Effect of Dentifrice Ingredients on Volume and Vitality of a Simulated Periodontal Multispecies Biofilm
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Size Calculation
2.2. Biofilm Preparation
2.3. Exposition to Non-Contact Brushing
2.4. Staining and Microscopical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2023, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Van der Weijden, F.; Doerfer, C.; Herrera, D.; Shapira, L.; Polak, D.; Madianos, P.; Louropoulou, A.; Machtei, E.; Donos, N.; et al. Primary prevention of periodontitis: Managing gingivitis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, F.A.; Slot, D.E. Efficacy of homecare regimens for mechanical plaque removal in managing gingivitis a meta review. J. Clin. Periodontol. 2015, 42, S77–S91. [Google Scholar] [CrossRef]
- Yaacob, M.; Worthington, H.V.; Deacon, S.A.; Deery, C.; Walmsley, A.D.; Robinson, P.G.; Glenny, A.M. Powered versus manual toothbrushing for oral health. Cochrane Database Syst. Rev. 2014, 6, CD002281. [Google Scholar] [CrossRef]
- Sharma, P.K.; Gibcus, M.J.; van der Mei, H.C.; Busscher, H.J. Influence of fluid shear and microbubbles on bacterial detachment from a surface. Appl. Environ. Microbiol. 2005, 71, 3668–3673. [Google Scholar] [CrossRef] [PubMed]
- Parini, M.R.; Pitt, W.G. Removal of oral biofilms by bubbles: The effect of bubble impingement angle and sonic waves. J. Am. Dent. Assoc. 2005, 136, 1688–1693. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Jager, D.; Finger, G.; Schaefer, N.; van der Mei, H.C. Energy transfer, volumetric expansion, and removal of oral biofilms by non-contact brushing. Eur. J. Oral Sci. 2010, 118, 177–182. [Google Scholar] [CrossRef]
- Pitt, W.G. Removal of oral biofilm by sonic phenomena. Am. J. Dent. 2005, 18, 345–352. [Google Scholar]
- Schmidt, J.C.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Schmidt, J.P.; Waltimo, T.; Weiger, R.; Walter, C. Efficacy of various side-to-side toothbrushes for noncontact biofilm removal. Clin. Oral Investig. 2014, 18, 793–800. [Google Scholar] [CrossRef]
- Schmidt, J.C.; Astasov-Frauenhoffer, M.; Waltimo, T.; Weiger, R.; Walter, C. Efficacy of various side-to-side toothbrushes and impact of brushing parameters on noncontact biofilm removal in an interdental space model. Clin. Oral Investig. 2017, 21, 1565–1577. [Google Scholar] [CrossRef]
- Schmidt, J.C.; Astasov-Frauenhoffer, M.; Waltimo, T.; Weiger, R.; Walter, C. Influence of the amplitude of different side-to-side toothbrushes on noncontact biofilm removal. Clin. Oral Investig. 2019, 23, 1951–1957. [Google Scholar] [CrossRef]
- Stanford, C.M.; Srikantha, R.; Kirchner, H.L.; Wu, C.D. Removal of supragingival plaque in an intraoral model by use of the Sonicare toothbrush. J. Int. Acad. Periodontol. 2000, 2, 115–119. [Google Scholar]
- Hope, C.K.; Wilson, M. Effects of dynamic fluid activity from an electric toothbrush on in vitro oral biofilms. J. Clin. Periodontol. 2003, 30, 624–629. [Google Scholar] [CrossRef]
- Roberts, F.A.; Hacker, B.M.; Oswald, T.K.; Mourad, P.D.; McInnes, C. Evaluation of the use of ultrasound within a power toothbrush to dislodge oral bacteria using an in vitro Streptococcus mutans biofilm model. Am. J. Dent. 2010, 23, 65–69. [Google Scholar]
- Adams, H.; Winston, M.T.; Heersink, J.; Buckingham-Meyer, K.A.; Costerton, J.W.; Stoodley, P. Development of a laboratory model to assess the removal of biofilm from interproximal spaces by powered tooth brushing. Am. J. Dent. 2002, 15, 12b–17b. [Google Scholar]
- Heersink, J.; Costerton, W.J.; Stoodley, P. Influence of the Sonicare toothbrush on the structure and thickness of laboratory grown Streptococcus mutans biofilms. Am. J. Dent. 2003, 16, 79–83. [Google Scholar]
- Hope, C.K.; Petrie, A.; Wilson, M. In Vitro Assessment of the Plaque-Removing Ability of Hydrodynamic Shear Forces Produced Beyond the Bristles by 2 Electric Toothbrushes. J. Periodontol. 2003, 74, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Magaz, V.; Llovera, B.; Martí, M.; Garre Contreras, A. Clinical Impact and Cosmetic Acceptability of Chlorhexidineenriched Toothpaste and Mouthwash Application on Periodontal Disease: A Randomized Clinical Study. J. Contemp. Dent. 2018, 19, 1295–1300. [Google Scholar] [CrossRef]
- Madrazo-Jiménez, M.; Rodríguez-Caballero, Á.; Serrera-Figallo, M.; Garrido-Serrano, R.; Gutiérrez-Corrales, A.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. The effects of a topical gel containing chitosan, 0.2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e696–e702. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sáez-Alcaide, L.M.; Molinero-Mourelle, P.; González-Serrano, J.; Rubio-Alonso, L.; Bornstein, M.M.; López-Quiles, J. Efficacy of a topical gel containing chitosan, chlorhexidine, allantoin and dexpanthenol for pain and inflammation control after third molar surgery: A randomized and placebo-controlled clinical trial. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e644–e651. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.; Marquardt, Y.; Huth, L.; Schmitt, L.; Prescher, K.; Winterhalder, P.; Steiner, T.; Hölzle, F.; Eble, M.; Malte Baron, J. Molecular effects of photon irradiation and subsequent aftercare treatment with dexpanthenol-containing ointment or liquid in 3D models of human skin and non-keratinized oral mucosa. Exp. Dermatol. 2021, 30, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, B.; Rashidipour, M.; Marzban, A.; Soroush, S.; Azadpour, M.; Delfani, S.; Ramak, P. Mentha piperita essential oils loaded in a chitosan nanogel with inhibitory effect on biofilm formation against S. mutans on the dental surface. Carbohydr. Polym. 2019, 212, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Shaheena, S.; Chintagunta, A.D.; Dirisala, V.R.; Sampath Kumar, N.S. Extraction of bioactive compounds from Psidium guajava and their application in dentistry. AMB Express 2019, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Schönknecht, K.; Surdacka, A.; Rudenko, L. Effectiveness of composed herbal extract in the treatment of gingivitis and oral and pharyngeal mucosa—A review of studies. Wiad. Lek. 2021, 74, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Sadrnia, M.; Arjomandzadegan, M.; Mohajerani, H.R. In vitro and in vivo evaluation of antibacterial and anti-biofilm properties of five ethnomedicinal plants against oral bacteria by TEM. Avicenna J. Phytomed 2021, 11, 180–189. [Google Scholar] [PubMed]
- Cvikl, B.; Lussi, A.; Gruber, R. The in vitro impact of toothpaste extracts on cell viability. Eur. J. Oral Sci. 2015, 123, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cvikl, B.; Lussi, A.; Moritz, A.; Gruber, R. Dentifrices for children differentially affect cell viability in vitro. Clin. Oral Investig. 2017, 21, 453–461. [Google Scholar] [CrossRef]
- Almohefer, S.A.; Levon, J.A.; Gregory, R.L.; Eckert, G.J.; Lippert, F. Caries lesion remineralization with fluoride toothpastes and chlorhexidine—Effects of application timing and toothpaste surfactant. J. Appl. Oral Sci. 2018, 26, e20170499. [Google Scholar] [CrossRef]
- Allan, I.; Newman, H.; Wilson, M. Antibacterial activity of particulate Bioglass® against supra- and subgingival bacteria. Biomaterials 2001, 22, 1683–1687. [Google Scholar] [CrossRef] [PubMed]
- Yotis, W.W.; Zeb, M.; Brennan, P.C.; Kirchner, F.R.; Glendenin, L.E.; Wu-Yuan, C.D. The action of selected agents on the accumulation of 18F by Streptococcus mutans. Microbios 1983, 36, 21–32. [Google Scholar] [PubMed]
- Schmidt, J.C.; Astasov-Frauenhoffer, M.; Waltimo, T.; Weiger, R.; Walter, C. Influence of the oscillation frequency of different side-to-side toothbrushes on noncontact biofilm removal. Clin. Oral Investig. 2018, 22, 2141–2147. [Google Scholar] [CrossRef] [PubMed]
- Astasov-Frauenhoffer, M.; Braissant, O.; Hauser-Gerspach, I.; Daniels, A.U.; Weiger, R.; Waltimo, T. Isothermal microcalorimetry provides new insights into biofilm variability and dynamics. FEMS Microbiol. Lett. 2012, 337, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Weiger, R.; Decker, E.M.; Krastl, G.; Brecx, M. Deposition and retention of vital and dead Streptococcus sanguinis cells on glass surfaces in a flow-chamber system. Arch. Oral Biol. 1999, 44, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.M.; Maier, G.; Axmann, D.; Brecx, M.; von Ohle, C. Effect of xylitol/chlorhexidine versus xylitol or chlorhexidine as single rinses on initial biofilm formation of cariogenic streptococci. Quintessence Int. 2008, 39, 17–22. [Google Scholar] [PubMed]
- Decker, E.M.; Weiger, R.; von Ohle, C.; Wiech, I.; Brecx, M. Susceptibility of planktonic versus attached Streptococcus sanguinis cells to chlorhexidine. Clin. Oral Investig. 2003, 7, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Hauser-Gerspach, I.; Kulik, E.M.; Weiger, R.; Decker, E.M.; Von Ohle, C.; Meyer, J. Adhesion of Streptococcus sanguinis to dental implant and restorative materials in vitro. Dent. Mater. J. 2007, 26, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.M.; Weiger, R.; Wiech, I.; Heide, P.E.; Brecx, M. Comparison of antiadhesive and antibacterial effects of antiseptics on Streptococcus sanguinis. Eur. J. Oral Sci. 2003, 111, 144–148. [Google Scholar] [CrossRef]
- Cho, S.B.; Nakanishi, K.; Kokubo, T.; Soga, N.; Ohtsuki, C.; Nakamura, T.; Kitsugi, T.; Yamamuro, T. Dependence of apatite formation on silica gel on its structure: Effect of heat treatment. J. Am. Ceram. Soc. 1995, 78, 1769–1774. [Google Scholar] [CrossRef]
- Tobler, D.; Braissant, O.; Waltimo, T.; Bornstein, M.M.; Astasov-Frauenhoffer, M. Stannous Source in Toothpastes Leads to Differences in Their Antimicrobial Efficacy. Oral Health Prev. Dent. 2023, 21, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, C.; Slot, D.E.; Bakker, E.W.P.; Van der Weijden, F.A. Does dentifrice use help to remove plaque? A systematic review. J. Clin. Periodontol. 2016, 43, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Sälzer, S.; Slot, D.; Dörfer, C.; Van der Weijden, G. Comparison of triclosan and stannous fluoride dentifrices on parameters of gingival inflammation and plaque scores: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2015, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Rajula, M.; Rao, K.; Ravishankar, P.; Albar, D.; Bahammam, M.; Alamoudi, A.; Alzahrani, K.; Alsharif, K.; Halawani, I.; et al. Antimicrobial Efficacy of Blended Essential Oil and Chlorhexidine against Periodontal Pathogen (P. gingivalis)—An In Vitro Study. Niger. J. Clin. Pract. 2023, 26, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Lea, S.C.; Khan, A.; Patanwala, H.S.; Landini, G.; Walmsley, A.D. The effects of load and toothpaste on powered toothbrush vibrations. J. Dent. 2007, 35, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Difloe-Geisert, J.; Astasov-Frauenhoffer, M.; Waltimo, T.; Weiger, R.; Walter, C. Impact of toothpaste slurry on noncontact biofilm removal—Preliminary results. In Proceedings of the 48. Jahrestagung der SSP, Baden, Switzerland, 19 September 2019. [Google Scholar]
- Hu, H.; Feng, C.; Jiang, Z.; Wang, L.; Shrestha, S.; Yan, J.; Shu, Y.; Ge, L.; Lai, W.; Hua, F.; et al. Effectiveness of remineralizing agents in the prevention and reversal of orthodontically induced white spot lesions: A systematic review and network meta-analysis. Clin. Oral Investig. 2020, 24, 4153–4167. [Google Scholar] [CrossRef]
- Pollard, A.J.; Khan, I.; Davies, M.; Claydon, N.; West, N.X. Comparative efficacy of self-administered dentifrices for the management of dentine hypersensitivity—A systematic review and network meta-analysis. J. Dent. 2023, 130, 104433. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, R.F.; Caneppele, T.M.F.; Scaramucci, T.; El Dib, R.; Maia, L.C.; Ferreira, D.; Borges, A.B. Protective effect of fluorides on erosion and erosion/abrasion in enamel: A systematic review and meta-analysis of randomized in situ trials. Arch. Oral Biol. 2020, 120, 104945. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.T.; Dos Reis, A.C.; da Costa Valente, M.L. Efficacy of Antimicrobial Agents in Dentifrices: A Systematic Review. Antibiotics 2022, 11, 1413. [Google Scholar] [CrossRef]
- Valkenburg, C.; Else Slot, D.; Van der Weijden, G.F. What is the effect of active ingredients in dentifrice on inhibiting the regrowth of overnight plaque? A systematic review. Int. J. Dent. Hyg. 2020, 18, 128–141. [Google Scholar] [CrossRef]
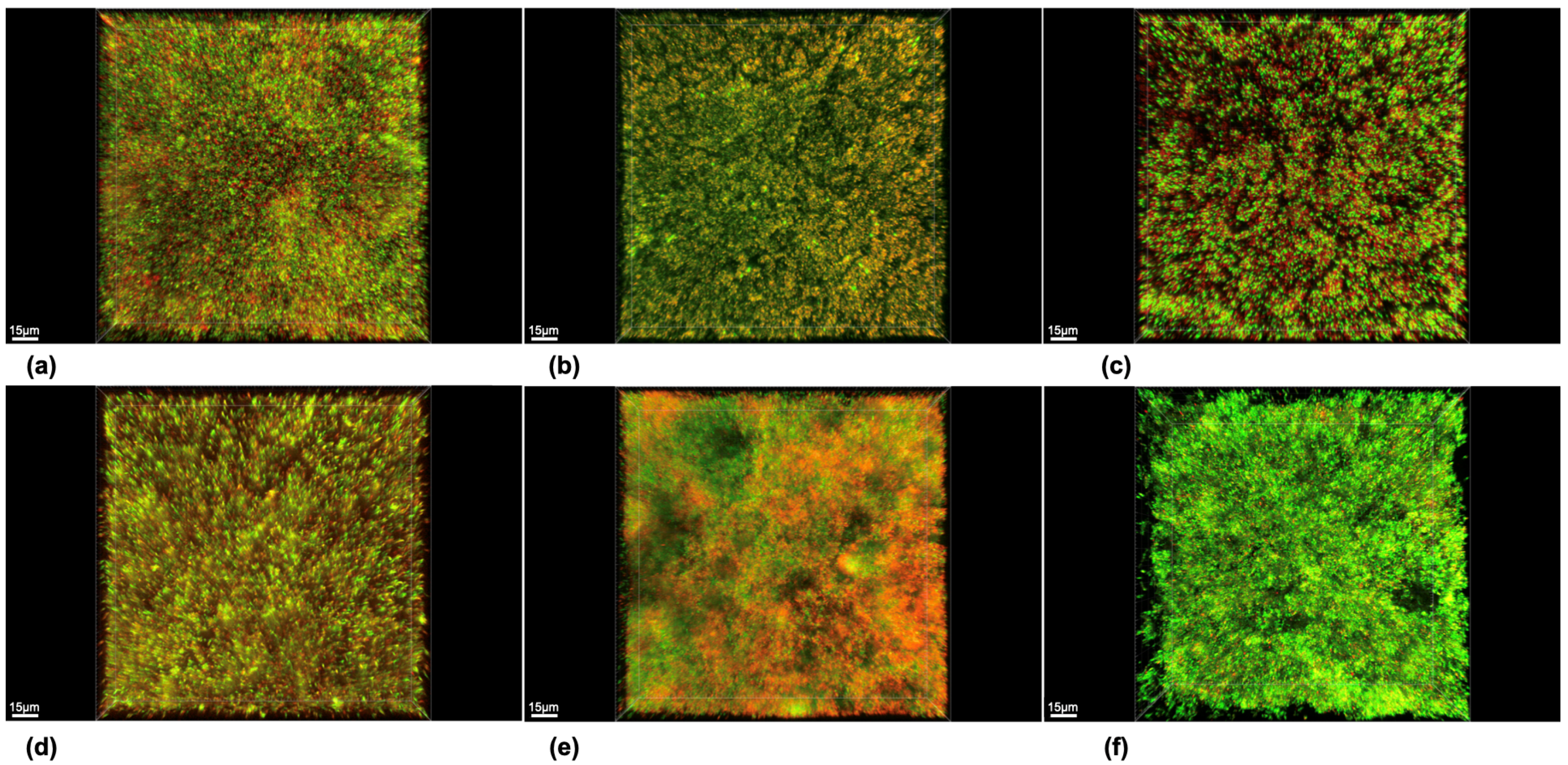
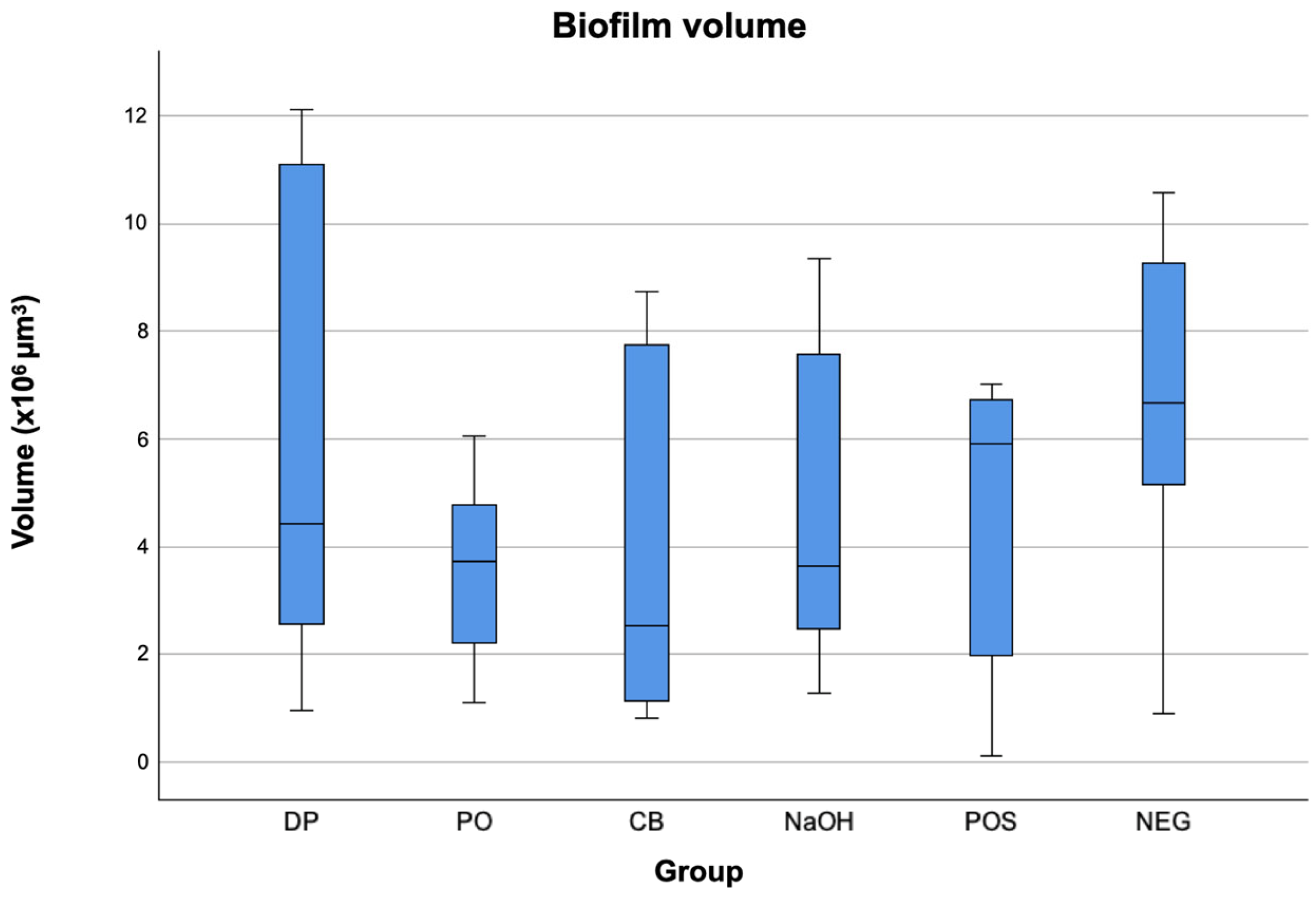
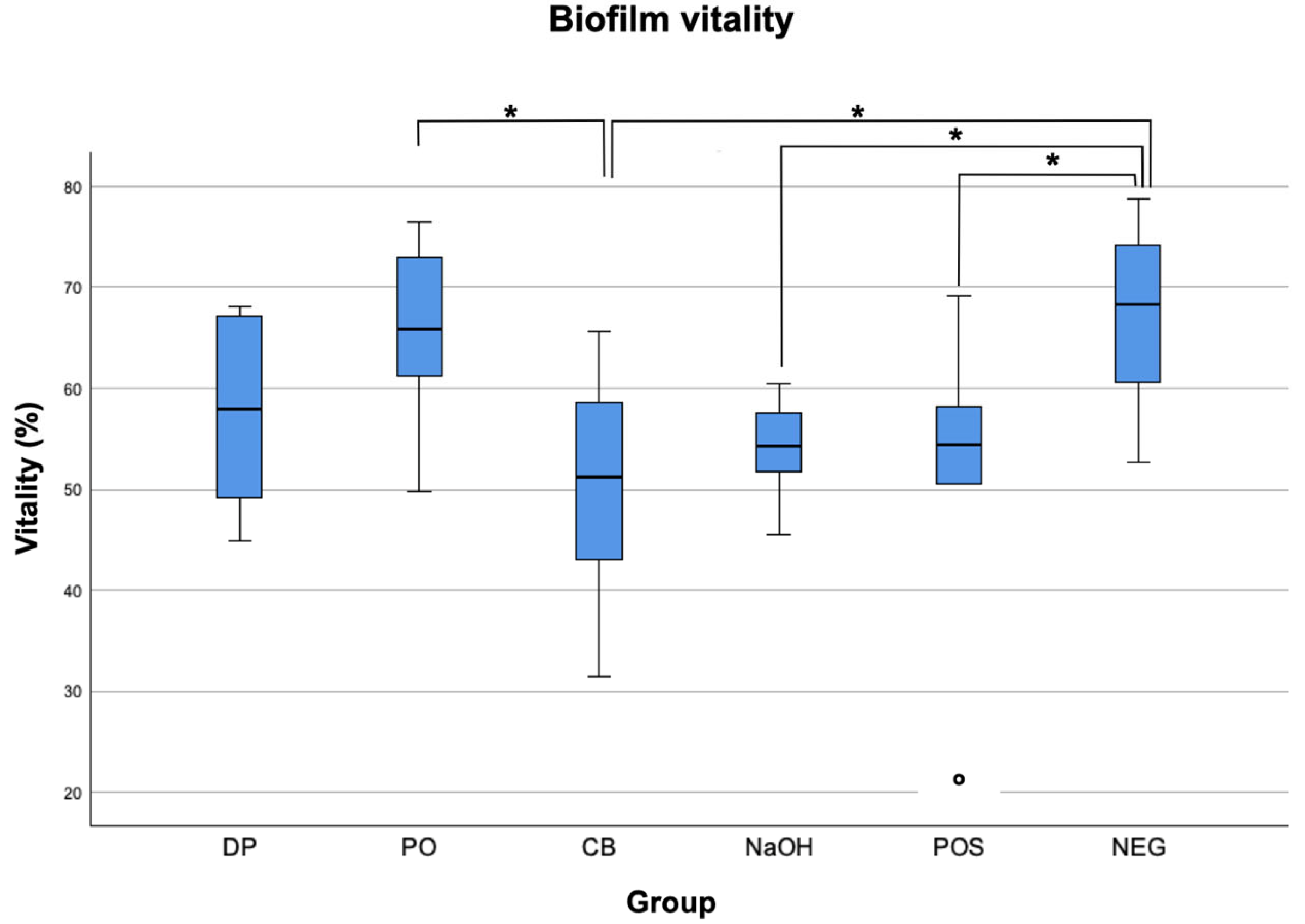
| Group | Biofilm Volume (×105 µm3) | Biofilm Vitality (%) | ||
|---|---|---|---|---|
| Medians | IQRs | Medians | IQRs | |
| DP 1 | 4.4 a | 9.2 | 58.00 a | 19.37 |
| PO 2 | 3.7 a | 3.2 | 65.91 ab | 15.51 |
| CB 3 | 2.5 a | 6.9 | 51.27 ac | 20.22 |
| NaOH 4 | 3.6 a | 5.8 | 54.35 abcd | 8.06 |
| POS 5 | 5.9 a | 5.3 | 54.40 abcde | 17.63 |
| NEG 6 | 6.6 a | 5.5 | 68.24 abf | 16.69 |
| DP 1 | PO 2 | CB 3 | NaOH 4 | POS 5 | NEG 6 | |
|---|---|---|---|---|---|---|
| DP | p = 0.171 | p = 0.443 | p = 0.661 | p = 0.701 | p = 0.089 | |
| PO | p = 0.171 | p = 0.033 | p = 0.071 | p = 0.08 | p = 0.742 | |
| CB | p = 0.443 | p = 0.033 | p = 0.742 | p = 0.701 | p = 0.014 | |
| NaOH | p = 0.661 | p = 0.071 | p = 0.742 | p = 0.956 | p = 0.033 | |
| POS | p = 0.701 | p = 0.08 | p = 0.701 | p = 0.956 | p = 0.037 | |
| NEG | p = 0.089 | p = 0.742 | p = 0.014 | p = 0.033 | p = 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karacic, J.; Ruf, M.; Herzog, J.; Astasov-Frauenhoffer, M.; Sahrmann, P. Effect of Dentifrice Ingredients on Volume and Vitality of a Simulated Periodontal Multispecies Biofilm. Dent. J. 2024, 12, 141. https://doi.org/10.3390/dj12050141
Karacic J, Ruf M, Herzog J, Astasov-Frauenhoffer M, Sahrmann P. Effect of Dentifrice Ingredients on Volume and Vitality of a Simulated Periodontal Multispecies Biofilm. Dentistry Journal. 2024; 12(5):141. https://doi.org/10.3390/dj12050141
Chicago/Turabian StyleKaracic, Jelena, Moritz Ruf, Johannes Herzog, Monika Astasov-Frauenhoffer, and Philipp Sahrmann. 2024. "Effect of Dentifrice Ingredients on Volume and Vitality of a Simulated Periodontal Multispecies Biofilm" Dentistry Journal 12, no. 5: 141. https://doi.org/10.3390/dj12050141
APA StyleKaracic, J., Ruf, M., Herzog, J., Astasov-Frauenhoffer, M., & Sahrmann, P. (2024). Effect of Dentifrice Ingredients on Volume and Vitality of a Simulated Periodontal Multispecies Biofilm. Dentistry Journal, 12(5), 141. https://doi.org/10.3390/dj12050141






