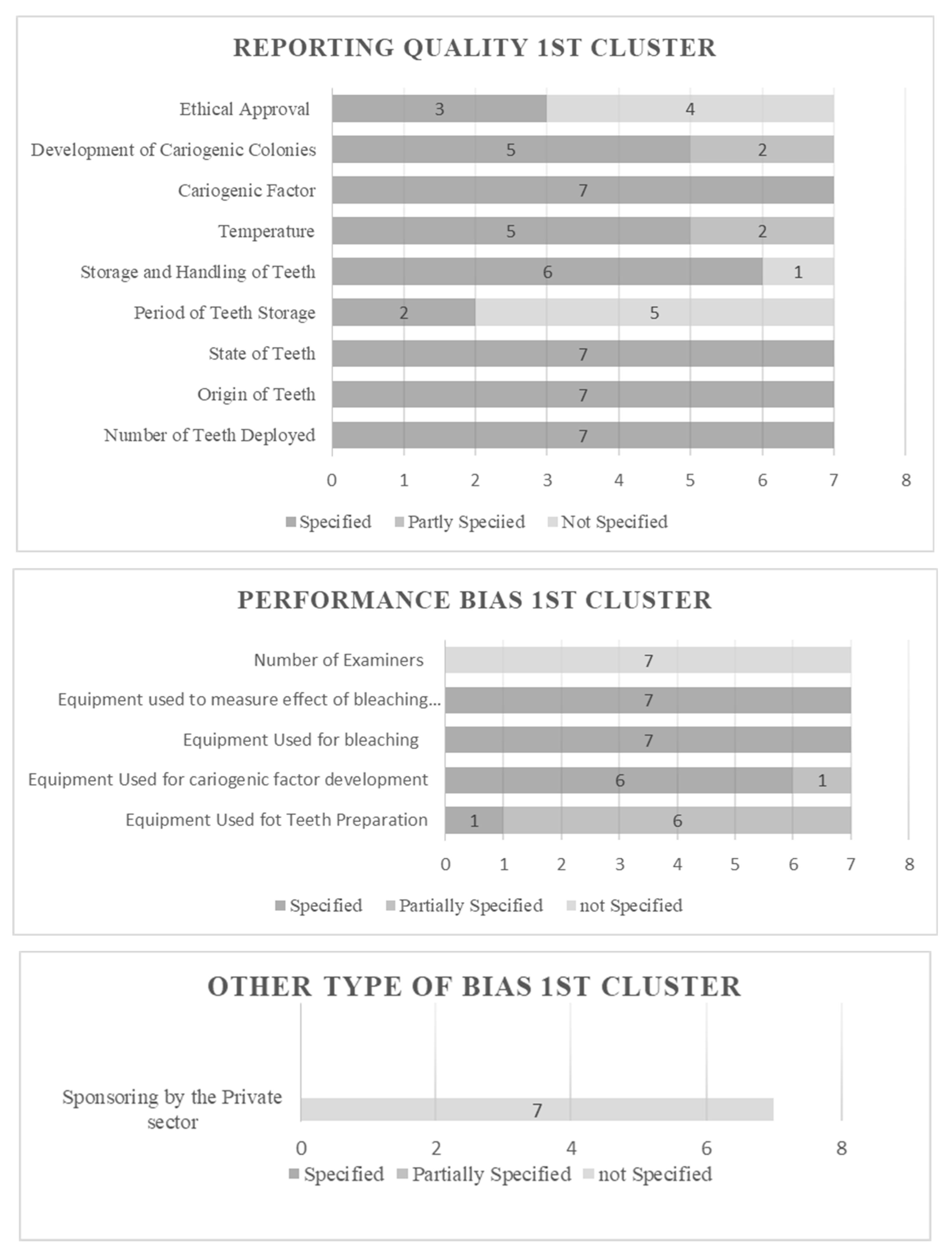
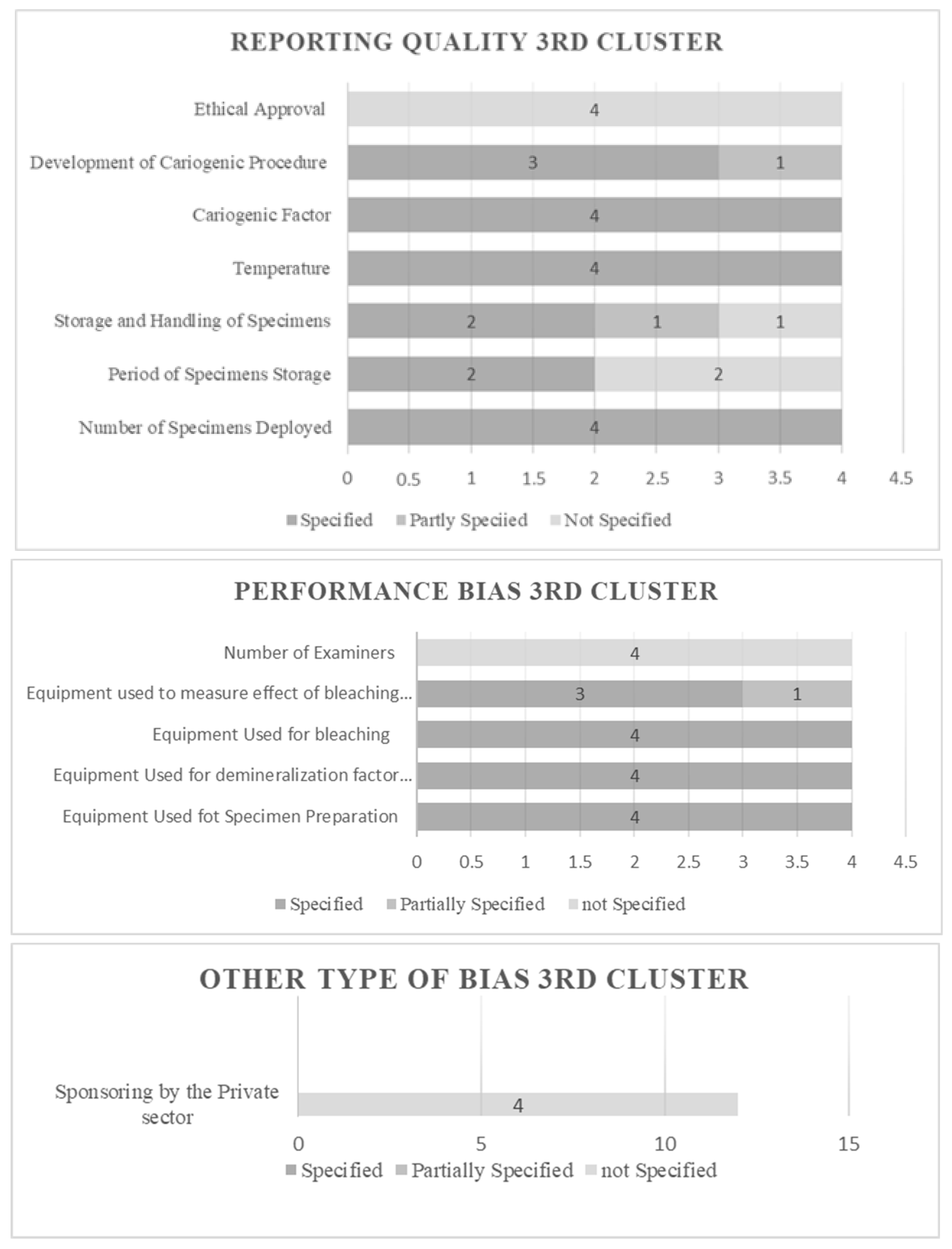
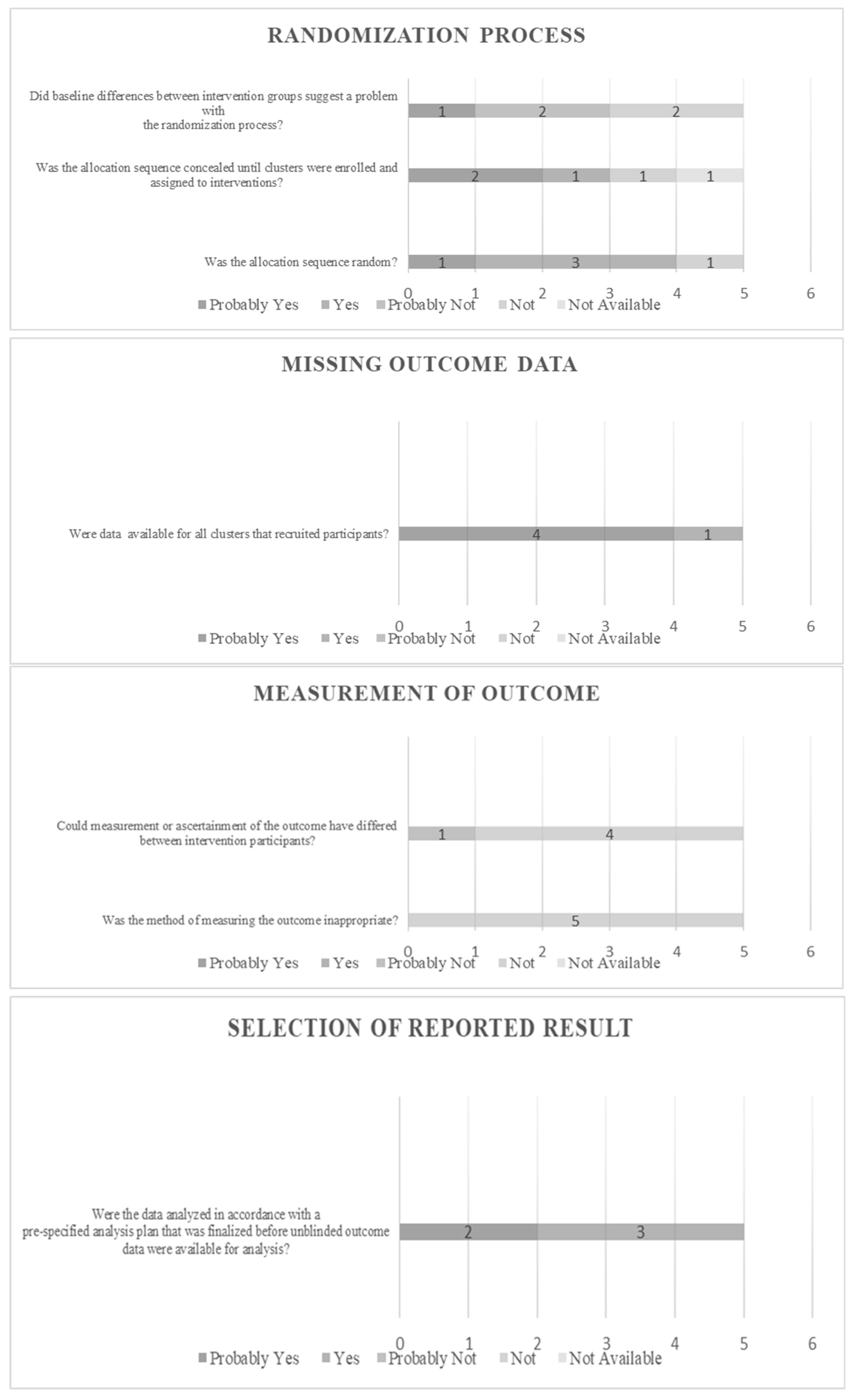
2.6. Risk of Bias Assessment (RoB)
3. Results
3.1. Results of Risk of Bias Assessment
3.2. Metanalysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kothari, S.; Gray, A.R.; Lyons, K.; Tan, X.W.; Brunton, P.A. Vital bleaching and oral-health-related quality of life in adults: A systematic review and meta-analysis. J. Dent. 2019, 84, 22–29. [Google Scholar] [CrossRef]
- Al-Zarea, B.K. Satisfaction with appearance and the desired treatment to improve aesthetics. Int. J. Dent. 2013, 2013, ID912368. [Google Scholar] [CrossRef] [PubMed]
- Fernández, E.; Bersezio, C.; Bottner, J.; Avalos, F.; Godoy, I.; Inda, D.; Vildósola, P.; Saad, J.; Oliveira, O.B., Jr.; Martín, J. Longevity, esthetic perception, and psychosocial impact of teeth bleaching by low (6%) hydrogen peroxide concentration for in-office treatment: A randomized clinical trial. Oper. Dent. 2017, 42, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Geus, J.L.; de Lara, M.B.; Hanzen, T.A.; Fernández, E.; Loguercio, A.D.; Kossatz, S.; Reis, A. One-year follow-up of at-home bleaching in smokers before and after dental prophylaxis. J. Dent. 2015, 43, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Moncada, G.; Sepúlveda, D.; Elphick, K.; Contente, M.; Estay, J.; Bahamondes, V.; Fernandez, E.; Oliveira, O.B.; Martin, J. Effects of light activation, agent concentration, and tooth thickness on dental sensitivity after bleaching. Oper. Dent. 2013, 38, 467–476. [Google Scholar] [CrossRef]
- Tay, L.Y.; Kose, C.; Loguercio, A.D.; Reis, A. Assessing the effect of a desensitizing agent used before in-office tooth bleaching. J. Am. Dent. Assoc. 2009, 140, 1245–1251. [Google Scholar] [CrossRef]
- Kina, J.F.; Huck, C.; Riehl, H.; Martinez, T.C.; Sacono, N.T.; Ribeiro, A.P.; Costa, C.A. Response of human pulps after professionally applied vital tooth bleaching. Int. Endod. J. 2010, 43, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Basso, F.G.; Hebling, J.; de Souza Costa, C.A. Concentrations of and application protocols for hydrogen peroxide bleaching gels: Effects on pulp cell viability and whitening efficacy. J. Dent. 2014, 42, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Soares, D.G.; Basso, F.G.; Pontes, E.C.; Garcia Lda, F.; Hebling, J.; de Souza Costa, C.A. Effective tooth-bleaching protocols capable of reducing H2O2 diffusion through enamel and dentine. J. Dent. 2014, 42, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Paula, E.A.; Kossatz, S.; Fernandes, D.; Loguercio, A.D.; Reis, A. Administration of ascorbic acid to prevent bleaching-induced tooth sensitivity: A randomized triple-blind clinical trial. Oper. Dent. 2014, 39, 128–135. [Google Scholar] [CrossRef]
- Rezende, M.; Loguercio, A.D.; Kossatz, S.; Reis, A. Predictive factors on the efficacy and risk /intensity of tooth sensitivity of dental bleaching: A multi regression and logistic analysis. J. Dent. 2016, 45, 1–6. [Google Scholar] [CrossRef] [PubMed]
- de Geus, J.L.; Wambier, L.M.; Kossatz, S.; Loguercio, A.D.; Reis, A. At-home vs In-office Bleaching: A Systematic Review and Meta-analysis. Oper. Dent. 2016, 41, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Maran, B.M.; Burey, A.; de Paris Matos, T.; Loguercio, A.D.; Reis, A. In-office dental bleaching with light vs. without light: A systematic review and meta-analysis. J. Dent. 2018, 70, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Maran, B.M.; Matos, T.P.; de Castro, A.D.S.; Vochikovski, L.; Amadori, A.L.; Loguercio, A.D.; Reis, A.; Berger, S.B. In-office bleaching with low/medium vs. high concentrate hydrogen peroxide: A systematic review and meta-analysis. J. Dent. 2020, 103, 103499. [Google Scholar] [CrossRef]
- Luque-Martinez, I.; Reis, A.; Schroeder, M.; Muñoz, M.A.; Loguercio, A.D.; Masterson, D.; Maia, L.C. Comparison of efficacy of tray-delivered carbamide and hydrogen peroxide for at-home bleaching: A systematic review and meta-analysis. Clin. Oral. Investig. 2016, 20, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Zanolla, J.; Marques, A.; da Costa, D.C.; de Souza, A.S.; Coutinho, M. Influence of tooth bleaching on dental enamel microhardness: A systematic review and meta-analysis. Aust. Dent. J. 2017, 62, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Höchli, D.; Hersberger-Zurfluh, M.; Papageorgiou, S.N.; Eliades, T. Interventions for orthodontically induced white spot lesions: A systematic review and meta-analysis. Eur. J. Orthod. 2017, 39, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Bourouni, S.; Dritsas, K.; Kloukos, D.; Wierichs, R.J. Efficacy of resin infiltration to mask post-orthodontic or non-post-orthodontic white spot lesions or fluorosis—A systematic review and meta-analysis. Clin. Oral. Investig. 2021, 25, 4711–4719. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Momoi, Y.; Fujitani, M.; Fukushima, M.; Imazato, S.; Kitasako, Y.; Kubo, S.; Nakashima, S.; Nikaido, T.; Shimizu, A.; et al. Evidence-based consensus for treating incipient enamel caries in adults by non-invasive methods: Recommendations by GRADE guideline. Jpn. Dent. Sci. Rev. 2020, 56, 155–163. [Google Scholar] [CrossRef]
- Attin, T.; Schmidlin, P.R.; Wegehaupt, F.; Wiegand, A. Influence of study design on the impact of bleaching agents on dental enamel microhardness. A Review. Dent. Mater. 2009, 25, 143–157. [Google Scholar] [CrossRef]
- Borges, B.C.; Borges, J.S.; de Melo, C.D.; Pinheiro, I.V.; Santos, A.J.; Braz, R.; Montes, M.A. Efficacy of a nivel at-home bleaching technique with carbamide peroxides modified by CPP-ACP and its effect on the microhardness of bleached enamel. Oper. Dent. 2011, 65, 521–528. [Google Scholar] [CrossRef] [PubMed]
- de Vasconcelos, A.A.; Cunha, A.G.; Borges, B.C.; Vitoriano Jde, O.; Alves-Júnior, C.; Machado, C.T.; dos Santos, A.J. Enamel properties after tooth bleaching with hydrogen carbamide peroxides in association with a CPP-ACP paste. Acta Odontolgoica Scand. 2012, 70, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Sánchez, I.; Oteo-Calatayud, J.; Serrano, J.; Martín, C.; Herrera, D. Changes in plaque and gingivitis levels after tooth bleaching: A systematic review. Int. J. Dent. Hyg. 2019, 17, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Review Manager (RevMan) [Computer Program], Version 5.4.1; The Nordic Cochrane Centre, The Cochrane Collaboration: Copenhagen, Denmark, 2020.
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Honda, K.; Iino, F.; Arai, T. Changes in enamel surface roughness and adhesion of Streptococcus mutans to enamel after vital bleaching. J. Dent. 2003, 31, 543–548. [Google Scholar] [CrossRef]
- Zhang, B.; Huo, S.; Liu, S.; Zou, L.; Cheng, L.; Zhou, X.; Li, M. Effects of Cold-Light Bleaching on Enamel Surface and Adhesion of Streptococcus mutans. Biomed. Res. Int. 2021, 2021, 3766641. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yuan, K.; Huang, Z.; Ma, R. Effects of Bleaching Associated with Er:YAG and Nd:YAG Laser on Enamel Structure and Bacterial Biofilm Formation. Scanning 2021, 20, 6400605. [Google Scholar] [CrossRef] [PubMed]
- Voina, C.; Delean, A.; Muresan, A.; Valeanu, M.; Mazilu Moldovan, A.; Popescu, V.; Petean, I.; Ene, R.; Moldovan, M.; Pandrea, S. Antimicrobial Activity and the Effect of Green Tea Experimental Gels on Teeth Surfaces. Coatings 2020, 10, 537. [Google Scholar] [CrossRef]
- Ittatirut, S.; Matangkasombut, O.; Thanyasrisung, P. In-office bleaching gel with 35% hydrogen peroxide enhanced biofilm formation of early colonizing streptococci on human enamel. J. Dent. 2014, 42, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Al-Qunaian, T.A. The effect of whitening agents on caries susceptibility of human enamel. Oper. Dent. 2005, 30, 265–270. [Google Scholar] [PubMed]
- Nam, S.H.; Ok, S.M.; Kim, G.C. Tooth bleaching with low-temperature plasma lowers surface roughness and Streptococcus mutans adhesion. Int. Endod. J. 2018, 51, 479–488. [Google Scholar] [CrossRef]
- Kim, Y.; Son, H.H.; Yi, K.; Ahn, J.S.; Chang, J. Bleaching Effects on Color, Chemical, and Mechanical Properties of White Spot Lesions. Oper. Dent. 2016, 41, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Al-Angari, S.S.; Lippert, F.; Platt, J.A.; Eckert, G.J.; González-Cabezas, C.; Li, Y.; Hara, A.T. Bleaching of simulated stained-remineralized caries lesions in vitro. Clin. Oral. Investig. 2019, 23, 1785–1792. [Google Scholar] [CrossRef] [PubMed]
- Briso, A.; Silva, Ú.; Souza, M.; Rahal, V.; Jardim Júnior, E.G.; Cintra, L. A clinical, randomized study on the influence of dental whitening on Streptococcus mutans population. Aust. Dent. J. 2018, 63, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.E.; Meyer-Lueckel, H.; Esteves-Oliveira, M.; Wierichs, R.J. Do bleaching gels affect the stability of the masking and caries-arresting effects of caries infiltration-in vitro. Clin. Oral. Investig. 2021, 25, 4011–4021. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.F.; Paes Leme, A.F.; Cavalli, V.; Giannini, M. Effect of 10% carbamide peroxide bleaching on sound and artificial enamel carious lesions. Braz. Dent. J. 2009, 20, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.B.; Pavan, S.; Dos Santos, P.H.; Giannini, M.; Bedran-Russo, A.K. Effect of bleaching on sound enamel and with early artificial caries lesions using confocal laser microscopy. Braz. Dent. J. 2012, 23, 110–115. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ogura, K.; Tanaka, R.; Shibata, Y.; Miyazaki, T.; Hisamitsu, H. In vitro demineralization of tooth enamel subjected to two whitening regimens. J. Am. Dent. Assoc. 2013, 144, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Okoye, L.O.; Lima, P.P.; Gakunga, P.T.; Amaechi, B.T. Investigation of the esthetic outcomes of white spot lesion treatments. Niger. J. Clin. Pract. 2020, 23, 1312–1317. [Google Scholar] [PubMed]
- Al-Angari, S.S.; Lippert, F.; Platt, J.A.; Eckert, G.J.; González-Cabezas, C.; Li, Y.; Hara, A.T. Dental bleaching efficacy and impact on demineralization susceptibility of simulated stained-remineralized caries lesions. J. Dent. 2019, 81, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.A.; Alves, F.K.; Campos Ede, J.; Mathias, P. Susceptibility to carieslike lesions after dental bleaching with different techniques. Quintessence Int. 2007, 38, e404–e409. [Google Scholar] [PubMed]
- Al-Shahrani, A.A.; Levon, J.A.; Hara, A.T.; Tang, Q.; Lippert, F. The ability of dual whitening anti-caries mouthrinses to remove extrinsic staining and enhance caries lesion remineralization—An in vitro study. J. Dent. 2020, 103S, 100022. [Google Scholar] [CrossRef] [PubMed]
- Pretty, I.A.; Edgar, W.M.; Higham, S.M. The effect of bleaching on enamel susceptibility to acid erosion and demineralisation. Br. Dent. J. 2005, 198, 285–290. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mor, C.; Steinberg, D.; Dogan, H.; Rotstein, I. Bacterial adherence to bleached surfaces of composite resin in vitro. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 1998, 86, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D.; Mor, C.; Dogan, H.; Zacks, B.; Rotstein, I. Effect of salivary biofilm on the adherence of oral bacteria to bleached and non-bleached restorative material. Dent. Mater. 1999, 15, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Wongpraparatana, I.; Matangkasombut, O.; Thanyasrisung, P.; Panich, M. Effect of Vital Tooth Bleaching on Surface Roughness and Streptococcal Biofilm Formation on Direct Tooth-Colored Restorative Materials. Oper. Dent. 2018, 43, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Gurgan, S.; Bolay, S.; Alaçam, R. Antibacterial activity of 10% carbamide peroxide bleaching agents. J. Endod. 1996, 22, 356–357. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.Y.; Pan, J.; Wang, L.; Zhang, C.F. Effects of hydrogen peroxide-containing bleaching on cariogenic bacteria and plaque accumulation. Chin. J. Dent. Res. 2011, 14, 47–52. [Google Scholar] [PubMed]
- Briso, A.L.; Gonçalves, R.S.; Costa, F.B.; Gallinari, M.d.e.O.; Cintra, L.T.; Santos, P.H. Demineralization and hydrogen peroxide penetration in teeth with incipient lesions. Braz. Dent. J. 2015, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Alkmin, Y.T.; Sartorelli, R.; Flório, F.M.; Basting, R.T. Comparative study of the effects of two bleaching agents on oral microbiota. Oper. Dent. 2005, 30, 417–423. [Google Scholar] [PubMed]
- Knösel, M.; Attin, R.; Becker, K.; Attin, T. External bleaching effect on the color and luminosity of inactive white-spot lesions after fixed orthodontic appliances. Angle Orthod. 2007, 77, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Franz-Montan, M.; Ramacciato, J.C.; Rodrigues, J.A.; Marchi, G.M.; Rosalen, P.L.; Groppo, F.C. The effect of combined bleaching techniques on oral microbiota. Indian J. Dent. Res. 2009, 20, 304–307. [Google Scholar] [PubMed]

| In Vitro | 23 | |
|---|---|---|
| Cluster 1 (Bacterial adherence on teeth) | 7 | |
| Cluster 2 (No bacteria involved) | 12 | |
| Cluster 3 (No teeth deployment) | 4 | |
| Clinical (Cluster 4) | 5 | |
| Total number of studies | 28 | |
| Study | Aim | Design | Sample Size and Basic Features | Findings | |
|---|---|---|---|---|---|
| 1. | “Changes in enamel surface roughness and adhesion of Streptococcus mutans to enamel after vital bleaching” Hosoya et al., 2003 [29] | To observe the influence of vital bleaching on the enamel surface and adhesion of S. mutans to tooth enamel. | In vitro |
| Teeth displayed increased adhesion of S. mutans colonies. |
| 2. | “Effects of Cold-Light Bleaching on Enamel Surface and Adhesion of Streptococcus mutans” Zhang et al., 2021 [30] | To investigate how cold-light bleaching influences enamel roughness and adhesion of S. mutans. | In vitro | 24 maxillary premolars, extracted for orthodontic purposes. |
|
| 3. | “Effects of Bleaching Associated with Er:YAG and Nd:YAG Laser on Enamel Structure and Bacterial Biofilm Formation” Hou et al., 2021 [31] | To compare the effects of bleaching associated with Er:YAG and Nd:YAG lasers on enamel structure and mixed biofilm formation on tooth surfaces. | In vitro | 68 enamel samples were prepared from sound permanent third molars. | The enamel surface structure significantly changed after bleaching both with and without laser treatment. |
| 4. | “Antimicrobial Activity and the Effect of Green Tea Experimental Gels on Teeth Surfaces” Voina et al., 2020 [32] | To evaluate an experimental green tea extract and an experimental green tea gel for enamel-restoring treatment after bleaching. To test the antibacterial and antifungal effect of the experimental extract against specific microorganisms. | In vitro | 28 healthy third molars, extracted for orthodontic purposes. |
|
| 5. | “In-office bleaching gel with 35% hydrogen peroxide enhanced biofilm formation of early colonizing streptococci on human enamel” Ittatirut et al., 2014 [33] | To compare the effects of 25% and 35% hydrogen peroxide in-office bleaching systems on surface roughness and streptococcal biofilm formation on human enamel. | In vitro | 162 enamel specimens were prepared from sound permanent anterior and premolar teeth. |
|
| 6. | “The Effect of Whitening Agents on Caries Susceptibility of Human Enamel” Al-Qunaian et al., 2005 [34] | Evaluated whether the treatment of human enamel with whitening agents containing different concentrations of carbamide or hydrogen peroxide changed the susceptibility of enamel to caries. | In vitro |
| Application of bleaching agents does not increase caries susceptibility of human enamel. Also, a bleaching agent-containing fluoride reduced caries susceptibility. |
| 7. | “Tooth bleaching with low temperature plasma lowers surface roughness and Streptococcus mutans adhesion” Nam et al., 2017 [35] | To evaluate the structural–morphological changes in enamel surface roughness and S. mutans adhesion after tooth bleaching with plasma activation in combination with a low concentration of 15% carbamide peroxide. | In vitro | 60 single-rooted human premolars with intact crowns extracted for orthodontic reasons. | The plasma activation did not cause changes in enamel. Combination methods using plasma and low concentrations of 15% carbamide peroxide caused even smaller changes in enamel. |
| Study | Aim | Design | Sample Size and Basic Features | Findings | |
|---|---|---|---|---|---|
| 1. | “Bleaching Effects on Color, Chemical, and Mechanical Properties of White Spot Lesions” Kim et al., 2016 [36] | To evaluate the effect of bleaching on teeth with white spot lesions. | In vitro | 20 human upper premolars extracted during dental treatments were used. | 10% carbamide peroxide of enamel with white spots minimized color disparities without altering mineral composition or microhardness. CPP–ACP remineralized the subsurface body lesion. |
| 2. | “Bleaching of simulated stained-remineralized caries lesions in vitro” Angari et al., 2018 [37] | To test the efficacy of bleaching on demineralized white spot lesion esthetic treatment. | In vitro | Enamel and dentin samples were sectioned from the buccal and lingual surfaces of human molars. | Stains that are nonmetallic respond better to bleaching. |
| 3. | “Demineralization and Hydrogen Peroxide Penetration in Teeth with Incipient Lesions” Briso et al., 2015 [38] | To evaluate the demineralization and hydrogen peroxide penetration in teeth with incipient lesions submitted to bleaching treatment. | In vitro | 100 sound permanent bovine incisors obtained from steers aged between 24 and 30 months were selected. | The bleaching treatment can increase the demineralization depth of incipient carious lesions. |
| 4. | “Do bleaching gels affect the stability of the masking and caries-arresting effects of caries infiltration—in vitro” Jansen et al., 2020 [39] | To evaluate the influence of different bleaching gels on the masking and arresting caries of infiltrated and non-infiltrated stained artificial enamel caries lesions. | In vitro | Bovine incisors were extracted from freshly slaughtered cattle (negative BSE test), cleaned, and preserved in 0.08% thymol. The teeth were separated in 400 enamel blocks. | The bleaching agents could improve the esthetic appearance of infiltrated and non-infiltrated stained enamel lesions. |
| 5. | “Effect of 10% Carbamide Peroxide Bleaching on Sound and Artificial Enamel Carious Lesions” Pinto et al., 2009 [40] | To evaluate the effect of 10% carbamide peroxide bleaching on Knoop surface microhardness and morphology of sound enamel and enamel with incipient caries after pH-cycling. | In vitro | 40 blocks with known surface microhardness were selected, made of extracted human third molars that had been erupted. | Carbamide peroxide bleaching demineralized sound enamel but not carious enamel. |
| 6. | “Effect of Bleaching on Sound Enamel and with Early Artificial Caries Lesions Using Confocal Laser Microscopy” Berger et al., 2012 [41] | To evaluate the effect of bleaching agents on sound enamel and incipient caries using confocal laser scanning microscopy. | In vitro | 20 extracted sound bovine incisors stored in 0.1% thymol solution, from which 80 (4 × 5 × 5 mm) samples of enamel were formed. | The bleaching treatments increased sound enamel demineralization, while the addition of Ca ions or ACP did not cause any changes. Incipient caries were not affected. |
| 7. | “In vitro demineralization of tooth enamel subjected to two whitening regimens” Ogura et al., 2013 [42] | To examine the level of demineralization of human tooth enamel after home or in-office bleaching with photo activation. | In vitro | 30 human premolars without stain or defect, extracted for orthodontic purposes. | In-office was favorable, according to demineralization. |
| 8. | “Investigation of the Esthetic Outcomes of White Spot Lesion Treatments” Lee 2022 [43] | To compare the ability of bleaching, resin infiltration, and microabrasion to mask existing white spot lesions (WSL). | In vitro | Extracted human maxillary incisor teeth—only teeth with WSLs were selected. | Among the three investigated treatment modalities, resin infiltration was most able to mask WSLs. |
| 9. | “Dental bleaching efficacy and impact on demineralization susceptibility of simulated stained-remineralized caries lesions” Al-Angari et al., 2018 [44] | To evaluate the color improvement of stained remineralized carious lesions with different bleaching systems and to evaluate the susceptibility of the bleached lesions to further demineralization. | In vitro | Human enamel samples (n = 21 per group). | At-home bleaching (15% CP) results in better esthetic results. |
| 10. | “Susceptibility to caries like lesions after dental bleaching with different techniques” Alves et al., 2007 [45] | To evaluate the effect of dental bleaching on the susceptibility of developing caries-like lesions. | In vitro | 30 unerupted human third molars. |
|
| 11. | “The ability of dual whitening anti-caries mouthrinses to remove extrinsic staining and enhance caries lesion remineralization—An in vitro study” Al-Shahrani et al., 2020 [46] | To investigate the ability of these mouthrinses to remove staining from artificially stained carious lesions and to improve remineralization. | In vitro |
| Artificially stained enamel caries lesions show reduced susceptibility to fluoride remineralization and whitening effects of commercial whitening and anti-caries mouthrinses. |
| 12. | “The effect of bleaching on enamel susceptibility to acid erosion and demineralization“ Pretty et al., 2005 [47] | To determine if bleaching with carbamide peroxide increases the risk of erosion or demineralization. | In vitro | 24 human incisors selected with specified criteria. | Tooth bleaching with carbamide (urea) peroxide (using commercially available concentrations) does not increase risk of acid erosion or caries. |
| Study | Aim | Design | Sample Size and Basic Features | Findings | |
|---|---|---|---|---|---|
| 1. | “Bacterial adherence to bleached surfaces of composite resin in vitro” Mor et al., 1998 [48] | To examine the effect of bleaching on bacterial adherence to composite resin restorations. | In vitro | 162 samples of Polofil Supra light-curing resin-based composite (Voco, Cuxhaven, Germany). | Carbamide peroxide and hydrogen peroxide can alter the adherence of microorganisms to polished surfaces of composite resin restorations. |
| 2. | “Effect of salivary biofilm on the adherence of oral bacteria to bleached and non-bleached restorative material” Steinberg et al., 1999 [49] | To examine the effect of in vitro biofilm on adherence of microorganisms on bleached and non-bleached restorative materials. | In vitro | 162 samples of Charisma Supra-light-curing resin-based composite. | Salivary biofilm on bleached restorations altered the adhesion of S. mutans, S. sobrinus, and A. viscosus. |
| 3. | “Effect of Vital Tooth Bleaching on Surface Roughness and Streptococcal Biofilm Formation on Direct Tooth-Colored Restorative Materials” Wongpraparatana et al., 2017 [50] | To compare the effect of bleaching with a 10% carbamide peroxide or a 40% hydrogen peroxide system on surface roughness of resin composite and resin-modified glass ionomer cement (RMGI) and the formation of biofilm. | In vitro | 2 restorative materials (a nanofilled resin composite material and an RMGI). 108 samples of each material in shade A2 were formed as disks of 5 mm in diameter and 2 mm thickness. | The bleaching systems used, 10% CP or 40% HP, significantly increased both the surface roughness and the streptococcal biofilm formation on resin composite and resin-modified glass ionomer cement. |
| 4. | “Antibacterial Activity of 10% Carbamide Peroxide Bleaching Agents” Gurgan et al., 1996 [51] | To examine the antibacterial properties of 3 carbamide peroxide bleaching agents (Nite White, Karisma, and Opalescence) on S. mutans, S. mitis, S. sanguis, Lactobacillus casei, and Lactobacillus acidophilus. | In vitro | S. mutans (type A, 10,919), S. mitis (type A, 4a), S. sanguis (type A, 6b), Lactobacillus casei (type 319), and Lactobacillus acidophilus (type A, 161). | All bleaching materials showed significant antibacterial effect when compared to 0.2% chlorhexidine solution. |
| Study | Aim | Design | Sample Size and Basic Features | Findings | |
|---|---|---|---|---|---|
| 1. | “Effects of Hydrogen Peroxide-Containing Bleaching on Cariogenic Bacteria and Plaque Accumulation” Zheng et al., 2011 [52] | To evaluate the effects of a bleaching agent containing 36% hydrogen peroxide on the plaque index and S. mutans and Lactobacilli counts in dental plaque and saliva. | In vivo/clinical | 20 medically fit adult volunteers. | S. mutans decreased significantly in plaque and saliva 4 weeks after bleaching. |
| 2. | “A clinical, randomized study on the influence of dental whitening on Streptococcus mutans population” Briso et al., 2017 [53] | To evaluate the influence of home bleaching on S. mutans counts in saliva, buccal mucosa, and plaque. | Clinical/in vivo | 30 individuals were selected, meeting the criteria for inclusion. | No change was observed when whitening was performed with carbamide peroxide. |
| 3. | “Comparative Study of the Effects of Two Bleaching Agents on Oral Microbiota” Alkmin et al., 2005 [54] | To evaluate the effects of bleaching with 10% carbamide peroxide (Platinum/Colgate) or 7.5% hydrogen peroxide (Day White 2Z/Discus Dental) on S. mutans. | In vivo | 30 volunteers who needed dental bleaching. | No changes were found in the S. mutans counts during bleaching treatment. |
| 4. | “External Bleaching Effect on the Color and Luminosity of Inactive White-Spot Lesions after Fixed Orthodontic Appliances” Knosel et al., 2007 [55] | To evaluate the effect of bleaching on the color and luminosity of inactive white spot lesions (WSLs) after orthodontic treatment. | In vivo/clinical | 10 patients with WSLs after orthodontic treatment. | External bleaching can satisfactorily mask WSLs. |
| 5. | “The effect of combined bleaching techniques on oral Microbiota” Franz-Montan et al., 2009 [56] | To evaluate the antimicrobial activity of 10% and 37% carbamide peroxide during 3 different techniques of dental bleaching. | In vivo | 32 patients assigned to 4 groups. | Carbamide peroxide when used at 37%, 10%, or in combination does not affect salivary microorganisms. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkavela, G.; Kakouris, V.; Pappa, E.; Rahiotis, C. Effect of Bleaching Agents on Healthy Enamel, White Spots, and Carious Lesions: A Systematic Review and Meta-Analysis. Dent. J. 2024, 12, 140. https://doi.org/10.3390/dj12050140
Gkavela G, Kakouris V, Pappa E, Rahiotis C. Effect of Bleaching Agents on Healthy Enamel, White Spots, and Carious Lesions: A Systematic Review and Meta-Analysis. Dentistry Journal. 2024; 12(5):140. https://doi.org/10.3390/dj12050140
Chicago/Turabian StyleGkavela, Grigoria, Vlassios Kakouris, Eftychia Pappa, and Christos Rahiotis. 2024. "Effect of Bleaching Agents on Healthy Enamel, White Spots, and Carious Lesions: A Systematic Review and Meta-Analysis" Dentistry Journal 12, no. 5: 140. https://doi.org/10.3390/dj12050140
APA StyleGkavela, G., Kakouris, V., Pappa, E., & Rahiotis, C. (2024). Effect of Bleaching Agents on Healthy Enamel, White Spots, and Carious Lesions: A Systematic Review and Meta-Analysis. Dentistry Journal, 12(5), 140. https://doi.org/10.3390/dj12050140









